文章信息
- Tang Ming, Chen Hui, Shang Hongsheng
- 唐明, 陈辉, 商鸿生
- MECHANISM OF VESICULAR-ARBUSCULAR MYCORRHIZAL FUNGI ENHANCED RESISTANCE OF POPLAR TO CANKER
- VA菌根真菌提高杨树抗溃疡病机制的研究
- Scientia Silvae Sinicae, 2000, 36(2): 87-92.
- 林业科学, 2000, 36(2): 87-92.
-
文章历史
Received date: 1999-05-27
-
作者相关文章
2. 西北农业大学 杨陵 712100
2. Northwest Agricaltural University Yangling 712100
The potential biocontrol of root diseases with mycorrhizal fungi is currently being investigated (Dehne et al. 1978; Hu Zhengjia and Wang Ping 1993; Liu Runjing and Qiu Weifan 1994), but there is less information on mycorrhizal interactions with trunk diseases of trees. Poplar canker is one of the important and widespread tree diseases in China. The bark structure, chemical composition and enzyme activity of poplar in relation to diseases resistance has been studied (Xang Yuing and Hua Xiaomei 1981; Yang Chuan-he et al. 1989; Hu Jingjiang et al. 1990; Zhu Wei and Jing Yao 1990), but there have been no reports of the effect of vesicular-arbuscular mycorrhizal (VAM) fungi on canker resistance of poplars.
Field investigations of the relation between mycorrhizal colonization and the disease index of poplar canker under natural conditions, suggested the inoculation of trees with VAM fungi could decrease the occurrence of canker (Tang Ming and Chen Hui 1994b). This study explores mechanisms for VAM fungusinduced changes in canker resistance, by investigation physiological and biochemical changes in poplar that could be related to canker resistance.
1 Materials and MethodsSandy soil collected in the Yangling area wasmixed with washed riversand (1:1v/v) and sterilised with formalin (Zhang Ling and Ran Chengxi 1989). The soil has the following properties:pH7.7 (in water); available phosphorus 10.4 ppm; available potassium 14.5ppm; total nitrogen 0.08%; and organic matter 1.08%.
One-year-old seedlings of Populus'bejingensis' (P. nigra L. var. italica (Moench.) Koenne × P. catherana Rehd.), a species which isoften infected by the canker fungus, were grown in potsin a greenhouse. The temperature range was kept between 10℃and 24℃, with a mean night minimum of 10℃ and a mean daily maximum of 24℃. Spores of the VAM fungus Glomus mosseae (Nichol. and Gerd.) Gerd. and Trappe were isolated from rhizosphere soil of P. 'beijingensis' by wet sieving and decanting (Gerdemann and Nicolson 1963) and propagated in pot cultures using clover (Trifolium repens L.) as the host. The infected root fragments, sporesand soil from these pot cultures were used as inoculum. The pathogenic fungus, Dothiorella gregaria Sacc., used in the experiments was isolated from canker tissue of poplar.
1.1 Determination of relative turgidity of barkThe potted transplants were divided into 4 different water content levels (A > B > C > D) by controlling their water supply, by watering to 20%, 15%, 10%or 5%soil water by weight respectively. Each water treatment group contained 30 seedlings, half of which were inoculated with G. mosseae and the remainderof which were used as controls. After 6 months bark samples were collected at 1m height to determine their relative turgidity (RT) using the following formula:
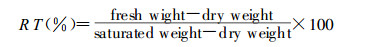
|
Quantification of mycorrhizal infection was done with root samples collected from 3 points on the lateral root system of each seedling by taking 4cm diameter×10~15cm deep soil cores. Rootswere removed from the soil then cleared with 10%KOHand stained with trypan blue (Phillips and Hayman 1970). The gridline intersect method was used to measure root colonization by mycorrhizal fungi (Giovanetti and Mosse 1980; Schenck 1982).
Determination of the disease index (DI) was performed using a graded standard of canker disease and a disease index was calculated according to Tang Ming and Chen Hui (1994b).
1.3 Biochemical determinationsPeroxidase (PO) and polyphenoloxidase (PPO) activities were determined using seedlings of C level water content inoculated with G. mosseae and/or D. gregaria (VAM + pathogen, VAM, or pathogen treatments). These plants were used to determine PO and PPO activities during the early growing stage of the second year. Annual brancheswere coolected and their bark was peeled off immediately after rinsing and cold storage, then fixed by freezing, before enzyme activities were determined (Hu Jingjiang et al., 1990).
Bark samples, collected at 1cm height from seedlings, were used for the determination of total phenolics, soluble sugars and available phosphorus contents. This material was rinsed, dried at 48℃~50℃, ground, sieved and kept in stoppered bottlesbefore use. These sampleswere then used to measure total phenilics-with phosphomolybdicacid-sodium tungstate colorimetry, soluble sugars-with flavone colorimetry, and available phosphorus-with molybde-num and antimony colorimetry (Zhu Wei and Jing Yao, 1990).
2 Results and Discussion 2.1 Effect of VAM fungi on turgidityRelative turgidity and mycorrhizal infection rate of seedlings inoculated with Glomus mosseae or uninoculated control treatments of different moisture contents are presented in Tab. 1.
|
|
The relative turgidity of VAM-inoculated seedlings was higher than that of the uninoculated control at all 4 water levels and followed the order of D < C < B < A. The influence of mycorrhizal associations on the absorption of moisture by seedlings was substantial at low water contents, butwas less when water supply was higher (Tab. 1). Mycor-rhizal colonization was inversely correlated with the water content of seedlings. The relative turgidity of bark also increased gradually as mycorrhizal formation increased at the same water content level.
Watercontentof plantsisanimportantinfluencing factoron disease resistance (Jing Yao and Tang Ming 1986; McPartland 1984; Xiang Yuing and Hua Xiaomei 1981). The research by Yang Chuanhe et al. (1989) pointed out that watercontentinfluencesphenylalanine ammonia-lyase (PAL) activity and further the synthesisof lignin, phenolics and flavonoidsin the phenylpropanoid metabolic pathway. Some phenolic constituents (e. g. catechol, p-hydroxybenzoic, protocatechuic acid) in bark decreased as the water content decreased. Increases in the relative turgidity of bark resulted in increases in catechol content and consequently in increased disease resistance. Resistance factors of the host are most effective in turgid tissues. The water content of living tissues in annual bark can be used as a canker-resistance index for poplar. Cankers are more likely to form when relative turgidity is less than 80% (Beir, 1964).
2.2 Effect of VAM and a pathogenic fungus on peroxidase and polyphenoloxidase activities in poplar barkThe results of enzyme activity determinations of tree seedlings inoculated with a VAM fungus and/or pathogen (Tab. 2) showed that the PO and PPO activities were highest in the dual inoculated plants. In single inoculated samples the enzyme activities (PO and PPO) were increased compared to controls, indicating that both the pathogenic and VAM fungi induced a higher enzyme activity of the host. Enzyme activities werehighest and disease index was lowest in dual inoculated seedlings (VAM+pathogen) but in seedlings inoculated with pathogen only the opposite was observed.
|
|
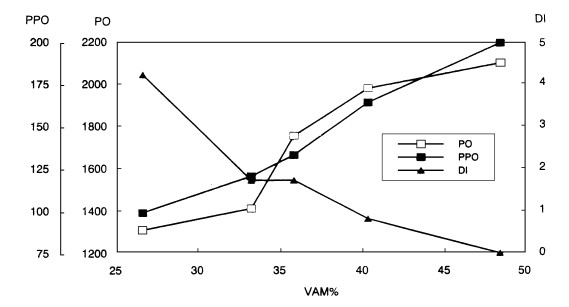
The relation betweenenzyme activitiesand disease indexshowed thatas the enzyme activitiesincreased the disease index decreased and the mycorrhizal infection rate increased (Fig. 1). Both mycorrhiza formation and enzyme activities were positively correlated with canker resistance in poplar. Peroxidase and polyphenoloxidase activities are known to be high in resistant cultivars of poplar (Hu Jingjiang et al., 1990). Canker resistance of poplars is positively correlated with mycorrhizal infection rate (Tang Ming et al., 1994b) and VAM fungi are able to induce the production of more than 10 pathogenesis-related proteins, promote terpenoid aldehyde contents and chitinase activity, thereby enhancing the resistivity of plants to pathogenic fungi(Liu Runjing et al., 1994; Dehne et al.1987)
|
Fig.1 Effect of mycorrhizal formation (VAM%) on peroxidase (PO) and polyphenoloxidase (PPO) optical density enzyme activity and the disease (DI) of poplar. |
The available phosphorus, total soluble sugars and total phenolic contents in mycorrhizal poplar bark were different from those of non-mycorrhizal controls. Available phosphorus and total phenolic contents in creased and total soluble sugars decreased in inoculated seedlings. Analysis of variance tests indicated that the differences in available phosphorus, total phenolicsand soluble sugarcontentswere all significantbetweeninoculated plantsand the controls (P > 0.05). Available phosphorus and total phenolic contentsincreased and total soluble sugars decreased asmycorrhizal formation increased in inoculated poplar seedlings (Fig. 2).

|
Fig.2 Effect of mycorrhizal formation (VAM%) on available phosphorus (AP%), total soluble sugars (TSS%) and total phenolics (TP%) in poplar bark. |
Mycorrhizal fungi are known to promote nutrient uptake and plant growth (Gou Xiuzhen and Bi Gouchang 1989; Tang Ming et al.1993). Increased phosphorus uptake would result in increasing phospholipid content and thus decreased permeability of plant cell membranes. These factors should reduce the susceptibility of plants to pathogenic fungi. We would expect that the growth of D. gregaria would be inhibited by higher phosphorus concentrations (Tang Ming and Chen Hui 1994). Therefore, it seems logical that VAM fungi would induce canker resistance of host plants by increasing their phosphorus and phenolic contents. Harris and Paul (1987) and Bevege et al. (1975) showed that 4%~14% of the total carbon resulting from photosynthesis is distributed to mycorrhizalfungi associated with the host plant, while total soluble sugars decreased. Conidia germination and colony growth of D. gregaria requires a high soluble sugar supply (Zhu Wei and Jing Yao, 1990). Therefore, reduced soluble sugar contents resulting from high mycorrhizal colonization rates, could reduce the impact of canker disease on poplar.
3 ConclusionsThe formation of VAM associations influences the physiological and biochemical aspects of poplar, including water uptake, phosphorus content, enzyme activities, and the content of substances that inhibit canker formation. Inoculation with VAM fungi apparently enhances wateruptake by seedlings, especially under conditions of low water supply. VAM associations also enhanced peroxidase and polyphenoloxidase activities in poplar bark, which were associated with reduced cankerdisease index. VAM fungusinoculation also caused increaesin the total phenolic content and decreasedin totalsoluble sugars thatare unfavourable to the growthof D. gregaria. VAM associationsalso increased canker resistance by promoting nutrients uptake and strengthening the vigor of trees, and by increasing the content of fungistatic substance (such as phenolics, lignin and phenylpropanoids) in bark. These mechanisms may work together to cause the VAM fungus-induced canker resistance of poplars that was observed.
Poplars are simultaneously infected by both VAMandectomycorrhizal fungi in nature and these associationsare negatively correlated with disease index of canker fungus. Infection by ectomycorrhizal fungi has shown a stronger direct influence on this disease than that of VAM fungi (Tang Ming et al., 1994a, c), and further study of the ef-fect of ectomycorrhizal associations on the induced resistance of poplars to the canker fungus is required.
Beir J E. 1964. The relation of some bark factors to canker-susceptibility. Phytopathology, 54: 250-253. |
Bevege D I, Bowen G D and Skinner M F. Comparative carbohydrate physiology of ecto-and endomycorrhizas. in Sanders I R, Mosse B and Tinker B (ed.), Endomycorrhizas, Academic Press, London, 1975, 149~174
|
Dehne H W, Schonbeck K, Baltrshat H. 1987. The influence of endotrophic mycorrhiza on plant disease 3. Chitinase activity and the ornithine cycle. Zeischrift fur Pflanzenkrankheiten un Pflanzenschutz, 65: 666-678. |
Gerdemann J W, Nicolson T H. 1963. Spores of mycorrhizal; Endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society, 46: 235-244. DOI:10.1016/S0007-1536(63)80079-0 |
Giovannetti M, Mosse B. 1980. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytologist, 84: 489-500. DOI:10.1111/j.1469-8137.1980.tb04556.x |
Guo Xiuzhen, Bi Gouchang. 1989. Forest mycorrhiza and applied technique. Beijing: Forestry Press in China.
|
Harris Y D and Paul EA. Carbon requirementsof vesicular-arbuscular mycorrhizae. in Safir G R(ed.), Ecophysoilogy of VA mycorrhizal phants, CRC Press, Boca Raton, 1987, 1987, 93~105
|
Hu Jingjiang, Jing Yao, Wen Jianlei, et al. 1990. The relation between peroxidase polyphenoloxidase and resistance to canker of poplars. Journal of the Northwesten College of Forestry, 5: 46-51. |
Hu Zhengjia and Wang Ping. The relation between VA mycorrhiza and plant diseases. in Li Fudi, Li Xueyuan, Liu Wuding and YuZiniu(ed.), Research advanced in a few fields of the life scienes and pedology, Agricultural Press, Beijing, 1993, 199~205
|
Jing Yao, Tang Ming. 1986. The biological characteristics of Dothiorella gregaria Sacc. of poplar and walnut. Forest Pest and Disease, 2: 4-7. |
Liu Runjing, Qiu Weifan. 1994. Recent advance in the study of endomycorrhizal (VAM) fungi on the induction of plant disease resistance. Acta Phytopathologica Sinica, 24: 1-4. |
McPartland J M. 1984. Hyphal morphology of Botrysphaeria dothidea in vessels of unstressed and drought stressed stems of Betula alba. Phytopathology, 74: 358-362. DOI:10.1094/Phyto-74-358 |
Phillips J M, Hayman D S. 1970. Improved procedures for clearing and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society, 55: 158-161. DOI:10.1016/S0007-1536(70)80110-3 |
Schenck N C. 1982. Methods and principles of mycorrhizal research. American Phytopathological Society, St. Paul.. |
Tang Ming, Chen Hui. 1994a. The morphological and anatomical characters of poplar ectomycorrhizae and their clasification. Acta Pedologica Sinica, 31(supplement): 177-181. |
Tang Ming, Chen Hui. 1994b. Relation between the poplar mycorrhizae and canker. Acta Pedobiologica Sinica, 31(supplement): 218-223. |
Tang Ming, Chen Hui. 1994c. Study on the species of ectomycorrhoizal fungi in Shaanxi Province. Scientia Silvae Sinicae, 30: 437-441. |
Tang Ming, Chen Hui, Zhang Boyong. 1993. Study on the VA mycorrhizae of Acer truncatum Bunge. Journal of the Northwest Forestry College, 8: 18-21. |
Xiang Yuing, Hua Xiaomei. 1981. Study on the canker-resistance and influential factors of poplar main varieties. Forest Pest and Disease, 1: 1-5. |
Yang Chuanhe, Yang Wang, Zhou Zhongming. 1989. The relation of bark phenolics and phenylalanine ammonialyase to the resistance of poplar canker. Scien tia Silvae Sinicae, 25: 311-316. |
Zhang Ling, Ran Chengxi. 1989. A study on the chemical sterilization method of culture soil of vesicular arbuscular mycorrhizas. Journal of Mianyang Agricultural College, 6: 24-27. |
Zhu Wei, Jing Yao. 1990. A preliminary study on the relation of the chemical compositions in the bark of poplar to Dothiorella gregaria Sacc. Shaanxi Forest Science and Technology, 3: 33-35. |
 2000, Vol. 36
2000, Vol. 36


