文章信息
- Dietrich Ewald, Hu Jianjun
- Dietrich Ewald, 胡建军
- EFFICIENCY IN THE FORMATION OF STABLE EMBRYOGENIC LINES AND SOMATIC EMBRYO REGENERATION IN NORWAY SPRUCE AND HYBRID LARCH
- 挪威云杉及杂种落叶松稳定胚性细胞系及体胚再生系统的建立
- Scientia Silvae Sinicae, 2003, 39(3): 53-62.
- 林业科学, 2003, 39(3): 53-62.
-
文章历史
Received date: 2000-06-07
-
作者相关文章
2. 中国林业科学研究院林业研究所 北京 100091
2. Research Institute of Forestry, CAF Beijing 100091
Somatic embryogenesis as a possible way of plant regeneration is by far one of the most efficient ways concerning the formation of embryos capable to germinate. The formation of plants via adventitious bud formation is often limited to responding genotypes, especially for conifers (Ewald et al., 1993). Thus somatic embryogenesis seems to offer a high potential for the propagation of rare plant material. e.g. seeds of selected conifer breeding material to overcome the problem of irregular seed formation. Because of these reasons we tried to elaborate methods to establish stable embryogenic lines and regeneration methods of several combinations derived from controlled pollinations in Norway Spruce and hybrid Larch. The number of publications in this field is still increasing that is why our aim was to determine the efficiency of this way of plant regeneration mainly from a practical point of view. Factors and problems which can influence the success were analysed to evaluate possibilities as well as risks at the present stage.
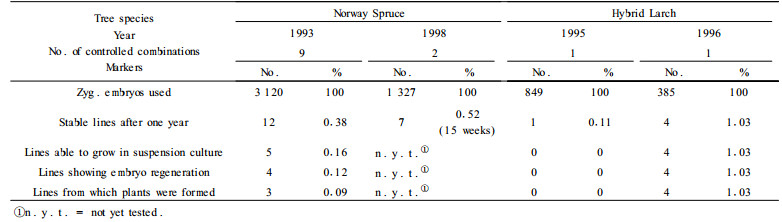
2 Material and Methods 2.1 Plant materialNorway Spruce, seeds which were used, originated from different controlled pollination. The donor trees, as a plantation of rooted cuttings (now 26 years old), derived from surviving Spruce plants in populations in the Saxon Ore Mountains heavily influenced by air pollution. Embryos were harvested either from ripe seeds (January 1992) or from seeds during ripening (August 1993). Although seeds were not totally ripened in August all embryos harvested were already fully developed (mature zygotic embryos). Nine combinations of controlled pollination were carried out in total, where two clones (I/47 and I/75) were combined with three pollen donor trees each and three additional combinations with other parents. In these experiments a total of 3 120 zygotic embryos were used. In 1998 it was possible to repeat the induction experiment with two of the most successful combinations referring to the formation of embryogenic lines. The formation of embryogenic lines was estimated with these seeds on a larger scale in this experiment (1 327 zygotic embryos). The influence of an antibiotic (cefotaxime 250 mg·L-1) was tested additionally, because a negative action of slowly growing endogenous bacteria onto callus growth and regeneration of somatic embryos was observed in former experiments (Ewald et al., 1997).
Hybrid Larch, seeds originated from controlled pollination of one European Larch clone (CS 657) as the mother tree and a Japanese Larch clone (W5) in a biclonal seed orchard. Offspring of this combination has shown an outstanding performance (Weiser, 1992). Controlled pollination was carried out in 1995 under plastic foil cover because of frost conditions and in 1996 in open air. Cones were harvested in June 1995 after the appearance of first globular zygotic embryos. Cones were stored at 6℃ after harvest. The stage of embryo development was recorded as follows: stage 1-without cotyledons, stage 2-cotyledons appear at a lateral position; stage 3-cotyledons fully developed and covering the shoot apex. In 1996 cone harvest started in July because of a long cool spring period. In total 849 zygotic embryos were used in 1995 and 385 in 1996.
2.2 Culture conditions 2.2.1 Norway Spruce(1) Formation of embryogenic lines Embryogenic lines were established on the SPE-nutrient medium according to Gupta and Durzan (1987) supplemented with 1 mg·L-1 (4.65 μmol) kinetin, 2 mg·L-1 (10.74 μmol) NAA, 450 mg·L-1 glutamic acid, 500 mg·L-1 casamino acids and 30 g·L-1 sucrose and solidified with 5.5 g·L-1 agar-agar (SERVA, high gel-strength). Cultures were transferred to fresh medium every fourth week and kept in the dark at 22℃ as described by Suess et al. (1990). Experiments to establish suspension cultures were carried out with these lines.
(2) Suspension cultures Callus parts of embryo-suspensor masses (ESM), at least 1 g fresh weight (FW), were transferred into 50 mL of the above mentioned liquid nutrient medium in 100 mL Erlenmeyer flasks on a rotary shaker at 75 r·min-1 in the dark. When continuous growth started, portions of 2 g cell mass of the embryo-suspensor masses were used as inoculum and subcultured every week. For regeneration experiments suspension was inoculated with the same density but cultured for two weeks under identical conditions. The influence of a combination of two carbon sources (CS) on the regeneration capacity was tested, adding either sucrose (30 g·L-1 = variant Z1), sucrose/starch (15 g·L-1 each = variant Z2) or sucrose/starch (7.5/22.5 g·L-1 = variant Z3) to the suspension culture medium. Soluble Toronto-starch was used.
(3) Regeneration of somatic embryos The ESM from main culture variants of two lines were harvested and resuspended in basal medium (SPE) containing 30 g·L-1 sucrose to induce embryo formation. The nutrient medium was supplemented with 8 mg·L-1 (30.26 μmol) abscisic acid (ABA) either filtersterile or autoclaved in combination with 7.5% polyethylene glycol 4 000 (PEG). Portions of 0.25 g were transferred onto viscose tissue sheets. The sheets with the attached ESM were placed onto the same medium (described above), but solidified with agar-agar in petri dishes. After 4 weeks the viscose sheets with cells and stage 1 and stage 2 embryos were transferred onto petri dishes with the SPE basal medium without phytohormones but with 0.5% activated charcoal (AC) sealed with cellophane to reduce air humidity gradually. Four weeks later the numbers of early stage embryos, mature embryos with cotyledons and precociously germinated embryos of these two stages were counted. Twelve to 24 viscose sheets were used per variant. The cultures were kept in the dark at 20℃. A 3-week-period of reduced relative air humidity (63% RH) was used to improve germination (Attree et al., 1995). The desiccated embryos were transferred for germination onto petri dishes with halfconcentrated Woody Plant Medium (WPM - Lloyd and McCown 1980) supplemented with 1 % activated charcoal (AC) and sealed with cellophane to reduce air humidity gradually during germination. After the formation of roots and the beginning of the apex development the embryos were transferred to Vitro Vent containers(DUCHEFA) with vermiculite saturated with a modified PEUKE nutrient medium (Peuke, 1987) containing 100 mg·L-1 arginine, 50 mg·L-1 glutamine, 1 g·L-1 sucrose at pH 5.8. Growing plants with a proper shoot axis and well developed root systems were transferred to Jiffy-7 peat pellets and acclimatised in the greenhouse before planting into pots. Germination on WPM medium was carried out at 23℃±2℃ under continuous red light. Vitro Vent containers with plants were subcultured at 17℃ and white light with a 16 h light-period per day.
To find out the influence of the factors tested SAS-package were used to analyse the data of somatic embryo regeneration.
2.2.2 Hybrid Larch(1) Formation of embryogenic lines In 1995 four different media were tested to establish embryogenic cultures of hybrid Larch. The basic medium was a modified MSG medium (Becwar et al., 1990) with the phytohormones used by Klimaszewska (1989): 0.45 μmol (0.1 mg·L-1) 2, 4 D + 2 μmol (0.45 mg·L-1) BAP + 2 μmol (0.43 mg·L-1) kinetin (MSG1). Additional growthregulator variants were MSG3 = 9 μmol (2 mg·L-1) 2, 4 D + 2.21 μmol (0.5 mg·L-1) BAP; MSG4 = 9 μmol (2 mg·L-1) 2, 4 D + 2 μmol (0.45 mg·L-1) BAP + 2 μmol (0.43 mg·L-1) kinetin; MSG5 = 10.74 μmol (2 mg·L-1) NAA + 2.32 μmol (0.5 mg·L-1) kinetin. All media were supplemented with 1 460 mg·L-1 glutamine. After 8 weeks growing embryogenic calli were transferred to an SPE basic medium according to Gupta and Durzan (1987) supplemented with 1.1 mg·L-1 (4.98 μmol) 2, 4D, 0.4 mg·L-1 (1.77 μmol) BAP, 0.4 mg·L-1 (1.86 μmol) kinetin, 450 mg·L-1 glutamic acid, 500 mg·L-1 casamino acids and 30 g·L-1 sucrose (S5).
In 1996 embryogenic lines were established only on the SPE-nutrient medium S5. Cultures were transferred to fresh medium every fourth week and kept in the dark at 22℃.
(2) Regeneration of somatic embryos At the end of a subculture interval callus pieces of ESM (approximately 0.25 g FW) were transferred to a nutrient medium (SPE) containing 16 mg·L-1 (60.52 μmol) filtersterile abscisic acid (ABA) in combination with 7.5% polyethylene glycol 4 000 (PEG) and 30 g·L-1 sucrose to induce embryo formation. The cultures were kept in the dark at 20 ℃. After 6 weeks mature embryos were collected. Embryos were desiccated and transferred to the soil in the same way as described for Norway Spruce.
(3) Statistics Comparison of frequencies as well as the determination of actions and interactions were carried out using the SAS package.
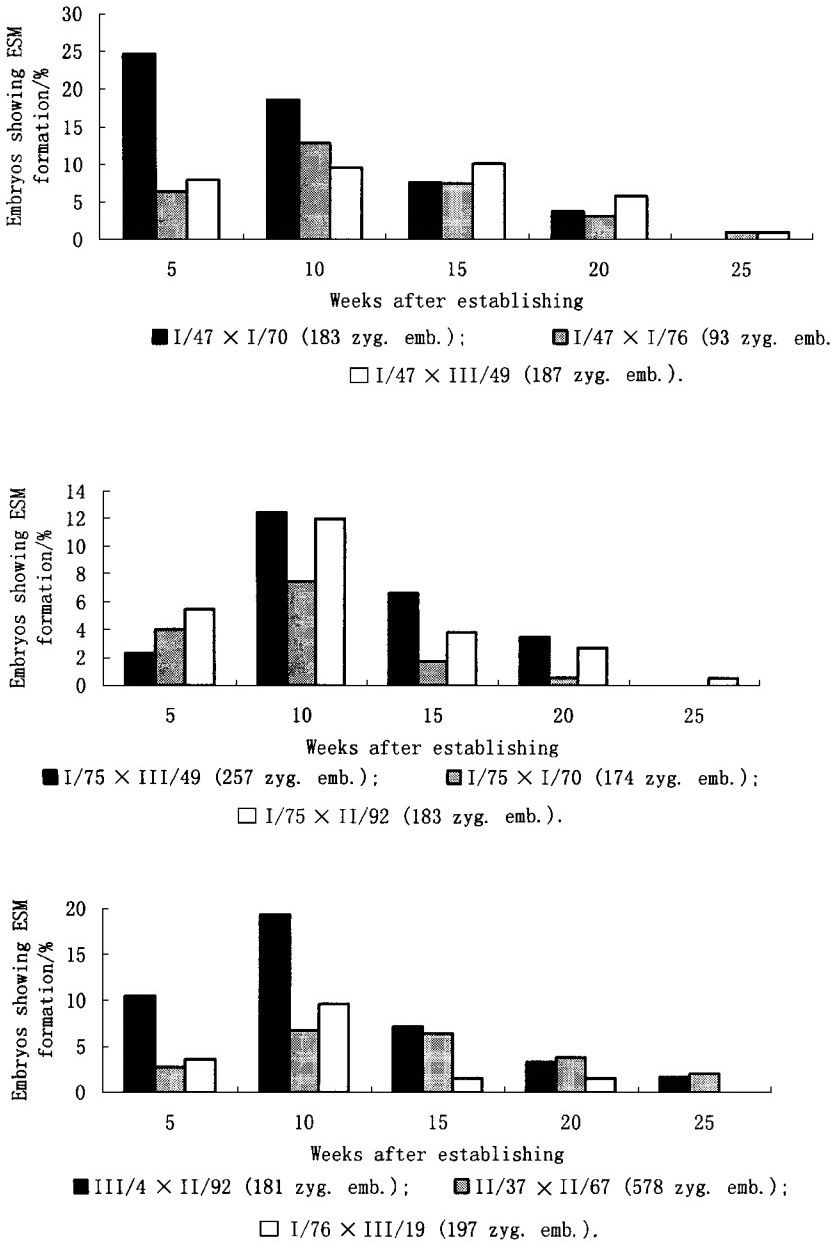
3 Results and Discussion 3.1 Norway Spruce 3.1.1 Formation of embryogenic linesConcerning the formation of embryogenic lines from different combinations (Fig. 1 and 2), we could confirm the results of other authors (von Arnold et al., 1995) concerning differences in induction frequencies among several parent tree combinations. Although almost all combinations, either directly used after embryo ripening in August or from stored seeds in January, showed a formation of embryogenic lines after four to eight weeks. Nevertheless only some combinations were able to produce stable lines with the ability of a continuous growth over a long time span. Starting from 3 120 zygotic embryos it was possible to get 12 embryogenic lines (0.38%) which could be propagated continuously. Similar results were observed in 1998 (Tab. 1). This is a very low amount of lines but it seems to be in accordance with the results of other authors (von Arnold et al., 1995).Higher induction frequencies were often measured only up to 10 weeks after induction and the number of lines used for elaboration of regeneration conditions was lower (Salopek et al., 1997-10 lines out of 127 initiated). The phenomenon of recrudescence, as the ability of a genotype to produce a new embryo-suspensor-mass after prolonged subculture, as it was described by Jokinen and Durzan (1994 - only 13% of their 53 lines showed this behaviour), was a factor which limits the number of sustainable embryogenic lines, especially from a practical point of view. The results observed in the repetition of our experiment in 1998 with only 2 combinations of controlled pollination confirmed the results of the first experiment (Tab. 2), as well showing that there was no improvement in enhancing the number of embryogenic lines by the addition of an antibiotic (cefotaxime) just from the culture initiation.

|
Fig.1 Influence of time on the appearance of embryo-suspensor-masses (ESM) in Norway Spruce starting with mature zygotic embryos of several crossbreeding combinations from stored seeds (January 1992) |
|
Fig.2 Influence of time on the appearance of embryo-suspensor-masses (ESM) in Norway Spruce starting with mature zygotic embryos of several crossbreeding combinations during the ripening of embryos (August 1993) |
|
|
|
|
Not all clones were able to propagate in liquid medium. Only 5 lines(41.7%) of 12 lines established on solid nutrient medium were able to grow in a suspension culture. The cell mass of suspension cultures was approximately doubled during a weekly interval.
3.1.3 Regeneration of somatic embryosSome factors were tested to measure their influence onto the formation of mature somatic embryos. The maximum number of mature embryos formed per 250 mg FW was 12.2±5.9 for clone 159 (onto nutrient medium Z3 + PEG + filtersterile ABA). For clone 9a the highest number of mature embryos was 31.8±9.1 and formed on the same medium combination. Early stage somatic embryos (without cotyledons) were formed at equal or lower amounts in the different variants. The number of precociously germinated embryos of both lines (159; 9a) did not exceed 20% of the number of mature somatic embryos formed by each line. Tab. 3 summarised the actions and interactions of the carbohydrates used in the suspension culture media, the presence or absence of PEG and the kind of ABA sterilisation during ripening period on the number of somatic embryos formed including precocious germination of these embryos. In this experiment the carbon source of the suspension culture medium showed only a limited influence, whereas the presence of PEG was a factor stimulating the regeneration of somatic embryos. Media with filtersterile ABA showed significantly better results compared with media where ABA was added before autoclaving. Interactions as far as it concerned the formation of early and mature somatic embryos were found for PEG and the mode of ABA sterilisation. Thus it was possible to summarise that the presence of PEG and the use of filtersterile abscisic acid was the optimal variant for embryo regeneration under the conditions described here. After one year in suspension culture only 4 lines regenerated somatic embryos. Some lines, although forming embryo-suspensor-masses, did not form any embryo even if the concentration of ABA was doubled. A restricted or lacking regeneration behaviour of some lines was also reported from several other groups (Jokinen et al., 1994; von Arnold et al., 1995). From one embryogenic line (clone 159) it was possible to transfer plants to the soil and to the nursery. Somatic embryos of the other lines showed no sufficient root development which might have been influenced by PEG as it was reported by Rutter (1998).
|
|
PEG was present in our experiment during the first 4 weeks of the maturation process. After one and a half year of continuous subcultures the regeneration capacity of the lines declined. Fourré et al. (1997) observed a high variability of mature embryo production from week to week and discussed it as long term alteration of the lines. The appearance of endogenous slow growing bacteria, difficult to detect, seemed to be another factor under our conditions which was responsible for the observed behaviour (Ewald et al., 1997). Experiments were carried out to determine the type of bacteria and to overcome their negative action by an antibiotic treatment (cefotaxime), but with a limited success (Ewald et al., 1998).
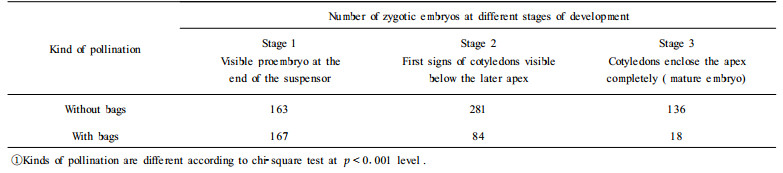
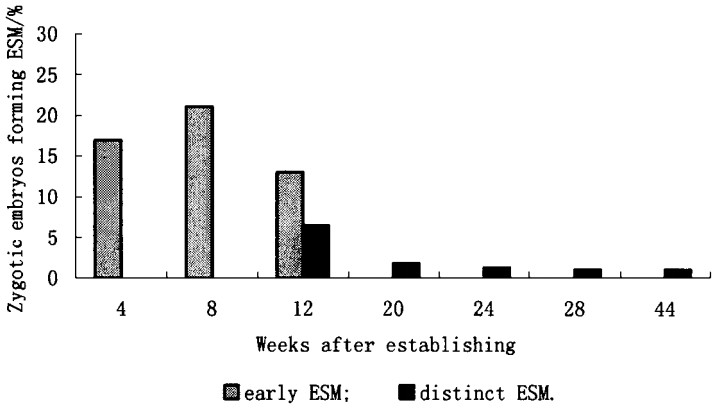
3.2 Hybrid Larch 3.2.1 Formation of embryogenic lines849 embryos were obtained from the material harvested in 1995. Embryos from controlled pollination with bags were 269 out of 69 cones, whereas 580 embryos out of 282 cones derived from pollination without pollination bags. Seed material obtained from the latter way(without bags) was checked by isozyme analysis and was found to be hybrids exclusively (Hertel 1996, pers. communication). The relation of the three embryo stages(1, 2, 3) from immature to fully mature embryos was 167:84:18 for controlled pollination, and 163:281:136 for open pollination (Tab. 4). It showed that there was a significant influence (chi-square, p < 0.001) of the pollination method on the later embryo development. Four nutrient media with different growthregulator combinations were tested in 1995 to induce embryogenic lines (Tab. 5). Although several types of callus were formed, only one stable embryogenic line was established. In 1996 the amount of immature somatic embryos was higher because of a cool spring and summer and a delayed period of seed ripening. In this experiment only one nutrient medium (S5) was used to induce embryogenic lines. Among 385 zygotic embryos used, 379 were counted as stage 1, 1 as stage 2 and 5 as stage 3, which meant fully mature. Thus it was clearly evident that the weather conditions during formation of the zygotic embryos could influence the velocity of embryo ripening and the success to find sufficient amounts of immature zygotic embryos over a longer time period. The results from 1995 confirmed this observation. The internal temperature conditions in pollination bags were different from the environment, which influenced the normal development of the zygotic embryos. In 1996 four stable embryogenic lines (1.03%) were established after a period of 44 weeks (Fig. 3). The number of early-detectable ESM directly at the embryo was higher compared with the number of ESM which could be propagated on nutrient medium after the separation from the zygotic embryo (distinct-detectable ESM). A similar decline in the number of embryogenic lines was observed by Thompson and von Aderkas (1992) for Western Larch. Their yield of sustainable embryogenic lines was (3%) which was in the same range determined by us for Norway Spruce (< 5%).
|
|
|
|
|
Fig.3 Influence of time on the appearance of embryo-suspensor-masses (ESM-early and distinct forms) in hybrid Larch from immature zygotic embryos of one crossbreeding combination, 385 zygotic embryos of Larch were used in total. |
The embryogenic line established in 1995 was not able to regenerate somatic embryos. Four of the embryogenic lines established in 1996 were able to form somatic embryos and plants later on, although the individual potential of each line was different. The remaining embryogenic lines were different concerning their ability to form mature somatic embryos, which was also reported by other authors (Klimaszewska, 1995; von Arnold et al., 1996), and it was in close correlation to the morphology of these lines. For haploid Larch lines, von Aderkas and Anderson (1993) observed fluctuations in embryo formation. The loss of the ability within one year of initiation was reported for one line. Despite reinvigoration experiments this line regained its ability spontaneously after a long time period. Slowly growing and difficult detectable bacteria as in Norway Spruce lines were found in our embryogenic cultures of Larch too and seemed to influence their regeneration potential after long-term subcultures. Visual selection and discarding of contaminated suspension cultures even over longer periods was no successful way. Using a special medium for the detection of these bacteria showed, that at least all embryogenic lines in suspension, but also on solid media, were contaminated with these highly specialised but often hidden bacteria (Ewald et al., 1997).
4 General conclusionsThe problems with Norway Spruce and hybrid Larch somatic embryogenesis in our experiments were similar, although the regeneration potential in Larch was higher. The main problem was to enhance the induction rate of embryogenic lines from immature or mature zygotic embryos and to get stable lines which could be handled without any loss of regeneration capacity over longer periods (Tab. 1). Cryopreservation could be a tool to rolve the problem of the loss of the regeneration capacity provoked by continuous subculturing. There were nevertheless possible influences and changes on the chromosome level in embryogenic cultures of conifers (Fourré et al., 1997; von Aderkas et al., 1993). Although there were still problems in the formation of somatic embryos, their germination and their successful transfer to the soil, these problems can be solved by optimising each subculture step. The appearance and action of endogenous bacteria in these cultures has yet not been well understood, which needs further research activities. Summarising these critical points it was at present difficult to provide large clone numbers of embryo forming lines of these two forest tree species. However such high clone numbers would be demanded according to the law for forest reproductive material in some of the EU-member-countries for practical forestry purposes like large scale afforestation. Therefore more emphasis has to be put on the improvement of methods to overcome the main bottlenecks of this regeneration way and using its promising perspectives for breeding and wood production.
Attree S M and Fowke L. Conifer somatic embryogenesis, embryo development, maturation drying, and plant formation. In: OL. Gamborg, G. C. Phillips (Eds. ) Plant Cell, Tissue and Organ Culture - Fundamental Methods, Springer-Verlag Berlin Heidelberg, 1995: 103-113
|
Ewald D, Suess R. 1993. A system for repeatable formation of elongating adventitious buds in Norway Spruce tissue cultures. Silvae Genetica, 42: 169-175. |
Ewald D, Naujoks G, Zaspel I and Szczygiel K. Occurrence and influence of endogenous bacteria in embryogenic cultures of Norway Spruce. In: A. C. Cassels (ed. ) Pathogen and Microbial Contamination in micropropagation, 1997: 149-154
|
Ewald D, Zaspel I and Behrendt U. Endogenous bacteria in tissue cultures of conifers - appearance and action. In: The eighth meeting of The Conifer Biotechnology Working Group, Rutgers University, June 1998: 7-11, Book of Abstracts
|
Fourré J L, Berger P, Niquet L, André P. 1997. Somatic embryogenesis and somaclonal variation in Norway Spruce: morphogenetic, cytogenetic and molecular approaches. Theor Appl Genet, 94: 159-169. DOI:10.1007/s001220050395 |
Gupta P K, Durzan D J. 1987. Biotechnology of somatic polyembryogenesis and plantlet regeneration in Loblolly Pine. Biotechnology, 5: 147-151. |
Jokinen K J, Durzan D. 1994. Properties of rescued embryonal suspensor masses of Norway Spruce determined by the genotype and the environment in vitro. Silva Fennica, 28: 95-106. |
Klimaszewska K. 1989. Plantlet development from immature zygotic embryos of hybrid Larch through somatic embryogenesis. Plant Science, 63: 95-103. DOI:10.1016/0168-9452(89)90105-2 |
Klimaszewaka K. Somatic embryogenesis in Picea mariana (Mill. ). In: S. M. Jain, P. K. Gupta and R. J. Newton (Eds). Somatic embryogenesis in Woody plants, Kluwer Academic Publishers, 1995, 3: 67-79
|
Lloyd G, Mccown B. 1980. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. In the Plant Propagators Soc., 30: 421-427. |
Peuke A D. Der Einfluβ von Schwefeldioxid-, Ozon-und Stickstoffdioxidbegasung auf den Stickstoffmetabolismus steril kultivierter Fichtensämlinge P. abies L. Karst. Dissertation, Gättingen, 1987
|
Rutter M R. The use of PEG in Loblolly Pine somatic embryo maturation. Problems and solution. In: The eighth meeting of The Conifer Biotechnology Working Group, Rutgers University, June 1988: 7-11, Book of Abstracts
|
Salopek B, Tramisak Milakovic T, Mihaljevic S, Jelaska S. 1997. Storage product accumulation during the maturation of Picea omorika (Panc.) Purk. somatic embryos. Periodicum Biologorum, 99: 117-124. |
Suess R, Ewald D, Matschke J. 1990. Erzeugung somatischer Embryonen aus Saatgut der gemeinen Fichte. Beitr. Forstwirtschaft, 24: 126-130. |
Thompson R G, Von Aderkas P. 1992. Somatic embryogenesis and plant regeneration from immature embryos of western Larch. Plant Cell Reports, 11: 379-385. DOI:10.1007/BF00234365 |
Von Aderkas P, Anderson P. 1993. Aneuploidy and polyploidization in haploid tissue cultures of Larix decidua. Physiol Plant, 88: 73-77. DOI:10.1111/j.1399-3054.1993.tb01762.x |
Von Arnold S, Egertsdotter U, Ekberg I, Gupta P, Mo H and Nörgaard J. 2. Somatic embryogenesis in Norway Spruce (Picea abies) In: S. M. Jain, P. K. Gupta and R. J. Newton (Eds). Somatic embryogenesis in woody plants, Kluwer Academic Publishers, 1995, 3: 17-37
|
Von Arnold S, Clapham D, Egertsdotter U, Mo L K. 1996. Somatic embryogenesis in conifers - A case study of induction and development of somatic embryos in Picea abies. Plant Growth Regulation, 20: 3-9. DOI:10.1007/BF00024050 |
Weiser F. 1992. Tree improvement of Larch at Waldsieversdorf: status and prospects. Silvae Genetica, 41: 181-188. |
 2003, Vol. 39
2003, Vol. 39