文章信息
- Shi Guanglu, Liu Suqi, Zhao Lilin, Cao Hui, Li Shiyou
- 师光禄, 刘素琪, 赵莉蔺, 曹挥, 李世友
- Community Structures and Diversity of Natural Enemies Between Integrated Pest Management and Conventional Management of Jujube Orchards
- 综合治理与常规防治枣园天敌昆虫的群落结构及其多样性
- Scientia Silvae Sinicae, 2005, 41(1): 100-108.
- 林业科学, 2005, 41(1): 100-108.
-
文章历史
Received date: 2003-05-27
-
作者相关文章
2. 大西洋森林中心加拿大林务局自然资源研究所 纽芬兰 A2H6J3
2. Natural Resources Canada, Canadian Forest Service, Atlantic Forestry Centre, Corner Brook Newfoundland, Canada A2H6J3
Jujube trees, Ziziphus jujuba, are widely cultured as an economic fruit crop in China, especially in northern parts of the country (Li et al., 1992). Because jujube fruit is rich in nutrition and has some medicinal function, the demand for supply is increasing (Chen et al., 1991). As a result of market demand, more and more farmers have expended their jujube plantations. The Chinese government is also promoting jujube cultivation to alleviate poverty in rural areas of north China (Shi et al., 1994). With the rapid expansion of plantations, insect pests in jujube orchards have become a serious problem in recent years. A number of insect pests damage foliage and trees; others feed directly on or in fruit, resulting in serious yield loss (Shi et al., 1998). To reduce the damage, farmers have relied on heavy applications of insecticides to control pests. However, using pesticides is becoming increasingly difficult because of insect resistance to synthetic chemicals and residue restrictions of chemicals in fruit (Shi et al., 1999).
Great efforts have been made in recent years to develop biological control-based integrated pest management (IPM) programs in jujube orchards. Preservation and utilization of natural enemies are important components of IPM programs (Shi et al., 1992). To utilize these natural enemies for pest management in jujube orchards, it is crucial to fully understand their basic biology, ecology and their interaction with hosts. However, very limited information is available regarding natural enemies in jujube orchards (Shi et al., 1992).
Under different management schemes, natural enemies may differ in composition, distribution and population dynamics. In the present study, we compared the differences in natural enemies between IPM and conventional management (CPM) in jujube sites. Through this study we hope to better understand how orchard management strategies affect communities of natural enemies. With this knowledge we hope to maximize the roles of natural enemies in suppression of pest population in jujube orchards.
1 Materials and Methods 1.1 Experimental SitesThe investigations were carried out from 1999 to 2001 in a jujube field located 2.5 km west of Taigu (112°8′E, 38°9′N, 780 m elevation). Trees were 18~20 yrs old and in full fruit production, with a height of 5 m. Both the IPM and CPM sites were located in the general area with similar natural conditions such as topography, geographical features and soil texture except for different management methods. Each IPM and CPM site was measured no less than 4 000 m2.
Before 1999, the IPM site was managed using conventional management methods as any other jujube orchards in the area. During the 3 yrs from 1999 to 2001, the integrated control approach was taken in the IPM site. To reduce overwintering insect pests in the soil or tree trucks, turning over the soil around trees and scraping bark were done before insects resumed their development in the spring. Trees were swayed to remove some pest insects from them and the insects were killed after dropping to the ground. An insect virus was also applied to control Chihuo zao. Sex pheromone traps were used to catch male adults in the site to reduce populations of Carposina niponensis and Ancylis(Anchylopera) sativa (Shi et al., 1994). Furthermore, the egg parasitoid Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae) was released to parasitize eggs of A. sativa. A bio-pesticide, avermectin, was applied to control spider mites and scale insects. Ground cover plants including legume and green manure were interplanted to provide habitat for natural enemies. If all these control methods failed, insecticides were applied to reduce insect damage. A total of 3 applications of insecticides were made in the IPM site each year (Shi et al., 1998).
In the CPM site, insecticides, acaricides and herbcides were used 7 times or more each year. Pesticides were applied when overwintering pests resumed activity. During the growing season, insecticides were sprayed 4 times to control insects that fed on buds, leaves, blooms and developing fruit. To control fruit borers, insecticides were sprayed in late July and the middle of August respectively. One additional insecticide and acaricide treatment were applied in 2000 compared to 1999. In 2001, all three pesticide types were applied 3 times more than in 1999 (Shi et al., 1998).
1.2 Sampling methodsIn each IPM and CPM site, five trees were chosen according to the chessboard sampling method to monitor population dynamics of natural enemies. The trees were monitored every 10 d from March 1st to October 30th each year. On each sampling date, all five trees at each site were observed from four different directions (East, West, South and North). At each direction, three levels of canopy (upper, middle and lower) were monitored. At each canopy level, the investigator spent approximately 2 min looking for natural enemies and recording numbers observed. Flitting natural enemies were captured with a sweep net (30 CPM in diameter, 100 CPM in depth, made from white nylon cloth). The net-captured species were brought back to the laboratory for identification. Furthermore, three 50 CPM twigs were chosen at each canopy level to check for the presence of natural enemies. From July to October, 15 fruits were checked at each canopy level at each sampling date to monitor the natural enemies parasitizing the borer pests of the fruit. We used non-destructive sampling protocols, i.e., no beneficial insects were removed from the sampling trees. With this sampling protocol, sometimes we encountered difficulty in identification of rare species. If that was the case, we located an identical specimen from other trees in the site and brought those specimens to the laboratory for identification. For unemerged parasitoids, parasitized hosts were brought back to the laboratory and reared in petri dishes (10 CPM in diameter, 2 CPM in height) under an ambient photoperiod of 13 h:11 h (L:D), with room temperature fluctuating between 18℃ and 23℃ and a relative humidity of 60%±10%. Once emerged, parasitoids were identified (Shi et al., 1993; Shi et al., 1998).
Besides sampling on trees, ground soil under the sampling trees was also checked for predators on each sampling date. Four samples were taken from each of the five sampled trees at each site. Each sample consisted of the top 20 CPM soil from a 100 by 100 CPM area. The sampled soil was observed for the presence of beetle predators, and then sieved. Any natural enemies extracted from the soil were recorded. After counting predators, the soil was put back to its original site (Shi et al., 2002).
1.3 Statistical analysesAll species and individuals of each species of beneficial insects observed during each sampling date in the 3 year were calculated as total numbers per 5 trees. The original data was converted into a monthly average per sampling site. The dominant degree index of Berger-Parker (Odum, 1983; Zhao et al. 1990; Jiang et al., 2000) was calculated: I = Nmax/NT, Nmax was the total number of the dominant species; NT was the total number in all species. The dominant concentration index of principal groups within insect communities throughout the sampling months was measured (Simpsom, 1949, Whittaker, 1965): C=∑(Ni/N)2, Ni was the total number in the ith species, N was the total number of all species, the same as NT in the dominant degree index formula. The Shannon-Wiener diversity index (H) was applied to measure the species diversity of the beneficial insect community (Simpsom, 1949; Simpson et al., 1995; Mu et al., 1997; Zhao et al., 1990): 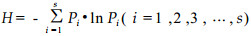
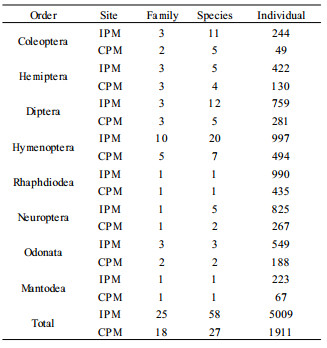
Over 3 years of study, 8 orders, 25 families, 58 species and 5 009 individuals of natural insect enemies were found in the IPM site while the corresponding numbers were 8, 18, 27, and 1 911 in the CPM site (Tab. 1). Of all the beneficial insects observed in the IPM and CPM sites, Hymenopterans were the most abundant followed by Dipterans. Predatory insects were found in 6 orders. Although Odonatans and Mantodeans were not abundant, they are thought to play a major role in the regulation of jujube insect pests (Li et al., 1992). Rhaphdiodeans and Neuropterans were major predators of spider mites, scale insects and foliage feeders, such as A. sativa, S. jujuba, and C. niponensis. Coleopteran species consisted of both ground and crown predatory beetles. However, the crown predators including some species of Coccinellidae also occasionally fed on the flowers of jujube.
|
|
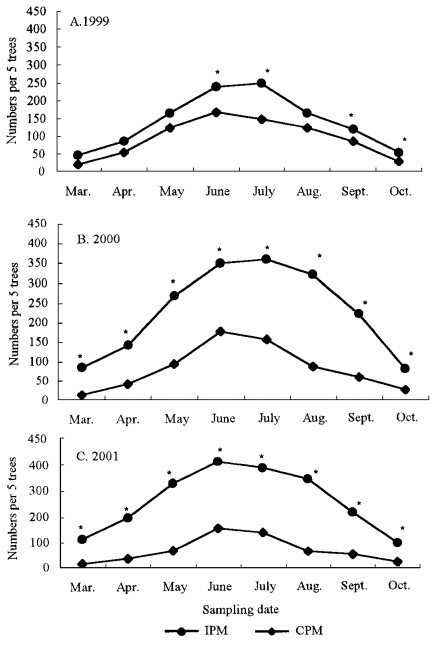
The number of natural enemies in the IPM and CPM sites were significantly different (Fig. 1). In the IPM site, legume and green manure plants provided nectariferous sources, coverage and living spaces for natural enemies. In the CPM, repeated applications of pesticides probably killed many of the beneficial insects. This would explain why natural enemy population in the IPM site were higher than that in the CPM site. The differences of natural enemies between the IPM and CPM sites were enlarged as time increased with IPM strategy implementation. The results indicated that there were significant differences (P < 0.05) in the total number of beneficial insects between the IPM and CPM sites from July 1999 to the end of that year except for August (t = 1.431, df = 4, P = 0.226) (Fig. 1, A). For all the samples in 2000 and 2001, significant differences in the total number of beneficial insects (P < 0.05) were found between the two sites (Fig. 1, B & C). Such differences enlarged with years. This might have been caused by carrying over effects of IPM and CPM methods on beneficial species.
|
Fig.1 Population dynamics of beneficial insects in the integrated pest management (IPM) and conventional management (CPM) jujube sites. An asterisk (*) indicates significant differences (P < 0.05) between the IPM and the CPM. |
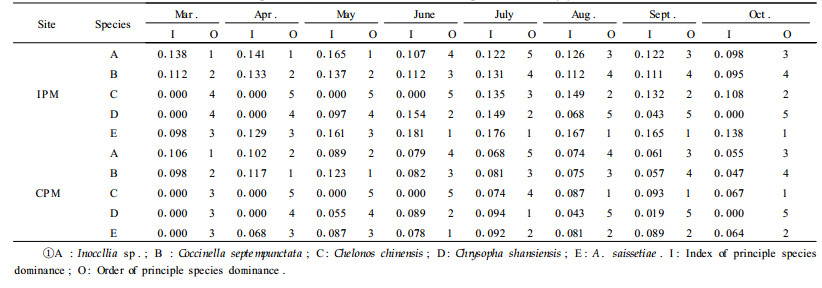
The results showed that the principal dominant species of beneficial insects were Inoccllia sp., Coccinella septempunctata, Chelonus chinensis, Chrysopa shansiensis and Anysis saissetiae (Tab. 2). Principal species dominances of beneficial insects were higher in the IPM site than in the CPM site, especially between July and August (P < 0.05). Among the five dominant species, the dominance differences of A. saissetiae were the biggest between the IPM and the CPM. The result, which A. saissetiae appeared one month earlier in the IPM than in the CPM site, indicated that IPM was important for the protection of beneficial parasitic insects. The dominance peak period of A. saissetiae, C. chinensis and C. shansiensis was also one month earlier in the IPM site than to in the CPM site.
|
|
Dominance of the dominant species was obviously bigger in the IPM site than in the CPM site because it had provided places for beneficial insects to breed, inhabit, survive, conceal and reproduce. The IPM techniques had reduced pesticide uses, which preserves and improves ecological quality. Beneficial insects not only had high species diversity, but also increased dominance.
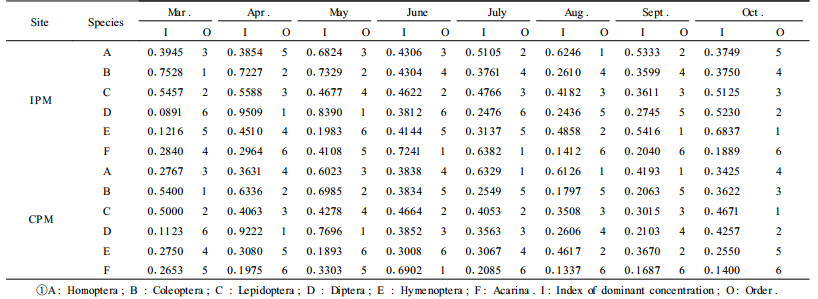
The results of dominant concentrations of principal groups of beneficial insects over time were shown in Tab. 3. The ecological dominant concentrations of both sites were almost the same. In March, the dominant concentrations of Coleopterans and Lepidopterans were higher; in April, Dipterans and Coleopterans; in May, Dipterans and Coleopterans in the IPM site, but Dipterans and Homopterans in the CPM site; in June and July, Acarina and Lepidopterans; in August and September, Hymenopterans and Homopterans; in October, Homopterans and Hymenopterans in the IPM site, but Acarina and Coleopterans in the CPM site. Comparison of the ranks of the results in Tab. 3 show that dominant concentrations of principal groups in the IPM site were always larger than those in the CPM site. Results indicated that the effects of integrated pest management were significant not only on species diversity, dominance of dominant species but also on dominant concentrations of principal groups.
|
|
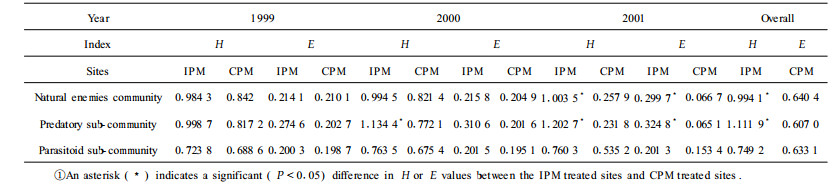
The beneficial insect communities consisted of predatory and parasitic sub-communities. The predatory sub-communities mainly included Lasius fuliginosus, Lasius niger, Inoccllia sp., C.septempunctata, Chilocorus rubidus, Chrysopa septempunctata, C. shansiensis, Tenodera capitata, Nabis inoferus, Melanostoma scalare, Paragus quadrifasciatus, and Syrphus nitens. The parasitic sub-community mainly included Frontina laeta, Cuphodera varia, Prosema siberita, Chaetexorista palpis, Barylypasp., Chlorocryptus purpuratus, Brulleia shibuensis, Megasty sp., A. saissetiae, A. sp., C. chinensis, and Clausenia purpuria. The differences in the species diversity and evenness values of beneficial insect communities between the IPM and the CPM sites were presented in Tab. 4.
|
|
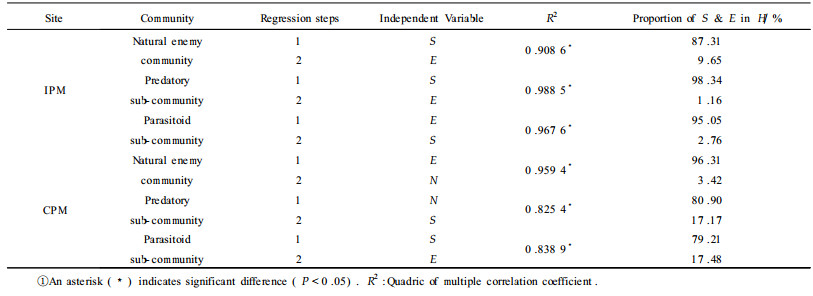
The results from Tab. 4 showed that the species diversity and the evenness of the beneficial insect community had no obvious discrepancy in the IPM site compared with those in the CPM site in 1999. However, species became more diversified in the IPM site (P < 0.05) than in the CPM site in 2000. Because IPM techniques had been applied for two years in the IPM site, the diversities and evenness of the beneficial insect communities and predatory sub-communities were obviously enhanced. Differences in the two communities were all significant (P < 0.05) between the IPM and CPM sites in 2001. Result indicates that the IPM techniques had beneficial effects on improving the richness of the beneficial insect species and the number of the beneficial insect sub-communities species population. The results also suggested that the structures and functions of beneficial insects species and groups were more rational and stable in the IPM site than in the CPM site.
2.4 Stepwise multiple regressive analysisThe results from Tab. 5 showed that richness was the principal element which affected community diversity in the IPM site. Species richness affected 87.31% of the variation of the beneficial insect community diversity in the IPM site, 98.34% in the predatory sub-community, and 95.05% in the parasitoid sub-community. Richness, evenness and the individual number were principal elements that affected community diversity in the CPM site. Evenness controlled 96.31% of the variation of the beneficial insect community diversity in the CPM site. Species richness affected 80.89% of the variation of the predatory sub-community diversity in the CPM site. Species richness devoted 79.2% in the parasitoid sub-community diversity in the CPM site.
|
|
The jujube orchards in Shanxi Province of China are rich in beneficial insects. More beneficial insect species were found in the IPM site than in the CPM site. A total of 58 species were found over 3 year in the IPM site, and only 27 in the CPM site. Inoccllia sp., C. septempunctata, C. chinensis, C. shansiensis, and A. saissetiae are dominant species. The ecological dominant concentrations of the natural enemy insect communities in both sites were similar.
The species diversity and the evenness in natural enemy insect communities in the IPM site were obviously higher than those in the CPM site. This means that IPM techniques not only played a positive role in reducing pollution and producing pesticide free jujube fruits, but also had beneficial effects protecting the ecological environment of the jujube site and increasing the species richness.
In conclusion the constitution, structure, function and the succession of the beneficial insect communities in the jujube areas greatly depended on the artificial disturbance such as the use of pesticides and other farm activities, and the complication of the environment. The diversity of the beneficial insect communities was directly affected by insecticides. The relations between species nutrition and energy in the community were changed by altering physical conditions such as the cropping of legume or herbage in jujube orchards. As a result, the community structure was formed. The results suggested that the inter-structures of the beneficial insect communities in the IPM site were more stable than those in the CPM site. The ability of beneficial insects to withstand the change of environment and then maintain a regulatory relationship within it was stronger in the IPM site. As well more species were included in the beneficial insect communities, and the distribution of the individual number of every species was even, and the beneficial insects in the IPM site could easily form the more complex interrelations with the insects of other species than those in the CPM site. There were more approaches for transferring nutrition then, and the community tended to be steady with the relative balance of different species in the community.
Little work has been done regarding the effects of natural enemy insects in the IPM and the CPM jujube orchard. The results from Shi et al., (2002) reported that the family, species and individuals of the insect community were 57, 108 and 13907 in the intercropped wheat jujube site using insecticides, while the corresponding numbers were 71 164 and 8 904 in the intercropped wheat jujube site without insecticides. The results from our study suggested that family, species and individuals of beneficial insects were all higher in the IPM site than in the CPM site. However, individual numbers of species in Dipterans, Hymenopterans, Rhaphdiodaens, Neuropterans, Odonatans and Mantodeans were much lower in the experimental site using insecticides than in the site without pesticide use (Shi et al., 2002). Our study clearly indicated the species individuals in these orders were higher in the IPM site than in the CPM site. The effects of the insecticides on the community of natural enemies were dramatic. Natural enemies in the canopies of trees treated with insecticides were only about one-quarter to one-third as abundant as in untreated trees (Michael et al., 2001). The results from our study also suggested that insecticides had obvious influences on the constitution, structure, function, and succession of natural insect enemies.
Pesticides can cause a significant reduction in abundance of the tiny parasitoids that attack scale insects. Populations of the three most common species of obscure scale parasitoids were suppressed 1 month after the application of insecticides (Michael et al., 2001). Our study clearly indicated that the dominance of A. saissetiae was the largest among the five dominant species between the IPM and the CPM site, and it appeared one month earlier in the IPM site than in the CPM site. These results also support previous findings that insecticides had major and lasting repercussions for beneficial insect communities (Morse et al., 1987). Outbreaks of scale insects have been documented in orchards where insecticides have reduced or eliminated populations of scale predators and parasites (De Back, 1974; Shi et al., 1997)
Rushton et al., (1989) found the frequent use of insecticides to be an important factor influencing the composition of ground beetle and spider communities in grasslands, where a few of highly invasive and colonizing species tended to dominate. Our data suggests that the community structures and ecoenvironment of the beneficial insects in jujube orchards could be improved by continued IPM techniques. However, the number and diversity of beneficial insects were adversely affected by insecticide applications in the CPM site. In addition, we also found a few invasive insects such as some species of the Syrphidae family, and that colonizing beneficial insects tended to dominate in the IPM site. Maximizing the impact of natural enemies already present in the agro-ecosystem could offset the effects of decreased chemical inputs on pest management (Sunderland et al., 1996). Conventional agricultural practices of the past half-century, including the cultivation of monocultures that allow for little habitat diversity, coupled with high chemical inputs, have resulted in a severe decrease in the abundance and biodiversity of natural enemies found in agro-ecosystems (Riechert et al., 1984; Altieri et al., 1987; Burn, 1989; Carcamo et al., 1995).
Results from Tao and Luo (1992) and Lawton (1983) indicated that wild or natural plants in citrus or apple orchards were beneficial for living and augmentation of beneficial insects. Our study also suggested that legume and green manure plants in the jujube orchards provided middle hosts and expended ecological capacities for beneficial insects. Therefore, interplanting crops, legumes and retaining weeds in jujube orchard should not be ignored because they were major components of community habitats.
To better prepare for future pest management in which some broad-spectrum insecticides may no longer be available, IPM practitioners need to become better informed about the natural enemy complexes already present in most agricultural ecosystems, and to what extent they may control economically important pests (Epstein et al., 2000). An increase in populations of natural enemies in jujube orchard systems, as a result of reduced chemical inputs, may prove to be a valuable component of the management program (Shi et al., 2002). Results from our investigation and previous work (Shi et al., 1998) clearly indicate that natural enemies in jujube orchards have great potential for control of jujube pests. Controlling jujube pests is nowadays primarily dependent on insecticides (Li et al., 1992). Although they are effective, their continued or repeated use has harmed natural enemies in jujube orchard ecosystems and has led to outbreaks of many jujube pests, undesirable effects on non-target organisms and environmental concerns. The negative effects of using pesticides in jujube orchards should highlight the need to develop new types of control alternatives.
Our study suggested that the IPM site had a significant (P < 0.05) more beneficial insects than the CPM site. However, using beneficial insects to control pests is not available operationally in jujube orchards. Other factors such as environmental conditions and inter-species competition also may be important in the population regulation of the beneficial insects in jujube orchards. Therefore, the ecological balance between jujube pests and beneficial insects should be considered carefully before new IPM practices are developed for jujube systems.
Acknowledgements
Authors thank all members of the Entomological Laboratory of the Department of Plant Protection at Shanxi Agricultural University in China for advice, discussion and technical assistance. We are grateful to Bruce Roberts for reviewing and significantly improving an earlier version of the manuscript. We also thank the Chinese National Natural Science Foundation Grant: 30170759 and the Education Department of Shanxi Province for financial support and the Shanxi Province Return Country People Foundation Grant 200447. The senior author would also like to express his appreciation to China Scholarship Council Project Grant 20814019 for funding to study in Canada where this manuscript was written, and to Canadian Forest Service for hosting. Helpful editorial comments from C. Ryan and D. M. Stone are highly appreciated.
Altieri M A, Anderson M K, Merrick L C. 1987. Peasant agriculture and the conservation of crop and wild plant resources. Conserv Biol, 1: 49-58. DOI:10.1111/j.1523-1739.1987.tb00008.x |
Burn A J. 1989. Long term affects of pesticides on natural enemies of cereal crop pests. In: Jepson P C eds. Pesticides and non-target invertebrates. Dorset, England, 177-193
|
Carcamo H A, Niemala J K, Spence J R. 1995. Farming and ground beetles: effects of agronomic practice on populations and community structures. Can Entomol, 21: 188-194. |
Chen Yijin, He Xiangsheng, Chen Molin. 1991. Chinese jujube research introduction. Beijing: China Science and Technology Publishing House.
|
De Bach P. 1974. Biological control by natural enemies. New York: Cambridge University Press.
|
Epstein D L, Zack R S, Brunner J F, et al. 2000. Effects of broad-spectrum insecticides on epigeal arthropod biodiversity in Pacific Northwest apple orchards. Environ Entomol, 29: 340-348. DOI:10.1093/ee/29.2.340 |
Jiang Guofan, Yan Zengguang, Cen Ming. 1993. Studies of insect community and diversity of mangrove forest in Yingluo harbour. Acta Appl Ecolog Sin, 2000, 11: 95-98
|
Lawton J H. Plant architecture and diversity of Phytophagous insects. Ann Rev Entomo, 28: 28-39. |
Li Lianchang, Li Lizhen, Fan Yongliang, et al. 1992. China jujube tree pest insects. Beijing: China Agricultural Publishing House.
|
Micheal J R, John J H, Clifford S, et al. 2001. Effects of cover sprays and residual pesticides on scale insects and natural enemies in urban forests. J Arboriculture, 27: 203-213. |
Morse J G, Bellows T S, Gaston L K, Iwata Y. 1987. Residual toxicity of acaracides to three beneficial species on California citrus. J Econ Entomol, 80: 953-960. DOI:10.1093/jee/80.4.953 |
Mu Jiyuan, Xu Hongtu, Li Huoju. 1997. Entomology ecology and forecast of agricultural pest. Beijing: China Agricultural Science and Technology Publishing House.
|
Odum E P. 1983. Basic ecology. New York: Saunders College Publishing.
|
Riechert S E, Lochley T. 1983. Spider as biological control agents. Annu Rev Entomol, 29: 299-320. |
Rushton S P, Luff M M, Eyre M D. 1989. Effects of pasture improvement and management on the ground beetle and spider communities of up land grasslands. J Appl Ecol, 26: 489-503. DOI:10.2307/2404076 |
Shi Guanglu, Cao Hui, Ge Feng, et al. 2002. Studies on the diversity and insect community in different intercropped and managed jujube area ecosystems. Sci Sil Sin, 38: 94-101. |
Shi Guanglu, Li Lianchang, Zhang Yumei. 1992. Initial studies on the natural enemies insect of jujube tree——Inoccllia sp.. J Shanxi Agric Univ, (Suppl.): 21-23. |
Shi Guanglu, Liu Xianqian, Li Jie. 1993. Studies on the sampling technique and spatial distribution of the Larva of Ancylis Sativa. Acta Agric Sin (Suppl.), 10: 249-252. |
Shi Guanglu, Liu Xianqian, Li Jie, et al. 1997. Studies on the bionomics of Quadraspidiotus perniciosus and its occurrence prediction. Sci Sil Sin, 33: 161-166. |
Shi Guanglu, Liu Xianqian, Wang Manquan, et al. 1998. Studies on the structures of the insect community and the effect of integrated pest management. Sci Sil Sin, 34: 58-64. |
Shi Guanglu, Liu Xianqian, Liu Xuedong. 1999. Studies on application of sex pheromone to control Carposina niponensis Walsinghan. In: China agricultural society ed., Research advancement of plant protection and plant nutrition. Beijing: China Agriculture Publishing House, 423-426
|
Shi Guanglu, Zheng Wangyi, Dang Zepu, et al. 1994. Fruit pests. Beijing: China Agriculture Publishing House.
|
Simpsom E H. 1949. Measurement of diversity. Nature, 163: 688. DOI:10.1038/163688a0 |
Simpson B B, Cracraft J. 1995. Systematics: the science of biodiversity. Bio Sci, 45: 670-672. |
SPSS Inc. 1999. SPSS Base 10.0 User's Guide. SPSS, Chicago, IL
|
Sundland K D, Bilbe T, Den L J, et al. 1996. Reproduction of beneficial predators and parasitoids in agrosystems in relation to habitat quality and food availability. In: Booij K, Den L eds. Arthropod natural enemies in arable land??: survival, reproduction and enhancement. Denmark: Aarhus University Press, 117-153
|
Tang Shouzheng. 1986. Poly-statistics analysis method. Beijing: China Forestry Publishing House.
|
Tao Zhengliang, Luo Zhiyi. 1992. Study of arthropod community of natural plant in citrus orchard. Acta Ecolog Sin, 12: 125-134. |
Whittaker R H. 1965. Dominance and diversity in land communities. Sci, 147: 250-260. DOI:10.1126/science.147.3655.250 |
Zhao Zhimo, Guo Yiquan. 1990. Principle and method of community ecology. Beijing: Science Technology Literature Publishing House.
|
 2005, Vol. 41
2005, Vol. 41