文章信息
- Shang Aiqin, Sun Zhenyuan, Zhao Liangjun
- 尚爱芹, 孙振元, 赵梁军
- High Efficient Regeneration in Vitro from Hypocotyl of Euonymus fortunei var. radicans
- 爬行卫矛下胚轴高频离体再生体系的建立
- Scientia Silvae Sinicae, 2009, 45(2): 136-141.
- 林业科学, 2009, 45(2): 136-141.
-
文章历史
- 收稿日期:2008-02-02
-
作者相关文章
2. 河北农业大学园艺学院 保定 071001;
3. 中国林业科学研究院林业研究所 国家林业局林木培育重点实验室 北京 100091
2. College of Horticulture, Hebei Agricultural University Baoding 071001;
3. Key Laboratory of Tree Breeding and Cultivation of State Forestry Administration Research Institute of Forestry, Chinese Academy of Forestry Beijing 100091
Euonymus fortunei var. radicans, an indigenous species of China, is a climbing, everygreen-woody plant. Having several desirable traits such as tolerance to sulfur oxids and dioxide, and wide adaptability to diverse ecological conditions (Pan et al., 2004), this species is widely grown as an ornamental plant in temperate and subtropical areas of China. Euonymus plants are often seriously destructed by aphis (Hodgson et al., 1999). E. fortunei var. radicans is often attacked by aphids (Myzus persicae) and coccid (Ceroplastes japonicus) too. Chemical treatment not only costs much but also leads to environmental pollution. Novel cultivars bred for insect resistance would greatly improve the usefulness of E.fortunei var. radicans.
Genetic improvement of woody plants by conventional methods is constrained by their long juvenile period, complex reproductive biology and high degree of heterozygosity (Vigouroux, 1992). Plant genetic transformation may help to solve these problems by introducing desirable traits into these species. To perform such a transformation, establishment of an efficient in vitro plant regeneration system must be developed.
Explant was one of the most important factors that influenced the efficient in vitro plant regeneration system. The types of explant, sampling time, explant age and polarity were all limiting factors to shoot regeneration. In Euonymus fortunei efficiency of adventitious shoots induction from stem was higher than that from leaf disc(Yin et al., 2004), but the regeneration frequency of adventitious shoots from hypocotyls was higher than that from stem (Wang et al., 2004). In pre-experiments, we found the shoots regeneration frequency from leaf disc was lower than that from hypocotyl (data not shown). Against these results, we used hypocotyl as explant to establish high efficient regeneration system of E. fortunei var. radicans.
Hypocotyl has been proven to be one of the most often employed explants for shoot regeneration in many woody species, such as Liquidambar styraciflua (Kim et al., 1997), Eucalyptus nitens and E. globulus (Bandyopadhyay et al., 1999), Psidium guajava (Singh et al., 2002), Annona squamosa (Nagori et al., 2004), Euonymus japonicus`Cuzhi' (Shang et al., 2005) and Feronia limonia (Vyas et al., 2005). To the best of our knowledge, shoot regeneration from hypocotyl of E. fortunei var. radicans has never been reported.
Direct shoot regeneration system has been used to genetic transformation. Cui et al. (2004) reported that the direct shoot regeneration system was successfully used to achieve the transformation of Antirrhinum majus in a short time period. So the goal of the study reported here was to establish an efficient plant regeneration system from hypocotyls segments of E.fortunei var. radicans, which could be employed for its transformation manipulation.
1 Materials and methods 1.1 Seed germinationSeeds of E.fortunei var. radicans collected during October, 2003 from Experimental Station of Chinese Academy of Forestry, Beijing, China were surface sterilized with 70% ethanol for 1 to 2 min and then treated with HgCl2 for 20 min, following by 4 or 5 times of washing in sterile distilled water. Sterilized seeds were inoculated on a basic medium (BM) composed of Murashige and Skoog (1962) (MS) medium containing 3% sucrose and 0.6% agar (w/v). The medium pH was adjusted to 5.8 prior to autoclaving at 121 ℃ for 15 min.
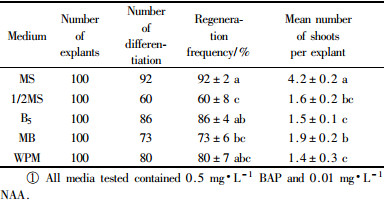
1.2 Shoot regenerationFour weeks after seed germination, hypocotyl explants (each 5 to 6 mm in length) were excised and used for shoot regeneration. Otherwise stated elsewhere, the explants were cultured horizontally on a shoot regeneration medium (SRM) composed of BM containing 0.5 mg·L-1 BAP and 0.01 mg·L-1 NAA for 4 weeks under the culture conditions as described for seed germination. After 30 days of culture, shoot regeneration was evaluated by regeneration frequency and mean number of shoots per explant. Regeneration frequency was defined as percentage of explants producing shoots longer than 5 mm. In this stage, 3 experiments were designed to optimize parameters for shoot regeneration. In the first experiment, the explants were cultured horizontally on BM supplemented with various concentrations and combinations of 6-benzylaminopurine (BAP), kinetin (KT), thidiazuron (TDZ) and naphthaleneacetic acid (NAA) (Tab. 1). In the second experiment, the explants were cultured horizontally on 5 different media: BM, half-strength BM, B5 (Gamborg et al., 1968), salts of MS plus B5's vitamines (MB) and woody plant medium (WPM) (Lloyd and McCown, 1980). All tested media contained 0.5 mg·L-1 BAP and 0.01 mg·L-1 NAA. In the third experiment, effect of orientation of explants on SRM on shoot regeneration was investigated. Explants were placed horizontally, vertically and vertically inverted, respectively, on SRM.
|
|
Hypocotyl explants cultured on SRM for various durations (4, 7 and 11days) were collected, fixed in formalin:acetic acid:alcohol, 1:1:18, for 24 h, dehydrated through an incremental ethanol series (50%, 70%, 85% and 95%) and stored in 100% ethanol. After embedding in paraffin, 10-μm-thick sections were cut by a microtome and stained (Jensen, 1962) with 1% safranine and 1% fast green. The sections were observed with an Olympus optical microscope.
1.4 Multiplication of shootsRegenerated shoots (2 to 3 cm long) were separated from explants and subcultured onto a shoot proliferation medium composed of BM containing 1.0 mg·L-1 BAP and 0.1 mg·L-1 NAA for shoot multiplication. Culture conditions were the same as for seed germination. Subculture was performed every 4 weeks.
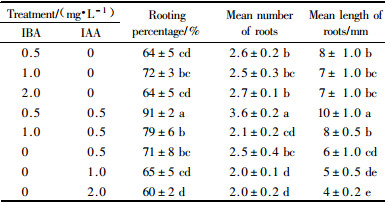
1.5 Rooting and acclimatizationOtherwise stated elsewhere, proliferating shoots about 2 to 3 cm in length were excised and transferred to half-strength MS supplemented with 100 mg·L-1 activated charcoal (AC), 0.5 mg·L-1 IBA and 0.5 mg·L-1 IAA, to induce root formation. One experiment was carried out to test effect of concentrations and combinations of auxin on root formation (Tab. 4). Percentage of rooting and root number per shoot were recorded after 30 days of culture. Culture conditions were the same as for seed germination. Plantlets with well-developed roots were removed from culture bottles and washed free of agar. After then, they were grown into black plastic pots containing a mixture of greenhouse compost at 24 ℃ under dim light conditions and covered with a plastic bag to maintain high humidity and prevent plantlets from wilting. After 2 weeks, the plastic bag was gradually opened to reduce humidity. After 4 weeks of hardening, the plants were moved to greenhouse conditions. Survival of plants was recorded after 4 weeks of transfer to greenhouse.
|
|
In all experiments, at least 100 explants were included in each of treatment of three replicates, and each experiments were carried out twice. Results were described by percentages of hypocotyl segments that regenerated shoots (regeneration frequency) (means±SE) and the number of shoots per regenerating explant (means±SE). Results were analyzed with ANOVA and Duncan's multiple range test (P=0.05) using the Statistical Analysis System package (SAS Institute v. 9.1).
2 Results 2.1 Influence of plant growth regulators on shoot regenerationOf the three cytokinins tested, BAP was most effective in induction of adventitious shoots, while TDZ completely failed to induce shoot regeneration, regardless of its concentration and combination with NAA (Tab. 1). TDZ at 0.5~2.0 mg·L-1 alone or combining with NAA at 0.01 mg·L-1 promoted only callus formation. Although either BAP or KT alone was able to induce formation of adventitious shoots, regeneration frequency was low: 15%~40%. Treatments combining BAP with NAA significantly improved shoot regeneration with the best results (92% of regeneration frequency and 4.2 of shoots per explant) obtained using 0.5 mg·L-1 BAP and 0.01 mg·L-1 NAA (Tab. 1). Compared with the other treatments by SSR, the results were significant. Therefore, shoot regeneration medium containing 0.5 mg·L-1 BAP and 0.01 mg·L-1 NAA was always used in all the following experiments.
2.2 Influence of the media on shoot regenerationAlthough hypocotyl explants inoculated on all the media tested were able to regenerate adventitious shoots, optimal regeneration frequency (92%) and highest number (4.6) of shoots per explants were obtained when MS medium was used (Tab. 2). This result was not significant difference compared to that of B5 and WPM media, but there was a significant difference compared to that of 1/2MS and MB media. Based on these results, MS was consistently adopted in the following experiments.
|
|
All three orientation treatments of hypocotyl explants on the medium were able to produce adventitious shoot formation. Both horizontal and vertically upright orientation on the medium produced comparable and significantly high shoot regeneration, compared with vertically inverted orientation (Tab. 3). When the explants were vertically inverted on the medium, callus formation was found at both proximal and distal parts, while adventitious shoots were regenerated only at distal part of the explants (Plate Ⅰ-1). The explants that had been vertically cultured with upright position on the medium produced both callus and adventitious shoots only at distal part (Plate Ⅰ-2). The explants, when placed horizontally on the medium, developed adventitious shoots at all parts of explants (Plate Ⅰ-3).
|
|

|
PlateⅠ 1. Vertically inverted orientation (bar= 1 mm); 2. Vertically upright orientation (bar = 1 mm); 3. Horizontal orientation (bar = 1 mm); 4. Active division of localized subepidermal cells forming meristematic zone at day 4 of culture (bar= 100 μm); 5. Organized development of meristematic nodule at day 7 of culture (bar =100 μm); 6. Adventitious buds a( day 11 of culture (bar = 500 μm); 7. Cultured for 15 d, showing a well-developed shoot bud and the leaves directly developed from the both sides of the bud primodium (bar=500 μm); 8. Shoot multiplication on shoot proliferation medium (bar = 1 cm): 9. Root formation on rooting medium (bar = 1 cm). |
When hypocotyl explants were cultured on SRM for 4 days, small and green protuberances were observed. These protuberances eventually developed into adventitious shoots in about 11 days without callus formation. Histological studies revealed that adventitious shoots directly generated from subepidermal cells of hypocotyl explants without a phase of callus formation. Meristematic activities occurred in subepidermal cells of the hypocotyl explants after 4 days of culture (Plate Ⅰ-4). Continuous division of these subepidermal cells formed meristematic nodules after 7 days of culture (Plate Ⅰ-5). Further differentiation of these meristematic cells eventually led to formation of well-developed bud primordium after 11 days of culture (Plate Ⅰ-6). Leaves developed directly from both sides of the shoot bud after 15 days of culture (Plate Ⅰ-7).
2.5 Multiplication of shoots and establishment of rooted cuttings in the greenhouseWhen adventitious shoots produced on the hypocotyl explants reached 2~3 cm in length, they were excised from the parent culture and transfered to BM containing 1.0 mg·L-1 BAP and 0.1 mg·L-1NAA for multiplication. After 30 days of culture, a 3~5 multiplication rate was obtained (Plate Ⅰ-8). A further increase in BAP concentration reduced multiplication rate, while an increased NAA promoted callus formation (data not shown). Treatments of either IBA (0.5~2 mg·L-1) or IAA (0.5~2.0 mg·L-1) were able to induce root formation of microshoots(Tab. 4). There was no difference of concentrations in root formation. The best results of root formation (91% of rooting percentage, 3.6 of mean root number and 11 mm of root length) were obtained using a treatment combining 0.5 mg·L-1 IBA and 0.5 mg·L-1 IAA (Plate Ⅰ-9). Ninety percent of plants survived hardening and were successfully established under greenhouse conditions.
3 DiscussionIn previous studies, the role of BAP in adventitious shoot bud differentiation has been demonstrated in a number of cases using different explants. With Annona squamosa, Nagori et al. (2004) compared effects of 3 cytokinins on adventitious shoot formation of hypocotyl explants, and found that the BAP was much more effective than TDZ and KT in shoot regeneration. Similar results of effects of BA on shoot regeneration from hypocotyl explants were also observed in Feronia limonia (Vyas et al., 2005) and Sesbania rostrata (Jha et al., 2002). Although BA alone was able to induce shoot regeneration, a combination of BA and auxin frequently evoked a significant response of treated explants to adventitious shoot formation. Vyas et al. (2005) reported in Feronia limonia, the response of medium containing 4.4 μmol·L-1 BA and 0.05 μmol·L-1 NAA was definitely better than that obtained with individual BA. It was also reported that the effects of BA and NAA on direct adventitious shoot bud formation were highly significant in Phragmites communis (Guo et al., 2004). A recent study by Shang et al. (2005) on regeneration from hypocotyl of Euonymus japonicus belonging to the same genus of E.fortunei var. radicans, also demonstrated a combination of BA at 1.5 mg·L-1 and NAA at 0.05 mg·L-1 resulted in the highest frequency of shoot regeneration. Results of the present study showed that addition of BAP to the shoot regeneration medium was able to induce adventitious shoot formation, and it was more effective than other cytokinins. We also observed that a combination of BAP (0.5 mg·L-1) with NAA (0.01 mg·L-1) significantly increased shoot regeneration of hypocotyl explants. These results were consistent with those of Nagori et al. (2004), Jha et al. (2002), Vyas et al. (2005), Guo et al. (2004), and Shang et al. (2005).
TDZ has been reported to promote adventitious shoot formation from hypocotyl explants of several plant species such as Liquidambar styraciflua (Kim et al., 1997), Linum (Mundhara et al., 2002) and Psidium guajava (Singh et al., 2002). In the present study, treatments using TDZ alone or combining with NAA completely failed to develop adventitious shoots, but only induce callus formation. Similar results were reported by Nagori et al. (2004), who used hypocotyl segments of Annona squamosa. These data again reflected that effect of TDZ on organogenesis depended on its concentration and plant species.
Orientation of explants on the medium was reported to influence adventitious shoot formation. Changing the orientation of explants from vertically upright to horizontal drastically decreased the frequency of shoot induction as well as the length of the shoot apical end (Saini et al., 2002). In a study of Vigna mungo (Saini et al., 2002), epicotyl explants inserted vertically in the medium or placed horizontally on the medium formed adventitious shoots, while all explants inserted in an inverted position did not regenerate shoots, but developed callus. Saini et al. (2002) suggested that the orientation might affect shoot regeneration by interacting with polarity of explants. Results from the present study also demonstrated that orientation of explants on the medium influenced adventitious shoot formation.
Type of medium had an effect on the adventitious shoot bud regeneration. In sweet and sour cherry (Prunus avium), Tang et al. (2002) reported that shoot regeneration was more efficient when using WPM medium as their basal medium than MS and other media. Whereas, with Annona squamosa, Nagori et al. (2004) found the highest frequency of shoot regeneration was obtained on B5 medium, the maximum number of shoot buds per explant was obtained on MS medium. MS was more suitable for shoot regeneration from hypocotyl explants of Euonymus japonicus (Shang et al., 2005). Results from the present study showed that MS was more efficient than any other media.
In conclusion, a highly efficient protocol for shoot regeneration of hypocotyl explants of E.fortunei var. radicans was developed in the present study. With the optimized parameters, 94% of regeneration frequency and 5.1 of shoots per explant were achieved. The regenerated shoots were micropropagated, rooted and finally established under greenhouse conditions.
Bandyopadhyay S, Cane K, Rasmussen G, et al. 1999. Efficient plant rengeneration from seedling explants of two commercially important temperate eucalypt species-Eucalyptus nitens and E. globulus. Plant Sci, 140: 189-198. DOI:10.1016/S0168-9452(98)00221-0 |
Cui Minlong, Ezura H, Nishimura S, et al. 2004. A rapid Agrobacterium-mediated transformation of Antirrhnum majus L. by using direct shoot regeneration from hypctocyl explants. Plant Sci, 166: 873-879. DOI:10.1016/j.plantsci.2003.11.021 |
Gamborg O L, Miller R A, Ojima K. 1968. Nutrient requirements of suspension culture of soybean cells. Exp Cell Res, 50: 150-158. |
Guo Y M, Yang Y G, Guo Y, et al. 2004. Adventitious shoot bud formation and plant regeneration from in vitro-cultured stem segments of reed (Phargmites communis Trin.). In Vitro Cell Dev Biol, 40: 412-415. DOI:10.1079/IVP2004553 |
Hodgson D J, Godfray H C J. 1999. The consequences of clustering by Aphis fabae foundresses on spring migrant production. Oecologia, 18(4): 446-452. |
Jensen W A. 1962. Botanical histochemistry. San Francisco: Freeman.
|
Jha S K, Prakash S, Jain N, et al. 2002. Production of adventitious shoots and plantlets from hypocotyl explants of Sesbania rostrata (Bremek & Obrem.). In Vitro Cell Dev Biol, 38: 430-434. DOI:10.1079/IVP2002313 |
Kim M K, Sommer H E, Bongarten B C, et al. 1997. High-frequency induction of adventitious shoots from hypocotyl segments of Liquidambar styraciflua L. by thidiazuron. Plant Cell Rep, 16: 536-540. |
Lloyd G, McCown B. 1980. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc Intl Plant Prop Soc, 30: 421-427. |
Mundhara R, Rashid A. 2002. Stimulation of shoot-bud regeneration on hypocoyl of Linum seedlings, on a transient withdrawal of calcium: effect of calcium, cytokinen and thidiazuron. Plant Sci, 162: 211-214. DOI:10.1016/S0168-9452(01)00541-6 |
Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant, 15: 473-497. DOI:10.1111/ppl.1962.15.issue-3 |
Nagori R, Purohit S D. 2004. In vitro plantlet regeneration in Annona squamosa through direct shoot bud differentiation on hypocotyl segments. Hort Sci, 99: 89-98. DOI:10.1016/S0304-4238(03)00084-0 |
Pan Qinghua(潘青华), Song Wan(宋婉), Lu Renqiang(鲁韧强), et al. 2004. Germplasm resource and variation of Euonymus fortunei. Journal of Beijing Forestry University(北京林业大学学报), 26(2): 58-62. http://en.cnki.com.cn/Article_en/CJFDTOTAL-BJLY200402013.htm
|
Saini R, Jaiwal P K. 2002. Age, position in mother seedling, orientation, and polarity of the epicotyl segments of blackgram (Vigna mungo L. Hepper) determines its morphogenic response. Plant Sci, 163: 101-109. DOI:10.1016/S0168-9452(02)00062-6 |
Shang Aiqin(尚爱芹), Cai Han(蔡汉), Yan Xiaojie(闫晓洁), et al. 2005. Plant regenertation from in vitro cultured hypocotyl explants of Euonymus japonicus 'Cuzhi'. Agri Sci in China(中国农业科学), 38: 2502-2507.
|
Singh S K, Meghwal P R, Sharma H C, et al. 2002. Direct shoot organogenesis on hypocotyl explants from in vitro germinated seedlings of Psidium guajava L. cv. Allahabad ssfeda. Hort Sci, 95: 213-221. DOI:10.1016/S0304-4238(02)00036-5 |
Tang H, Ren Z L, Reustle G, et al. 2002. Plant regeneration from leaves of sweet and sour cherry cultivars. Hort Sci, 93: 235-244. DOI:10.1016/S0304-4238(01)00328-4 |
Vigouroux A. 1992. Preliminary results for obtaining a plane tree resistant to canker stain and adapted to European conditions. Acta Hort, 320: 91-96. |
Vyas S, Joshi N, Tak K, et al. 2005. In vitro adventitious shoot bud differentiation and plant regeneration in Feronia limonia L. (Swingle). In Vitro Cell & Dev Biol, 41(3): 296-302. |
Wang Maoliang(王茂良), Zhao Liangjun(赵梁军), Ren Guifang(任桂芳), et al. 2004. Adventitious bud regenerating system of Euonymus fortunei. Acta Horticulturae Sinica(园艺学报), 31(2): 241-244. http://en.cnki.com.cn/Article_en/CJFDTOTAL-YYXB200402027.htm
|
Yin Shuping(尹淑萍), Jin Wanmei(金万梅), Lu Renqiang(鲁韧强), et al. 2004. Shoot regeneration and agrobacterium-mediated transformation of Euonymus fortunei Hand. -Mazz//Zhang Qixiang (张启翔). Advances in ornamental horticulture of China(中国观赏园艺研究进展). Beijing: China Forestry Press (北京: 中国林业出版社), 185-188.
|
 2009, Vol. 45
2009, Vol. 45