文章信息
- 孙山, 张立涛, 杨兴华, 高辉远.
- Sun Shan, Zhang Litao, Yang Xinghua, Gao Huiyuan
- 板栗幼叶展叶过程的反射光谱和叶绿素荧光动力学
- Spectral Reflectance and Chlorophyll Fluorescence Kinetics of Young Leaves at the Various Stages of Leaf Expansion in Field-Grown Chestnut Plants
- 林业科学, 2009, 45(4): 162-166.
- Scientia Silvae Sinicae, 2009, 45(4): 162-166.
-
文章历史
- 收稿日期:2008-02-01
-
作者相关文章
2. 山东农业大学生命科学学院 泰安 271018
2. College of Life Science, Shandong Agricultural University Tai'an 271018
植物幼叶的叶绿素含量随叶片形态结构的发育建成而逐渐积累(Drumm-Herrel et al., 1985),幼叶的光合能力显著低于成熟叶片。有研究将幼叶较低的光合能力归因于光合机构发育不健全(Jiang et al., 2006)。由于板栗(Castanea mollissima)幼叶的光合速率低,加之通常位于树冠的外围,受到强烈日光的直晒,会使幼叶中比完全展开叶存在更多的过剩激发能。叶片中过剩激发能如果不能被及时耗散掉,就会导致活性氧的过量产生,破坏光合机构(Muller et al., 2001)。在自然条件下,发育过程中的幼叶极少因为过多的激发能而不能正常发育,说明幼叶在发育过程中能及时建立起光保护的相关机制。植物耗散过剩激发能的形式包括非辐射能量耗散(主要是热耗散)、荧光发射(仅占整个去激途径的较小比例,约3%)等,光能利用和耗散方式之间存在着相互竞争关系(武海等,1993)。光合作用中的过量激发光能主要以非辐射能量耗散的形式耗散, 它是植物避免强光对光合器官造成损伤的一种主要的防御机制(Gilmore et al., 1991)。有关板栗幼叶发育过程中光合能力、光化学活性和光保护的研究尚未见报道。在许多板栗品种的新抽生幼叶中,常可以观测到花青素的存在;而新抽生幼叶中花青素的出现与光破坏防御之间的关系却很少被关注。阐明板栗叶片发育过程中光合特性和光破坏防御的机制变化,对板栗研究及生产实践具有重要的理论和实际意义。
作为叶绿体色素之一的类胡萝卜素,除了参与光能的捕获外,本身也是一种强抗氧化剂,参与单线态氧的猝灭(Edge et al., 1997)。叶黄素循环在植物耗散过剩光能保护光合机构免受强光破坏中的作用逐渐受到重视(吴长艾等, 2001)。存在于叶片液泡中的花青苷可以通过遮光、作为抗氧化剂来减轻过剩激发能对植物的伤害(Close et al., 2003)。本试验以板栗为试材,研究幼叶发育过程中原初光化学反应、过剩激发能耗散的动态变化,以期了解幼叶发育过程中光合能力和光化学活性的变化,以及类胡萝卜素、花青素在耗散过剩光能中的作用,从而为板栗的育种实践提供理论依据。
1 材料与方法 1.1 试验材料试验于2007年5月中旬在山东泰安国家板栗种质资源圃进行。试验树为5年生实生本砧的“8019”(C. mollissima cv. 8019),植株生长健壮,株行距为2 m×3 m,管理水平中等。从树冠的南向外围,选择色泽相似、无损伤的不同发育阶段的叶片用于测定。以完全展开的叶片的面积作为100%,其他各发育阶段叶片的面积换算成完全展开叶片面积的百分比。叶片面积用LI-3000A手持叶面积仪(Licor, 美国)测定。
1.2 光合速率的测定用便携式光合测定系统CIRAS-2(PP Systems, 英国)于晴天的上午10:00—12:00时进行连体叶片测定。光强、CO2浓度和叶温采用CIRAS-2光合测定系统分别控制在1 200 μmol·m-2·s-1、360 μmol·mol-1和30 ℃。每种叶面积梯度测定5个叶片,选择叶片中部的相同部位测定(下同)。
1.3 快速叶绿素荧光诱导动力学测定参考Schansker等(2003)的方法,每次测定前叶片先暗适应30 min,然后暴露在饱和脉冲光(3 000 μmol·m-2·s-1 PFD)下1 s,用连续激发式荧光仪Handy-PEA(Hansatech, 英国)测量快速叶绿素荧光诱导动力学曲线(O-J-I-P荧光诱导曲线)。
1.4 JIP-test分析获得的O-J-I-P荧光诱导曲线用JIP-test进行分析(Strasser et al., 1995),得到以下参数:1) PSⅡ最大量子效率φPo;2)捕获的光能用于电子传递的量子效率(φEo);3)单位面积吸收的能量(ABS/CS);4)单位面积有活性的反应中心的密度RC/CS;5)单位反应中心吸收(ABS/RC)、用于电子传递(ETO/RC)及热耗散(DIO/RC)的能量。
1.5 反射光谱测量用配有叶片专用测量探头的Unispec田间便携式光谱仪(PPSystem,美国),测量连体叶片的反射光谱。光谱仪的测定波段范围为310~1 100 nm,光谱采样间隔(波段值)为3.3 nm,光谱分辨率 < 10 nm。按以下公式计算各光谱指数。中午强光下叶黄素组分脱环化比例(ΔPRI)的测定,根据Gamon等(1999)的方法原理进行设计,即黎明前测定充分暗适应的反射光谱,用记号笔圈定测定位置,然后于12时测定相同部位的光下反射光谱。
1) 叶绿素含量:Chl NDI= (R750-R705)/(R750+R705)(Gamon et al., 1999);2)类胡萝卜素/叶绿素:PSRI=(R680-R500)/R750(Merzlyak et al., 1999);3)花青苷:Red/Green=∑Ri/∑Rii (i=600~699, ii=500~599) (Gamon et al., 1999);4)叶黄素组分脱环化比例:ΔPRI=PRID-PRIL,PRI=(R531-R570)/(R531+R570),PRID为暗适应光化学反射指数,而PRIL为中午光下的光化学反射指数。
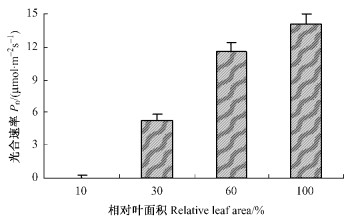
2 结果与分析 2.1 幼叶展开过程中叶片光合能力的变化从图 1看出,相对叶面积(RLA)约为10%时,净光合速率(Pn)接近0,然后光合速率随叶面积的增加而逐步提高。相对叶面积达到60%左右时,净光合速率已超过完全展开叶片的80%,接近完全展开叶片。板栗叶片的相对叶面积可能为其发育成熟程度的可靠标志之一。
|
图 1 板栗叶片展开过程中光合速率的变化 Figure 1 Changes in net photosynthetic rate (Pn) of field chestnut leaves from emergence to full expansion 以完全展开叶片的面积作100%,未完全展开叶的面积用相对百分比表示。每个点为5次独立测量的平均值,下同。 The area of fully expanded leaf was taken as 100%, and the relative areas of those unexpanded leaves were expressed as percentage of fully expanded leaf. Values are means±SE, each data is the average of 5 independent measurements. The same below. |
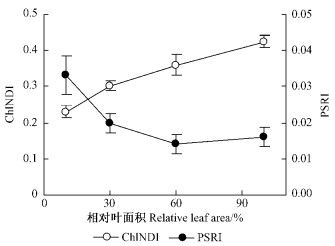
从图 2看出,叶片展开过程中,叶绿素相对含量(ChlNDI)不断上升。而类胡萝卜素和叶绿素比值(PSRI)的变化趋势与叶绿素含量的变化完全相反,幼叶明显高于完全展开叶。随着叶片的展开,类胡萝卜素与叶绿素含量的相对比值逐渐变小,但在相对叶面积达到60%后,基本不再改变。
|
图 2 板栗叶片展开过程中叶绿素相对含量(ChlNDI)和类胡萝卜素/叶绿素(PSRI)的变化 Figure 2 Changes of ChlNDI and PSRI during the expansion of chestnut leaves |
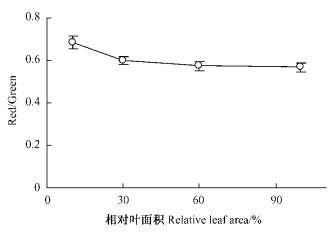
从图 3看出,相对叶面积10%左右的幼叶中,花青素的相对含量(Red/Green)明显较高,随后迅速下降,相对叶面积达30%时已与完全展开叶差异不显著。
|
图 3 板栗叶片展开过程中花青苷相对含量(Red/Green)的变化 Figure 3 Changes of Red/Green during the expansion of chestnut leaves |
从叶绿素荧光诱导动力学曲线可以看出,叶片展开过程中,初始荧光(FO)变化较小,而最大荧光(FM)显著增加,但当相对叶面积达到60%后,基本不再改变(图 4)。

|
图 4 板栗叶片展开过程中叶绿素诱导动力学曲线的变化 Figure 4 Changes of the chlorophyll a fluorescence transients during the expansion of chestnut leaves |
植物快速叶绿素荧光诱导曲线中包含着大量关于PSⅡ反应中心原初光化学反应的信息。为了解展叶过程中板栗叶片光系统Ⅱ(PSⅡ)的变化,对叶绿素荧光诱导曲线进行JIP-Test分析。
在展叶过程中,叶绿素荧光诱导动力学曲线有很大的改变,但反映原初光化学反应量子效率的指标(φPo)仅有较小的增加(< 12.2%);而叶片捕获的光能用于电子传递的量子效率(φEo)却随叶面积的增加而明显提高(图 5)。

|
图 5 板栗叶片展开过程中原初光化学反应量子效率(φPo)和捕获光能用于电子传递量子效率(φEo)的变化 Figure 5 Changes of φPo and φEo during the expansion of chestnut leaves |
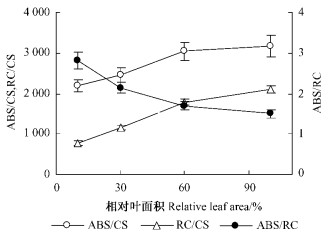
展叶过程中,单位面积吸收的光能(ABS/CS)、单位面积反应中心的数目(RC/CS)的变化趋势与光合速率(Pn)基本相似,随叶面积的增加而上升,当相对叶面积达到60%左右时,已接近完全展开叶片;而单位反应中心吸收的光能(ABS/RC)一直下降(图 6)。
|
图 6 板栗叶片展开过程中单位面积吸收的光能(ABS/CS)、单位面积反应中心的数目(RC/CS)、单位反应中心吸收的光能(ABS/RC)的变化 Figure 6 Changes of ABS/CS, RC/CS and ABS/RC during the expansion of chestnut leaves |
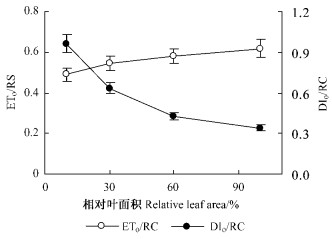
幼叶中单位反应中心用于电子传递的能量(ETO/RC)略低于完全展开叶,但其单位反应中心的热耗散(DIO/RC)随相对叶面积的增加而逐步减少(图 7)。
|
图 7 板栗叶片展开过程中单位反应中心用于电子传递的能量(ETO/RC)、单位反应中心的热耗散(DIO/RC)的变化 Figure 7 Changes of ETO/RC and DIO/RC during the expansion of chestnut leaves |
从整个叶片展开过程看,中午强光下叶黄素库脱环化程度(ΔPRI)不断降低,但相对叶面积10%左右的幼叶略低于30%叶片(图 8)。

|
图 8 板栗叶片展开过程中中午强光下叶黄素库脱环化程度(ΔPRI)的变化 Figure 8 Changes of ΔPRI during the expansion of chestnut leaves |
板栗幼叶的原初光化学反应量子效率(φPo)和单位反应中心用于电子传递的能量(ETO/RC)仅略低于完全展开叶(图 5,7),表明相对叶面积达到10%后,幼叶的原初光化学反应已基本建成。而光合能力的提升是逐步完成的,相对叶面积达到60%时,净光合速率才达到完全展开叶片的80%左右,说明幼叶的光合能力主要受碳同化暗反应的限制。
板栗幼叶的叶绿素含量较低,单位面积吸收的光能(ABS/CS)少,但因单位面积有活性的反应中心的数目(RC/CS)低,反而导致单位反应中心吸收的光能(ABS/RC)更多,使幼叶中比完全展开叶存在更多的过剩激发能(DIO/RC)。对于过剩的激发能,植物主要是通过叶黄素循环进行热耗散掉(Muller et al., 2001; Kulheim et al., 2002)。ΔPRI为反映叶黄素循环脱环化色素相对含量的指标(Gamon et al., 1999),较高的ΔPRI表明板栗幼叶通过叶黄素循环耗散过剩激发能(图 8)。因此,板栗幼叶中相对较高的类胡萝卜素含量,其主要功能应是为避免过剩激发能对光合系统的破坏。
研究表明:当植物叶片暴露在强光下,或逆境抑制叶片对光能的利用从而产生过量激发能时,与花青苷生成有关的关键酶表达上调(Christie et al., 1994; Chalker-Scott, 1999)。花青苷主要通过遮光、作为抗氧化剂来减轻过量光能对植物的伤害(Feild et al., 2001; Hoch et al., 2001; Gould et al., 2002; Close et al., 2005)。刚抽生的板栗幼叶花青苷含量在整个发育阶段最高(图 3),我们认为这是幼叶的一种重要的光保护防御措施。酶促反应的叶黄素循环也随发育时间而完善,相对叶面积10%幼叶的ΔPRI与30%叶片相当甚至略低(图 8),此时花青苷的出现可加强对刚抽生幼叶的光保护。
本研究表明:相对于成熟叶片,板栗幼叶的原初光化学反应已基本建成,且有较好的保护防御机制保护光合机构。光合速率除受到光化学反应的影响外,也受光合暗反应碳同化能力的影响。板栗幼叶展开过程中,暗反应碳同化能力随发育时间如何变化,还有待于进一步的深入研究。
吴长艾, 孟庆伟, 邹琦. 2001. 叶黄素循环及其调控. 植物生理学通讯, 37(1): 1-5. |
武海, 许大全. 1993. 依赖叶黄素循环的非辐射能量耗散在防御珊瑚树叶片光抑制破坏中的作用. 植物生理学报, 19(2): 181-187. DOI:10.3321/j.issn:1671-3877.1993.02.013 |
Chalker-Scott L. 1999. Environmental significance of anthocyanins in plant stress responses. Photochemistry and Photobiology, 70: 1-9. DOI:10.1111/php.1999.70.issue-1 |
Christie P J, Alfenito M R, Walbot V. 1994. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta, 194: 541-549. DOI:10.1007/BF00714468 |
Close D C, Beadle C L. 2003. The Ecophysiology of foliar anthocyanin. The Botanical Review, 69(2): 149-161. DOI:10.1663/0006-8101(2003)069[0149:TEOFA]2.0.CO;2 |
Close D C, Bealdle C L. 2005. Xanthophyll-cycle dynamics and rapid induction of anthocyanin synthesis in Eucalyptus nitens seedlings transferred to photoinhibitory conditions. Journal of Plant Physiology, 162: 37-46. DOI:10.1016/j.jplph.2003.10.001 |
Drumm-Herrel H, Mohr H. 1985. Photosensitivity of seedlings differing in their potential to synthesize anthocyanin. Physiologia Plantarum, 64: 60-66. DOI:10.1111/ppl.1985.64.issue-1 |
Edge R, McGarvey D J, Truscotte T G. 1997. The carotenoids as anti-oxidants. Journal of Photochemistry and Photobiology B: Biology, 41: 189-200. DOI:10.1016/S1011-1344(97)00092-4 |
Field T S, Lee D W, Holbrook N. 2001. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiology, 127: 566-574. DOI:10.1104/pp.010063 |
Gamon J A, Surfus J S. 1999. Assessing leaf pigment content and activity with a reflectometer. New Phytologist, 143: 105-117. DOI:10.1046/j.1469-8137.1999.00424.x |
Gilmore A M, Yamamoto H Y. 1991. Zeaxanthin formation and energy dependent fluorescence quenching in pea chlorlplasts. Plant Physiology, 96: 636-643. |
Gould K S, Mckelvie J, Markham K R. 2002. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell Environment, 25: 1261-1269. DOI:10.1046/j.1365-3040.2002.00905.x |
Hoch W A, Zeldin E L, McCown B H. 2001. Physiological significance of anthocyanins during autumnal leaf senescence. Tree Physiology, 21: 1-8. DOI:10.1093/treephys/21.1.1 |
Jiang C D, Jiang G M, Wang X Z, et al. 2006. Increased photosynthetic activities and thermostability of photosystem Ⅱ with leaf development of elm seedlings (Ulmus pumila) probed by the fast fluorescence rise OJIP. Environmental and Experimental Botany, 58: 261-268. DOI:10.1016/j.envexpbot.2005.09.007 |
Kulheim C, Agren J, Jansson S. 2002. Rapid regulation of light harvesting and plant fitness in the field. Science, 297: 91-93. DOI:10.1126/science.1072359 |
Merzlyak M N, Gitelson A A, Chivkunova O B, et al. 1999. Non-destructive optical detection of leaf senescence and fruit ripening. Physiologia Plantarum, 106: 135-141. DOI:10.1034/j.1399-3054.1999.106119.x |
Muller P, Li X P, Niyogi K K. 2001. Non-photochemical quenching: a response to excess light energy. Plant Physiology, 125: 1558-1566. DOI:10.1104/pp.125.4.1558 |
Schansker G, Srivastava A, Govindjee, et al. 2003. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Functional Plant Biology, 30: 785-796. DOI:10.1071/FP03032 |
Strasser B J, Strasser R J. 1995. Measuring fast fluorescence transients to address environmental questions: the JIP test//Mathis P. Photosynthesis: from light to biosphere. Dordrecht: Kluwer Academic Publishers, 5: 977-980.
|
 2009, Vol. 45
2009, Vol. 45

