文章信息
- Jin Youju, Li Jiquan, Li Jianguang, Luo Youqing, Stephen A. Teale
- 金幼菊, 李继泉, 李建光, 骆有庆, Stephen A Teale
- Olfactory Response of Anoplophora glabripennis to Volatile Compounds from Ash-leaf Maple(Acer negundo) Under Drought Stress
- 光肩星天牛对干旱胁迫下复叶槭挥发物的嗅觉反应
- Scientia Silvae Sinicae, 2004, 40(1): 99-105.
- 林业科学, 2004, 40(1): 99-105.
-
文章历史
Received date: 2001-10-25
Revised date: 2003-07-15
-
作者相关文章
2. 河北农业大学林业资源与工程学院 保定 074200;
3. 北京林业大学资源与环境学院 北京 100083;
4. 纽约州立大学环境科学与林业学院
2. Forestry Resource & Engineering College, Hebei Agricultural University Baoding 074200;
3. Resourses and Environment College, Beijing Forestry University Beijing 100083;
4. College of Environmental Science and Forestry, State University of New York Syracuse, NY 13210
Asian longhorned beetle (ALB), Anoplophora glabripennis Motshulsky, (Coleoptera: Cerambycidae) is a major pest of hardwood trees in China (Luo et al, 1999; 2000). Larvae bore into the living trunk and branches from oviposition sites in the bark and feed in the phloem and xylem, causing deterioration or death of the tree. Serious economic and ecological losses chronically occur in Northwest China. Ash-leaf maple (Acer negundo) is the most preferred host tree for ALB in China and has been studied as a source of chemical attractants of ALB. Volatiles from ash-leaf maple have been identified and shown to elicit antennal responses from ALB (Li et al, 1999). Due to the arid climate in northern China, especially in the Northwest, only limited tree species, mainly Populus spp., can be used to establish shelterbelts. Poplar trees planted in the drought-prone regions of Northwest China are damaged by ALB more seriously than those in other areas. Thus it appears that there is a relationship between the ALB host selection and drought stress.
Plants are sedentary organism that respond to external stimuli metabolically. When plants are exposed to adverse environmental conditions such as drought, volatile compounds emitted from plants alter in composition and concentration (Mattson et al, 1987). Three groups of volatile organic compounds from plants under drought stress have been well studied, including isoprene (Sharkey et al, 1993), monoterpenes (Gershenzon, 1984; Clark et al, 1980; Bryant et al., 1983), and oxygenated volatile organic compounds (Kimmerer et al, 1982). Lorio et al. (1995) showed that monoterpene concentrations in plants increase in response to drought. Similarly, water deficit results in production of large amounts of acetaldehyde and ethanol from plants, but not until the water potential of leaves is quite low and leaves are severely wilted (Kimmerer et al, 1982; Robert et al, 1995).
The volatile compounds are the major contributors to the characteristic leaf odor for various classes of plants, and they are also utilized as attractants by some insects (Sekiya et al, 1983; Visser et al, 1978). The various volatile compounds induced by water stress have a significant impact on the behavioral responses of insects including attraction to plants and their specific host preference. The purpose of this work is to identify chemical cues that mediate ALB host selection behavior by studying the electrophysiological and behavioral responses to chemicals emitted from water-stressed ash-leaf maple.
2 Methods and materials 2.1 Controlled growth conditionsThree-year-old ash-leaf maples were used for all experiments and ranged in height from 1.2 m to 1.5 m. The seedlings were potted in 25 L plastic pots with equal quantities of loam. Pots were watered to saturation daily. The seedlings were grown in the field under natural conditions.
2.2 Volatile emission under drought stressThe experiment was carried out in July 1999 at Beijing Forestry University. In July, the temperature ranged from 35° to 45° and the photosynthetic photon flux was from 800 μmol·m-2s-1 to 1 000 μmol·m-2s-1 at noon, and at night the temperature ranged from 20° to 30°. Two drought treatments were used in July. In one experiment the water content of the soil decreased from 100% to 30% of the field capacity (FC) in two days due to high transpiration rate, and then back to 100% FC for 20 h after re-watering. In the second experiment the water content of the soil was kept at 35% FC for three days by compensating for the water losses at noon and 7 p.m. after it was decreased from 100%FC to 35% FC. Water loss was determined by weighing. Every treatment was replicated 3 times.
2.3 Collection of headspace volatilesThe odor source was placed in a gas-tight glass box (90 cm×90 cm×90 cm). A shade net with 80% transparency was suspended over the box to prevent the leaves from being burned, by the higher temperature inside the box. A small fan was put in the glass box to mix the air and the volatile compounds from the seedlings. The in-coming air at the top of the box was filtered through activated carbon. The tops of the two seedlings were inserted through holes in the bottom of the box for half an hour to allow accumulation of volatiles. Subsequently, the headspace volatiles from the treatments and air controls were simultaneously collected on Tenax TA packed in a glass tube (15 cm×0.3 cmID) for an hour between 10:00—11:00 a.m. with an airflow rate of approximately 90 mL·mm-1.
2.4 Analysis of headspace volatilesCompounds were identified and quantified by GC-MS (Trace 2000-Voyager, Finnigan, Thermo-Quest). The instrument was equipped with a CP-4020 TCT device (Chrompack, The Netherlands), a split/splitless injector and a capillary column (CP-Sil 5CB low bleed/MS30 m×0.32 mm ID, film thickness of 1 μm). The pressure of the carrier gas (helium) was adjusted to 6 kPa. The MS condition was EI of 70 eV, full scan (m/z from 10 to 400) with a scanning rate at 0.4 sec·scan-1.
Volatiles were desorbed from the Tenax TA by heating in the thermal desorption device (Chrompack, The Netherlands) at 250° for 10 min, and cryo-focused in a cold trap at -100°, then quickly heated to 200° in 1 min to transfer the volatile compounds into the GC-MS (Trace 2000-Voyager, Finnigan, Thermo-Quest) using the following temperature program: initial temperature 20°; 10°·min-1 to 250°, hold 10 min, total time 39 min. Compounds were identified by searching the NIST library in the Xcalibur data system (Finnigan), and by comparison of retention index(RI). We expressed the intensity of the MS detector response as the value per liter of sampled air and per gram of Tenax TA.
2.5 InsectsALB adults were reared at the USDA APHIS PPQ, Otis Methods Development Center in Massachusetts from infested hosts collected in the New York City area and express shipped to Syracuse. Beetles were stored at 22° and allowed to feed on sugar maple until antennae were used for analysis.
2.6 Gas chromatography with electroantennographic detection (GC-EAD)GC-EAD was conducted at the College of Environmental Science and Forestry. Antennae were removed and inserted into a saline-filled glass well fitted with Ag-AgCl pellets. Potential differences were recorded over time by amplification of the signal with a Grass P18 amplifier, analog to digital conversion with a PowerLab 2 (AD Instruments) and storage and display on a PC computer. 1 μL of each standard chemical was blended with methanol, and then the total volume was complemented with methanol to 50 μL. A 3 μL aliquot was injected into the GC (Hewlett Packard model 5?890) and eluted on a HP-Wax capillary column (30 m×0.25 mm ID, 0.25 μL film thickness) equipped with flame ionization detector. The carrier gas was He and the temperature program was: splitless injection at 220°, initial oven temperature 40° for 1 min., then 5°·min-1 until 210°.
2.7 Field trapping testField trapping experiments were conducted in a forest in Qingtongxia City, Ningxia Province, with about 7-year-old poplar trees [including Populus opera (P. simonii×P. pyramidalis) and P. alba var. pyramidalis Bunge]. Two experiments were conducted, one in 1999; and one in 2000. All trials were conducted during the season of adult emergence, from the beginning of July to the end of August.
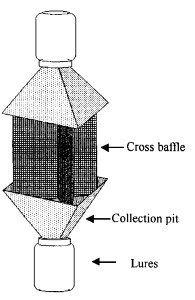
Intercept traps with 2 collection pits are used in field trapping test. The intercept trap was designed during the fields test. The trap was made of 2 beetle containers in the upper and down part that joined with cross-shaped plastic board by 2 funnels (Fig. 1).
|
Fig.1 Intercept trap used in the field test |
Traps were hung on the trunk at a height of about 1.5 m from the ground level on July 26 in 1999, and in 2000 started on July 17. In 1999, the experiment ended on August 26, and in 2000, the experiment ended on August 24. Five traps were used for each chemical and control in 1999 and in 2000. Trap spacing was a minimum of 6 m. The positions of trap with replicates of every treatment were distributed randomly in the forest. Microcentrifuge dispensers with cotton wicks were used for releasing chemicals. The dispensers were placed on the center of the trap. When there was more than one compound being released from a single trap, those compounds are placed in a separate tube. When chemicals were replaced weekly, the position of trap of every treatment was also changed.
2.8 Statistical AnalysisData were analyzed by using the SPSS (Statistical Package for the Social Science). Data were subjected to Levene's test for homogeneity of variances, and then were analyzed with one-way analyses of variance (ANOVA). Means separations were accomplished with a Duncan test at P < 0.05.
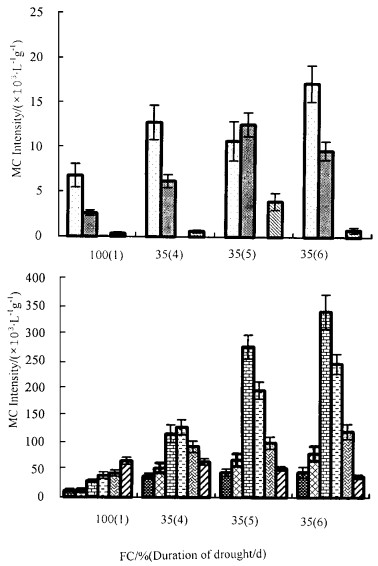
3 results 3.1 Volatiles emission under drought stressIn July, Ten compounds showed significant quantitative differences between 100% FC and the drought treatments, including butyl alcohol, pentyl alcohol, trans-2-hexen-1-al, cis-3-hexen-1-ol, pentanal, pentanoic acid, hexanal, hexanoic acid, acetophenone and longifolene.
In both water stress treatments, most of the volatiles released from the ash-leaf maple increased in amount except longifolene which decreased. The degree and duration of the drought had significant effects on the plant volatiles in both quantity and quality. For example, trans-2-hexen-1al was not detected in the 35% FC treatment(Fig. 2), but showed a large increase in the 30% FC treatment (Fig. 3). At 35% FC, all volatile chemicals increased to a different degree when the duration of the drought was prolonged, except for longifolene which decreased gradually. When the 35% FC treatment was carried out for three days, hexanal and hexanoic acid increased by eight times and six times respectively as compared to pre-stress. However, after the plants were re-watered for 20h, all volatiles decreased except for acetophenone that increased slightly. Besides the ten compounds above, drought stress conditions also induced the ash-leaf maple to produce ethanol, isobutyl and trans-2-hexen-1-ol etc. in small amounts.
|
Fig.2 Changes of relative abundance of volatiles from ash-leaf maple with soil water content of 100% and 35% FC in July In the fig. 2~3, 1: Butyl alcohol(m/z31); 2:Pentyl alcohol(m/z31); 3:E-2-hexen-1-al(m/z55); 4:Z-3-hexen-1-ol(m/z82); 5:Pentanal(m/z44); 6:Pentanoic acid(m/z60); 7:Hexanal(m/z44); 8:Hexanoic acid(m/z60); 9: Acetophenone(m/z120); 10:Longifolene(m/z161) 1×103·L-1·g-1 of the mass chromatogram intensity was taken as a unit to compare the same volatile component emitted from different treatment seedlings, which was converted to the value of per liter of pumping gas and per gram of dry leaf. |

|
Fig.3 Changes of relative abundance of volatiles from ash-leaf maple after serious drought stress to 30% FC and rewatering in July In the fig. 2~3, 1: Butyl alcohol(m/z31); 2:Pentyl alcohol(m/z31); 3:E-2-hexen-1-al(m/z55); 4:Z-3-hexen-1-ol(m/z82); 5:Pentanal(m/z44); 6:Pentanoic acid(m/z60); 7:Hexanal(m/z44); 8:Hexanoic acid(m/z60); 9: Acetophenone(m/z120); 10:Longifolene(m/z161) 1×103·L-1·g-1 of the mass chromatogram intensity was taken as a unit to compare the same volatile component emitted from different treatment seedlings, which was converted to the value of per liter of pumping gas and per gram of dry leaf. |
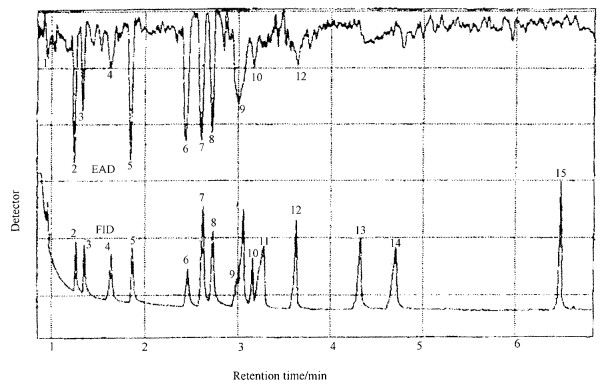
Gas chromatographic (GC) analysis of volatiles with flame ionization detector (FID) and electroantennographic detector (EAD) revealed that six compounds elicited strong antennal responses (Fig. 4). 2-Pentanol gave the strongest antennal response, followed by 1-pentanol, 1-hexenol, cis-3-hexen-1-ol, 2-butoxy-ethanol and 1-butanol.
|
Fig.4 Flame ionization (FID) and electroantennographic detector (EAD) responses to chemical standards 1:α-pinene; 2:2-pentanol; 3:1-butanol; 4:limonene; 5:1-pentanol; 6:1-hexanol; 7:cis-3-hexen-1-ol; 8:2-butoxy-ethanol; 9:1-Octen-3-ol; 10: linalool oxide; 11:α-longipinene; 12:linalool; 13:1-nonanol; 14:1, 3-butanediol; 15:4-isopropyl benzyl alcohol. |
Trap catches of beetles were examined with various combinations of test chemicals in various places (Tab. 1 and Fig. 5). In trial1, the results showed that cis-3-hexen-1-ol, hexanol, 1-hexenol and trans-2-hexen-1-al could catch beetles, and there were differences among those compounds in 1999 (F=5.125; Df=3, 16; P < 0.05);(Tab. 1). Relative comparison of trap catches among the test compounds showed that cis-3-hexen-1-ol was more attractive to ALB. The results of trial 2 also showed significant differences among those compounds tested in 2000 (F=19.78; Df=5, 24; P < 0.001);(Fig. 5). Interestingly, only a few beetles were caught in traps baited with 1-butanol, 1-pentanol and 2-pentanol respectively. However, the attractiveness to ALB adults was greatly enhanced in field when all the 3 alcohols are mixed together (Fig. 5).
|
|

|
Fig.5 ALB adults caught per intercept trap baited with chemical standards in 2000 "Mixture" means chemical mixture by 1-butanol, 1-pentanol and 2-pentanol at the ratio of 1:1:1. Different letters above the bars indicate significant differences (P < 0.05, Duncan's multiple range test), Levene's test assuming equal variances (P=0.675). |
Herbivorous insects are greatly influenced by water stress of their host plants (Holtzer, 1988; Takabayashi et al, 1994). Many volatile compounds, e.g. ethylene, ethanol, ethane, acetaldehyde and other leaf volatiles, are released from plants under drought stress, as a result of changes in plant metabolism (Kimmer et al, 1982).
Plants under water stress can be induced to produce an approximately proportional increase in hydrogen peroxide free radical and lipid peroxidase activity(Jiang, 1999; Jiang et al, 1991; 1993). Lipid peroxidation in vitro can lead to production of several aldehydes (Schcuenstein et al, 1977; Sekiya et al, 1979) that would lead to alcohol and carboxylic acid synthesis. Visser et al. (1978) reported that the GLVs are formed mainly by oxidative degradation of linolenic acid and linoleic acid; the constituents of leaf lipids. The mechanism of formation of other volatiles from plant is not well understood. On average, the temperature and the photosynthetic photon flux in the daytime in July were much higher than those in other months of the year. Charron et al. (1996) pointed out that the emission of cis-3-hexenal, cis-3-hexenol and cis-3-hexenyl acetate from lettuce grown under high photosynthetic photon flux was higher than that at low photosynthetic photon flux.
In our study, many GLVs were identified from ash-leaf maple under drought stress, including saturated or mono-unsaturated six carbon aldehydes, alcohols, and carboxylic acids, e.g. hexenal, cis-3-hexen-1-ol, trans-2-hexen-1-al, and hexanoic acid. Among them, cis-3-hexen-1-ol and trans-2-hexen-1-al elicited high EAG activity (Li et al, 1999).
In GC-EAD tests of chemical standards, our results clearly showed that five alcohols, 1-pentanol, 1-butanol, cis-3-hexen-1-ol emitted from drought-stressed maple and 2-pentanol, 2-butoxy-ethanol induced a very strong EAD response by ALB adults(Fig. 4), in agreement with the previous results (Li et al, 1999). Among the compounds with strong EAD responses, 1-pentanol, 1-butanol and cis-3-hexen-1-ol were characteristic of water stressed ash-leaf maple (Fig. 3). This suggests that water stress significantly affects volatile production of ash-leaf maple.
GLVs released from plants have been assigned several roles in insect behavior, including: (1) host plant finding by phytophagous insects (Visser, 1986); (2) enhancement and interruption of attraction to insect pheromone (Dickens et al, 1990; 1992), and (3) host finding by parasitoids (Nettles, 1980; Turlings et al, 1990). Trees growing under water stress are damaged more seriously than unstressed trees by ALB. Our field trap test confirmed that volatiles from stressed ash-leaf maple could attract ALB adults. However, in this preliminary study we have only tested partial compounds induced by drought stress. Further field tests on others drought-stress induced compounds, such as cis-3-hexen-1-al, 1-pentanal, 1-hexanal, pentanoic acid, should be conducted further more. Additionally, it is evident from these results that volatiles released from plants under water stress play an important role in insect behavior, but volatile compounds from plants are also influenced by many other factors, e.g. light, temperature, nutrients and physical damage. Thus in the future, we are also planning to study the volatile compounds released from plants under different conditions, and how these compounds will influence the behavior of ALB in the lab and in the field.
Bryant J, Chapin F, KLEIN D. 1983. Carbon/nutrient balance of boreal plants in relation to herbivory. Oikos, 40: 357-368. DOI:10.2307/3544308 |
Charron C S, Cantliffee D J, Wheeler R M, et al. 1996. Photosynthetic photon flux, photoperiod and temperature effects on emissions of Z-3-hexenal, Z-3-hexenol and Z-3-hexenyl acetate from lettuce. J Amer Soci Horti Sci, 121: 488-494. DOI:10.21273/JASHS.121.3.488 |
Clark R, Menary R. 1980. The effect of irrigation on the yield and composition of peppermint oil. Aust J Agri Res, 31: 489-498. DOI:10.1071/AR9800489 |
Dickens J C, Bullings R F, Payne T L. 1992. Green leaf volatiles interrupt aggregation pheromone response in bark beetles infecting pines. Experimentia, 48: 523-524. DOI:10.1007/BF01928180 |
Dickens J C, Jang E B, Light D M, et al. 1990. Enhancement of insect pheromone responses by green leaf volatiles. Naturwissenschaften, 77: 29-31. DOI:10.1007/BF01131792 |
Gershenzon J. 1984. Changes in the levels of plant secondary metabolites under water and nutrient stress. Rec Adv Phyto, 18: 273-320. |
Holtzer T O. Host plant suitability in relation to water stress. In: Heinrichs E A eds. Plant stress-insect interaction. New York: Joln Wiley & Sons, 1988, 111-137
|
Hsiao T C. 1973. Plant responses to water stress. Ann Rev Plant Physi, 24: 519-570. DOI:10.1146/annurev.pp.24.060173.002511 |
Jiang Mingyi. 1999. Generation of hydroxyl radicals and its relation to celluar oxidative damage in plants subjected to water stress (in Chinese). Acta Botania Sinica, 41: 229-234. |
Jiang Mingyi, Jing Jiahai. 1993. Generation of hydyoxyl radical in plants and its relation to the initiation of lipid peroxidation (in Chinese). Plant Physiology Communication, 29: 300-305. |
Jiang M Y, Jing Jiahai, Wang Shaotang. 1991. Effect of osmotic stress on photosynthetic pigment and level of membrane-lipidperxidation in rice seedlings (in Chinese). Acta Universitatis Agriculturals Boreali-Occidentalis, 19: 79-84. |
Kimmer T W, Kozlowski T T. 1982. Ethylene, ethane, acetaldehyde and ethanol production by plant under stress. Plant Physi, 69: 840-847. DOI:10.1104/pp.69.4.840 |
Li Jianguang, Luo Youqing, Jin Youju. 1999. Electroantennogram activity of ash-leaf maple (Acer negundo) volatiles to Anoplophora glabripenns (in Chinese). Journal of Beijing Forestry University, 21(4): 1-5. |
Lorio P, Stephen F, Paine T. 1995. Environment and ontogeny modify Loblolly Pine response to induced water deficits and Bark Beetle attack. For Ecol Mana, 73: 97-110. DOI:10.1016/0378-1127(94)03500-V |
Luo Youqing, Huang Jingfang, Li Jianguang. 2000. Main achievements and problems and prospects on research of Poplar Longhorn Beetles in China(in Chinese). Insect Knowledge, 37(2): 116-122. |
Luo Youqing, Li Jianguang. 1999. Strategy on applied technology and basic studies of Poplar Longhorn Beetle management(in Chinese). Journal of Beijing Forestry University, 21(4): 6-12. |
Mattson W J, Haack R A. 1987. The role of drought in outbreaks of plant-eatting insects. Bioscience, 37: 110-118. DOI:10.2307/1310365 |
Nettles W C Jr. 1980. Adult Eucelatoria sp.: response to volatiles from cotton and okra plants and from larvae of Heliothes virescens, Sposopters eridania, and Estigmene acrea. Environ Ent, 9: 759-763. DOI:10.1093/ee/9.6.759 |
Robert C E, James P, David A B. 1995. Drought stress of apple trees alters leaf emissions of volatile compounds. Physiologia Plantarum, 93: 709-712. DOI:10.1111/j.1399-3054.1995.tb05120.x |
Schcuenstein E H, Esrerbauer H Z. 1977. Aldehydes in biological systems.Their natural occurrence biological activities. London: Pion Ltd: 199-221.
|
Sekiya J, Kajiwara T, Hatanaka A. 1979. Volatile C6-aldehyde formation via hydroperoxides from C18-unsaturated fatty acids in etiolated alfalfa and cucumber seedlings. Agricultural and Biological Chemistry, 43: 969-980. |
Sekiya J, Kajiwara T, Munechika K, et al. 1983. Distribution of lipoxygenase and hydroperoxidelyase in the leaves of various plant species. Phytochem, 22: 1867-1869. DOI:10.1016/0031-9422(83)80003-X |
Sharkey T D, Loreto F. 1993. Water stress, temperature, and light effect on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia, 95: 328-333. DOI:10.1007/BF00320984 |
Takabayashi J, Dicke M, Posthumus M A. 1994. Volatile herbivore induced terpenoids in plant-mite interactions: Variation caused by biotic and abiotic factors. J Chem Ecol, 20: 1329-1354. DOI:10.1007/BF02059811 |
Tingey D T, Rosemary E, Gumpertz M. 1981. Effects of environmental conditions on isoprene emission from live oak. Planta, 152: 565-570. DOI:10.1007/BF00380829 |
Turlings T C J, Tumlinson J H, Lewis W J. 1990. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science, 250: 1251-1253. DOI:10.1126/science.250.4985.1251 |
Visser J H. 1986. Host odor perception in phytophagous insects. Annu Rev Entomol, 31: 121-144. DOI:10.1146/annurev.en.31.010186.001005 |
Visser J H, Ave D A. 1978. General green leaf volatiles in the olfactory orientation of the colorado beetle (Leptiotarsa decemlineata). Entomologia experimentalis et applicata, 24: 738-749. DOI:10.1111/j.1570-7458.1978.tb02838.x |
 2004, Vol. 40
2004, Vol. 40