文章信息
- 吴楚, 范志强, 王政权.
- Wu Chu, Fan Zhiqiang, Wang Zhengquan.
- 氮磷供应状态对水曲柳幼苗氮磷吸收与生长的影响
- Influences of Nitrogen and Phosphorus Supply on Their Absorption and Growth of Fraxinus mandshurica Seedlings
- 林业科学, 2005, 41(5): 196-200.
- Scientia Silvae Sinicae, 2005, 41(5): 196-200.
-
文章历史
- 收稿日期:2004-01-06
-
作者相关文章
2. 东北林业大学森林资源与环境学院 哈尔滨150040
2. School of Forest Resources & Environment, Northeast Forestry University Harbin 150040
氮(N)和磷(P)是植物所需的最重要的2种营养元素,其可利用性对植物的生长发育产生重大影响,如生长与光合(Whitehead et al., 1997; Masarovièová et al.,2000;Broadley et al., 2001)、生物量分配(Masarovièová et al., 2000)、根系统结构(Linkohr et al., 2002)以及细根的形成和寿命(Majdi,2001)等。氮磷决定了植物生长,并在影响植物生长过程相互作用(Groot et al., 2003)。在土壤中,氮和磷的可利用性的空间异质性变化很大,如在北美艾灌丛草原群落中,仅在10 cm的距离内,氮变化9倍,磷变化3倍(Jackson et al., 1993)。因此在土壤中,有些小斑块营养富集。这种富集斑块的出现是动物活动(Anderson et al., 1981)、相邻植物的影响(Norton,1991)以及微生物活动(Paul et al., 1989)的结果。土壤中氮磷富集的程度不同,导致有些小斑块中硝酸盐多于磷酸盐,另一些则磷酸盐多于硝酸盐(Yanai et al., 1996)。此外,大气氮素沉降导致局部森林土壤中氮素过高,对森林植物的生长、光合作用和营养状态产生影响(Nakaji et al., 2001)。
土壤中绝大多数磷是以不溶于水的钙、铁和镁等无机盐形式和有机P形式存在,(Bieleski et al., 1983;Barber, 1984),即使是肥沃的土壤,土壤溶液中磷很少超过8 mol· L-1(Barber et al., 1962),而且磷酸盐在土壤中向根系吸收的位置扩散速率非常小(Smith et al., 2003),而植物对磷酸盐的吸收又较快,因而在根的周围形成一个磷的耗竭带(depletion zone)。土壤中无机氮尤其是硝酸盐易溶于水,扩散速率大,易淋溶(陈怀满等,2002),同时,在土壤中,硝酸盐比磷酸盐的扩散速率至少高4个数量级(Nye et al., 1977)。这种扩散速率的巨大差异导致植物根际氮磷可利用性不平衡。许多证据表明,营养不平衡会引起营养的吸收与利用,如磷缺乏会抑制硝酸盐的吸收(Neumann et al., 2000;Le Bot et al.,1990;Rufty et al., 1990;Pilbeam et al., 1993;Gniazdowska et al., 1999)。此外,磷供给会影响氮向Rubisco的分配(Warren et al., 2002),从而影响光合作用。
氮磷不平衡供应对多年生草本沙生冰草(Agropyron desertorum)和灌木三齿蒿(Artemisia tridentata)的氮吸收没有产生显著的影响,但对三齿蒿磷吸收产生显著影响,对沙生冰草的磷吸收没有显著影响(Cui et al., 1998)。氮磷不平衡供应对这2种植物总生物量没有显著影响,表明这2种植物具有整合土壤营养资源的生理功能。水曲柳(Fraxinus mandshurica)是否也具有整合土壤营养资源的生理功能呢?本文以沙培方式,对水曲柳幼苗进行2种营养处理:氮供给充足而磷供给不足、磷供给充足而氮供给不充足,以氮磷平衡供给为对照,进而研究水曲柳幼苗对氮磷的吸收与利用以及总生物量的影响。
1 试验材料和方法 1.1 材料培养经过低温层积处理的水曲柳种子经过消毒后播种于培养基。当幼苗长到2片真叶时,于5月20日移植到塑料盆(直径30 cm,高27 cm)中。桶中事先装有河沙(用稀盐酸浸泡、水冲洗)。每盆4棵幼苗。幼苗供给如下平衡营养液:NH4NO3,8 mmol·L-1;KH2PO4,1 mmol·L-1;KCl,1 mmol·L-1;CaCl2·6H2O,1 mmol·L-1;MgSO4·7H2O,0.6 mmol·L-1;FeCl 3·6H2O,0.02 mmol·L-1;MnCl2·4H2O,6 μmol·L-1;H3BO3,0.016 mmol·L-1;ZnCl2,0.3 μmol·L-1;CuCl2·2H2O,0.3 μmol·L-1;NaMoO4·2H2O,0.3 μmol·L-1;必要时用Ca(OH)2或H2SO4把pH值调整到5.5~6.0。1月后,一部分材料进行N浓度处理:1.0和4.0 mmol·L-1,即N1和N2处理。在进行N浓度处理时,其他营养成分的浓度不变;一部分材料进行P浓度处理:0.125和0.5 mmol·L-1,即P1和P2处理。进行P浓度处理时,其他营养成分的浓度不变。以平衡营养为对照(即CK)。每种处理10盆。每个处理每2天浇1次营养液(每盆每次浇100 mL),浇灌营养液时间在上午8:00—9:00进行。每天8:00—9:00和17:00—18:00分2次浇水,每次每盆约200 mL。温室内昼夜温度分别为30 ℃和18 ℃,相对湿度80%以上,光照平均14h·d -1。
1.2 试验方法1) 材料收获 植株收获于9月12日进行。收获时,首先把植株从栽培基质中取出,操作时避免叶片和根系损伤。后用水冲洗干净,拿到实验室。每一种处理随机取10株幼苗,分别分株摘下叶片,以叶面积仪(Li-3000型,Li-Cor,Co.Ltd,Lincoln,Nebraska,USA)测定单株叶面积,然后,以利刃从根颈处分开茎和根。把根、茎、叶放在80 ℃下烘至恒重,然后以分析天平进行称量,确定单株根、茎、叶各部位生物量及总生物量。2)氮磷含量分析根、茎、叶片中总N和P含量分析均采用凯氏消化。测定总N时,称取0.1 g样品,在消化管中与H2SO4溶液、由K2SO4和CuSO4配置的催化剂一起消化,在如下温度各消化0.5 h:50、150、300和420 ℃。然后以凯氏定氮仪测定(Horneck et al., 1998)。测定总P时,称取样品0.15 g,在如下温度各消化0.5 h:100、150、300 ℃。消化后,以比色法测定消光度(董鸣,1996)。3)生长参数的计算比叶面积(specific leaf area,SLA)描述了叶片的几何形态,表示单位叶片干重叶片面积大小(Masarovicova,1997),是衡量叶片解剖特征的重要指标。叶重比(leaf mass ratio,LMR)和根重比(root mass ratio,RMR)分别衡量了叶和根在整体植株中的比例。在收获时,以叶面积仪(Li-3000型,Li-Cor,Co.Ltd,Lincoln,Nebraska,USA)测定单株叶面积,然后在在80 ℃下烘至恒重,以分析天平称量。SLA计算根据Masarovicova(1997)的方法进行。LMR和RMR按照Groot等(2001)的方法计算。
1.3 统计分析所有数据的统计分析采用SPSS for Windows软件(SPSS Inc,Chicago,Illinois,USA)。方差分析(ANOVA)用来比较不同处理之间的显著性,P < 0.05为差异显著。
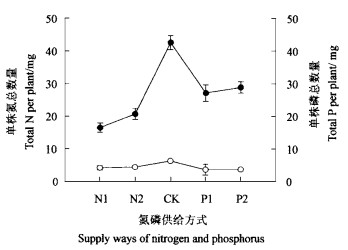
2 试验结果 2.1 氮磷不平衡供给对氮磷吸收的影响氮磷在树木生长过程具有重要的作用,其吸收就会影响树木的生长和发育。在氮磷不平衡供应条件下,水曲柳幼苗单株含氮磷数量发生明显的变化(图 1)。氮磷平衡供应时单株氮数量显著高于氮磷不平衡供应时的单株氮数量(P < 0.05),而且磷缺乏影响到氮的吸收,因为在P1和P2处理时N的供给与对照是相同的(图 1),这表明氮磷平衡供应有利于氮在水曲柳幼苗体内累积,外界氮的供应影响氮的累积。氮磷平衡供应时单株磷数量也显著高于氮磷不平衡供应时的单株磷数量(P < 0.05),同时在氮缺乏时单株磷数量高于磷缺乏时单株磷数量,这表明氮磷平衡供应有利于磷在水曲柳幼苗体内的累积,外界磷的供给影响磷的累积。
|
图 1 氮磷不同供应方式下单株氮 (●)和磷(○) 的数量变化 Fig. 1 Changes in amounts of N (●) and P (○) in single seedlings under different supply ways of N and P |
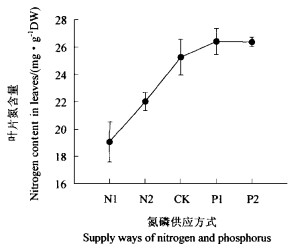
外界氮的供应影响到水曲柳幼苗叶片内的氮的累积(图 2)。在N1和N2处理下,叶片中氮的浓度显著低于氮磷平衡供应时和氮多磷少时(即P1和P2处理)。在P1和P2处理时,氮的供应与氮磷平衡供应时氮的供应一样,因此叶片中氮的浓度没有显著差别(P>0.05),尽管在这2种处理下叶片氮浓度稍高于氮磷平衡供应时的叶片氮浓度(图 2)。
|
图 2 氮磷不同供给方式下叶片氮含量的变化 Fig. 2 Changes in N contents in leaves of seedlings under different supply ways of N and P |
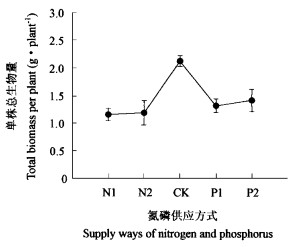
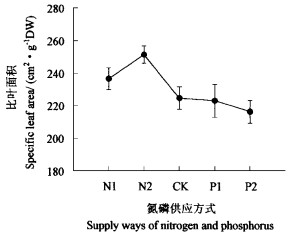
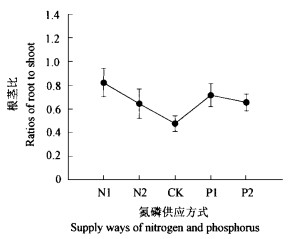
氮磷不平衡对水曲柳幼苗的生长产生影响。氮磷平衡供应时水曲柳幼苗单株总生物量显著高于氮磷不平衡供应(N1、N2、P1和P2处理)时的单株总生物量(P < 0.05),氮缺乏(N1和N2处理)和磷缺乏(P1和P2处理)时单株总生物量没有差异(P>0.05,图 3)。在氮缺乏(N1和N2处理)时SLA明显高于氮供应充足(对照、P1和P2处理),但只有在N2处理时其SLA显著高于氮供应充足时的SLA(P < 0.05,图 4),这表明氮的供应影响了水曲柳幼苗叶片的生长,对其解剖结构产生影响。同时氮磷不平衡供应对生物量的分配产生影响。在氮磷平衡供应时,LMR显著高于氮磷不平衡时的LMR(图 5),而且磷缺乏(P1和P2处理)时虽然N的供应水平与对照一样,但LMR还低于氮缺乏(N1和N2处理),这表明在氮充足而磷相对缺乏时磷能降低水曲柳幼苗叶片生物量的比例。同时与P1和P2处理时的RMR相比,氮磷平衡供应时RMR显著小些(P < 0.05);P1和P2处理时的RMR高于N1和N2处理时的RMR,这表明磷更能使水曲柳幼苗的生物量向根系分配(图 5)。从根茎比来看,氮磷平衡供应时根茎比最小(0.476),氮最缺乏(1 mmol·L-1)时根茎比最大,显著高于氮磷平衡供应时的根茎比(P < 0.05)。磷缺乏(P1和P2处理)时,其根茎比高于N2处理时的根茎比,但低于N1处理时的根茎比(图 6)。
|
图 3 氮磷不同供给方式下单株总生物量的变化 Fig. 3 Changes in total biomass per seedling under different supply ways of N and P |
|
图 4 氮磷不同供给方式下比叶面积的变化 Fig. 4 Changes in SLA of seedlings under different supply ways of N and P |

|
图 5 氮磷不同供应方式下叶重比和根重比的变化 Fig. 5 Changes in LMR and RMR of seedling under different supply ways of N and P |
|
图 6 氮磷不同供应方式下根茎比的变化 Fig. 6 Changes in ratios of root to shoot of seedlings under different supply ways of N and P |
水曲柳幼苗在氮磷不平衡供给(氮多磷少或磷多氮少)时,单株幼苗所拥有的氮和磷均显著地小于氮磷平衡供给时幼苗所拥有的氮磷数量(图 1),这表明水曲柳幼苗在氮磷平衡供给和不平衡供给时获得氮磷能力存在明显的差异。在磷多氮少时,磷并没有促进氮的吸收;在氮多磷少时,磷却降低氮的吸收(图 1)。磷缺乏降低氮的吸收的原因在于磷缺乏会抑制硝酸盐的吸收。Schjørring(1986)的试验结果表明,缺乏磷的大麦(Hordeum vulgare)、荞麦(Fagopyrum esculentum)和油菜(Brassica napus)都表现硝酸盐吸收速率下降。这种现象同样出现在鹰嘴豆(Cicer arietinum)(Le Bot et al., 1990)、白羽扇豆(Lupinus albus)(Neumann et al., 2000)等植物上。尽管磷缺乏时叶片中氮含量高于氮磷平衡供给和氮缺乏时叶片中氮含量(图 2),但磷缺乏会影响氮向Rubisco的分配(Warren et al., 2002),从而影响光合作用,最终导致单株总生物量显著下降(图 3)。在氮磷平衡供给时单株总生物量显著高于氮磷不平衡供给时单株总生物量(图 3)。在氮磷不平衡供给时水曲柳幼苗单株氮磷总数量和总生物量的显著下降,这表明水曲柳幼苗在氮磷不平衡供给时并不能调整氮磷营养。
同时,氮磷供给失衡也引起了水曲柳幼苗形态发生变化。氮少磷多时SLA大于氮磷平衡供给和氮多磷少时的SLA(图 4),表明氮缺乏导致叶片变薄。氮多磷少时SLA增大与叶片中氮含量多(图 2)有关。
在氮磷平衡供给时,LMR比氮磷不平衡供给时的LMR显著大,而RMR略小于氮少磷多时的RMR,显著小于磷少氮多时的RMR(图 5),同时根茎比也小于氮磷不平衡供给时的根茎比(图 6),这些结果表明,在氮磷不平衡下生物量的分配格局发生变化。这种变化是为了达到植物体内碳—营养平衡(Bryant et al., 1983)或生长分化平衡(Herms et al., 1992)。
4 结论在营养缺乏时,植物能调整碳的分配格局,从而最大限度地获得限制植物生长的营养成分。氮磷供给失衡是一种相对营养缺乏,有些植物能调节自身的形态和生理功能而使总生物量达到高水平。本文试验得出如下结论:水曲柳幼苗在氮磷供给失衡的条件下,不能调整氮磷营养资源,单株总生物量显著下降。
陈怀满, 郑春荣, 周东美, 等. 2002. 土壤中化学物质的行为与环境质量. 北京: 科学出版社, 238-282.
|
董鸣. 1996. 陆地生物群落调查观测与分析. 北京: 中国标准出版社, 249-250.
|
Anderson R V Coleman D C.Cole C V. 1981. Effects of saprotrophic grazing on net mineralization. Ecological Bulletin, 33: 201-215. |
Barber S A.1984.Soil Nutrient Bioavailability: A Mechanistic Approach.John Wiley and Sons, Inc, New York, 165-173
|
Barber S A, Walker J M, Vasey E H.1962.Principles of ion movement through the soil to the plant root.Trans Joint Meeting Commission Ⅳ&Ⅴ Internat Soil Sci, New Zealand, 121-124
|
Bieleski R L, Ferguson I B.1983.Physiology and metabolism of phosphate and its compounds.In: Läuchli A, Bieleski R L, eds.Encyclopedia of Plant Physiology.Vol.15A.Berlin: Springer Verlag, 422-449
|
Broadley M R, Escobar-Gutirrez A J, Burns A, et al. 2001. Nitrogen-limiting growth of lettuce is associated with lower stomatal conductance. New Phytol, 152: 97-106. DOI:10.1046/j.0028-646x.2001.00240.x |
Bryant J P, Chapin F S, Klein D R. 1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos, 40: 357-368. DOI:10.2307/3544308 |
Cui M, Caldwell M M. 1998. Nitrate and phosphate uptake by Agropyron desertorum and Artemisia tridentata from soil patches with balanced and unbalanced nitrate and phosphate supply. New Phytol, 139: 267-272. DOI:10.1046/j.1469-8137.1998.00188.x |
de Groot C C, Marcelis L F M, van den Boogaard R, et al. 2003. Interaction of nitrogen and phosphorus nutrition in determinating growth. Plant Soil, 248: 257-268. DOI:10.1023/A:1022323215010 |
de Groot C C, Marcelis L F M, van den Boogaard R, et al. 2001. Growth and dry-mass partitioning in tomato as affected by phosphorus nutrition and light. Plant Cell Environ, 24: 1309-1317. DOI:10.1046/j.0016-8025.2001.00788.x |
Gniazdowska A, Krawczak A, Mikulska M, et al. 1999. Low phosphate nutrition alters bean plants ability to assimilate and translocate nitrate. Journal of Plant Nutrition, 22: 551-563. DOI:10.1080/01904169909365651 |
Herms D A, Mattson W J, et al. 1992. The dilemma of plants:to grow or defend. Q Rev Biol, 67: 283-335. DOI:10.1086/417659 |
Horneck A D, Miller R O.1998.Determination of total nitrogen in plant tissue.In: Kalra Y P ed.Handbook of Reference Methods for Plant Analysis.New York: CRC Press, 75-83
|
Jackson R B.Caldwell M M. 1993. The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology, 74: 612-614. DOI:10.2307/1939320 |
Le Bot J, Alloush G A, Kirkby E A, et al. 1990. Mineral nutrition of chickpea plants supplied with NO3 or NH4-N.Ⅱ.Ionic balance in relation to phosphorus stress. Journal of Plant Nutrition, 13: 1591-1601. |
Linkohr B I, Williamson L C, Fitter A H, et al. 2002. Nitrogen and phosphate availability and distilbution have different effects on root system architecture of Arabidopsis. Plant J, 29: 751-760. DOI:10.1046/j.1365-313X.2002.01251.x |
Majdi H. 2001. Changes in fine root production and longevity in relation to water and nutrient availability in a Norway spruce stand in northern Sweden. Tree Physiol, 21: 1057-1061. DOI:10.1093/treephys/21.14.1057 |
Masarovicova E.1997.Measurements of plant photosynthetic activity.In: Handbook of Photosynthesis.Pessarakli M.ed.New York: Marcel Dekker, 769-801
|
Masarovièová E, Welschen R, Lux A, et al. 2000. Photosynthesis biomass partitioning and peroxisomicine A1 production of Karwinskia species in response to nitrogen supply. Physiol Plant, 108: 300-306. DOI:10.1034/j.1399-3054.2000.108003300.x |
Nakaji T, Fukami M, Dokiya Y, et al. 2001. Efiects of high nitrogen load on growth, photosynthesis and nutrient status of Cryptomeria japonica and Pinus densiflora seedlings. Trees, 15: 453-461. |
Neumann G, Massonneau A, Langlade N, et al. 2000. Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Annals of Botany, 85: 909-919. DOI:10.1006/anbo.2000.1135 |
Norton J M.1991.Carbon and nitrogen dynamics in the rhizosphere of Pinus ponderosa seedfings.Ph.D.Dissertation.University of California Press, Berkeley, CA.USA.
|
Nye P H, Tinker P B.1977.Solute movement in the soil-root system.Berkeley CA.USA: University of California Press, 193-204
|
Paul E A, Clark F E.1989.Soil microbiology and biochemistry.New York, USA: Academic Press, 223-235
|
Pilbeam D J, Cakmak I, Marschner H, et al. 1993. Effect of withdrawal of phosphorus on nitrate assimilation and PEP carboxylase activity in tomato. Plant and Soil, 154: 111-117. DOI:10.1007/BF00011079 |
Rufty T W, MacKnown C T.Israel D W. 1990. Phosphorus stress effects on assimilation of nitrate. Plant Physiol, 94: 328-333. DOI:10.1104/pp.94.1.328 |
Schjørring J K. 1986. Nitrate and ammonium absorption by plants growing at a sufficient or insufficient level of phosphorus in nutrient solutions. Plant and Soil, 91: 313-318. DOI:10.1007/BF02198114 |
Smith F W, Mudge S R, Rae A L, et al. 2003. Phosphate transport in plants. Plant Soil, 248: 71-83. DOI:10.1023/A:1022376332180 |
Warren C R, Adams M A. 2002. Phosphorus affects growth and partitioning of nitrogen to Rubisco in Pinus pinaster. Tree Physiol, 22: 11-19. DOI:10.1093/treephys/22.1.11 |
Whitehead S J, Caporn S J M, Press M C. 1997. Effects of elevated CO2, nitrogen and phosphorus on the growth and photosynthesis of two upland perennials: Calluna vulgaris and Pteridium aquilinum. New Phytol, 135: 201-211. DOI:10.1046/j.1469-8137.1997.00651.x |
Yanai J, Linehan D J, Robinson D, et al. 1996. Effects of inorganic nitrogen application on the dynamics of the soil solution composition in the root zone of maize. Plant Soil, 180: 1-9. DOI:10.1007/BF00015405 |
 2005, Vol. 41
2005, Vol. 41

