文章信息
- Zhang Cunxu, Zhang Huanling, Jia Xiaoming, Dong Jianguo
- 张存旭, 张焕玲, 贾小明, 董建国
- Proliferation, Maturation and Germination of Somatic Embryos in Quercus variabilis
- 栓皮栎体胚的增殖、成熟和萌发
- Scientia Silvae Sinicae, 2008, 44(6): 39-44.
- 林业科学, 2008, 44(6): 39-44.
-
文章历史
Received on: Apr., 23, 2007
-
作者相关文章
Quercus variabilis is one of the most valuable forest species with multiple uses. Besides as a timber supplier, the tree is a primary source of cork for industrial uses. The acorn cups serve as a source of tannin extract. The logs are used for fuel, burning charcoal, cultivation of mushroom for rural development. It is known that Q. variabilis population has been decreasing in China mainly due to overexploiting in the past decades. Therefore, it is urgent to develop technologies for conservation, genetic improvement and regeneration of this broadleaf species.
Since oaks are mainly reproduced through seed production, the problems in tree improvement programs through conventional methods are the long reproductive cycle, the variations in acorn production and the difficulty in seed storage. Unfortunately conventional asexual methods have been unsuccessful. Many efforts have been made in propagating oak species through somatic embryogenesis. The successful species include Q. rubra(Gingas et al., 1989), Q. robur (Chalupa, 1990), Q. petraea(Jörgensen, 1993), Q. suber(Manzanera et al., 1993), Q. acutissima(Kim et al., 1997) and Q. ilex(Mauri et al., 2003). Somatic embryogenesis in Q. variabilis was induced from zygotic embryos by Kim et al.(1995) and Zhang et al.(2005b).
A major problem in somatic embryogenesis in woody plants is the low rate for somatic embryos to convert into plants. The same problem in Quercus species(Bueno et al., 2000; Cuenca et al., 1999) was possibly due to poor quality, lack of maturation and desiccation tolerance in somatic embryos(Etienne et al., 1993). In addition to osmotic agents, carbohydrates have been used to improve embryo quality before germination for some species(Xing et al., 1999; Robichaud et al., 2004). In our previous study(Zhang et al., 2005a) embryogenic lines of Q. variabilis were established from leaf explants. However the previous research was focused on induction of somatic embryo only. The objective of the current research was to develop protocol for proliferation, maturation and germination of somatic embryos in Q. variabilis.
1 Materials and methods 1.1 Plant material and culture conditionEmbryogenic cultures were initiated from leaf explants as previously described(Zhang et al., 2005a). Briefly, leaf segments of one genotype collected from one-month-old seedlings were cultured in 70 mm × 90 mm screw-capped jars containing MS(Murashige et al., 1962) basal medium supplemented with 1.0 mg·L-1 α-naphthaleneacetic acid(NAA) and 0.5 mg·L-1 6-benzyladenine(BA). Embryogenic cultures were transferred to fresh medium once in 4 weeks. Cultures were maintained under a 16-h(light)/8-h(dark) photoperiod(50 μmol·s-1 m-2) provided by fluorescent lamps(Philips, China). Cultures were grown at day temperature of(25±1)℃ and(20±1)℃ at night. Unless otherwise indicated, the media contained 3% sucrose and 0.5% agar(Japan). Media pH was adjusted to 5.8 before autoclave. Media were sterilized by autoclave for 20 min at 121 ℃. Abscisic acid(ABA) was filter-sterilized with 0.22 μm filters and added to media after autoclave and when media cooled down to about 50 ℃.
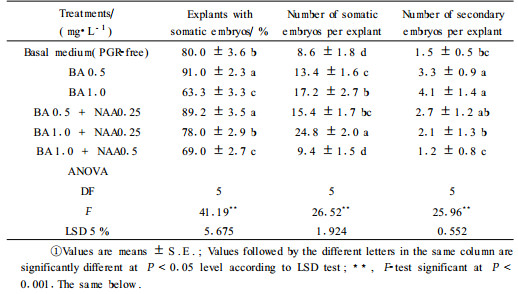
1.2 Somatic embryos proliferationProliferation of somatic embryos in proembryogenic masses(PEMs) was achieved by culturing 50~60 mg callus explants on MS medium without plant growth regulators(PGRs) or supplemented with BA at different concentrations(0.5 mg·L-1 and 1 mg·L-1) and 0.25 mg·L-1 NAA. After 6 weeks, the number of explants with somatic embryos, the number of somatic embryos per embryogenic callus, as well as the number of secondary embryos per explant were recorded.
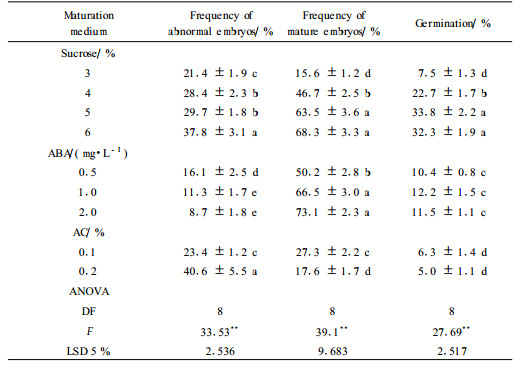
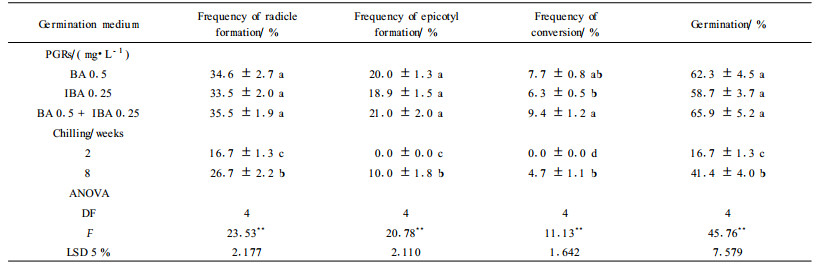
1.3 Somatic embryos maturation and germinationIn maturation experiments, white opaque or pale yellow cotyledonary somatic embryos were isolated from embryogenic cultures and cultured on different maturation media, which were MS medium with 0.5 mg·L-1 polyvinylpyrrolidone(PVP) supplemented 1) 3%, 4%, 5% or 6% sucrose; 2) 0.5, 1 or 2 mg·L-1 ABA; and 3) 0.1% and 0.2% activated charcoal(AC). After 4 weeks, the rates of mature embryos, explants with secondary embryos and abnormal embryos were recorded. The mature embryos were embryos consisting of an elongated hypocotyl with radicle, two cotyledons, and an epicotyl node. They were then transferred into germination medium(MS basal medium with 3% sucrose). The rates of germination were determined in 8 weeks. A somatic embryo was considered germinated when its radicle elongated to more than 5 mm. To test PGR effects on somatic embryo germination, mature embryos were selected and germinated on half-strength(1/2MS) medium containing 0.5 mg·L-1BA, 0.25 mg·L-1 indolebutyric acid(IBA) respectively or both. When the pre-germination cold treatments were tested, mature embryos were transferred to MS medium and placed at 4 ℃ in the dark for 2 or 8 weeks before transferring to germination medium. After 8 weeks on germination medium, performance was evaluated for embryo conversion, i.e. the percentages of embryos developed both roots and shoots.
1.4 Statistics analysisAll experiments were arranged in a completely randomized design. Each treatment was applied to 20 replicates. Five explants were cultured in each jar with 25 mL medium. The experiments were repeated three times. The number of somatic embryos per explant and percentages of proliferation, maturation and germination were analyzed by single factor analysis of variance following the General Linear Model procedure of the SAS statistical package(SAS Institute Inc., 1985) and significant differences between treatments were determined by LSD test at P < 0.05. In the case of percentages, a previous arcsine transformation of the variable was performed.
2 Results 2.1 Proliferation of somatic embryosExplants of PEMs(50~60 mg) with a white-creamy nodular-friable callus(Plate Ⅰ-1) which were induced on MS medium supplemented with 1.0 mg·L-1 NAA and 0.5 mg·L-1 BA, were further subcultured on fresh MS medium free or containing PGRs for somatic embryos development and proliferation(Plate Ⅰ-2, 3). Secondary somaticembryos mainly originated at the root pole and also on the cotyledons of the primary embryos(Plate Ⅰ-4). Anomalous morphologies such as the somatic embryos fused along their axes(Plate Ⅰ-5) and the embryos with three cotyledons were also observed(Plate Ⅰ-6).

|
图版Ⅰ |
After 6 weeks on embryo proliferation medium, significant differences(P < 0.001) between the treatments(Tab. 1) were observed in somatic embryo formation. BA in low concentration NAA(0.25 mg·L-1) was noted to be more productive in embryo proliferation process than the control(PGR-free)(P < 0.05 LSD test). The frequency of somatic embryo formation from explants ranged from 69% to 91%, with the mean number of embryos per explant from 8.6 to 24.8. The best result was obtained on 1 mg·L-1 BA and 0.25 mg·L-1 NAA. The capacity of somatic embryos for secondary embryogenesis was relatively low. The best treatment was the one including 1 mg·L-1 BA.
|
|
When embryos reached cotyledonary stage(Plate Ⅰ-7) on proliferation medium, they were transferred to maturation medium. The experiments have been carried out to achieve germination and conversion by promoting embryo maturation through adjusting sucrose, ABA and AC at different levels. An analysis of variance showed significant difference(P < 0.001) for all parameters between treatments(Tab. 2).
|
|
Sucrose concentration had a significant influence on morphology of somatic embryos at cotyledonary stage on various maturation media. Especially sucrose at high concentrations enhanced formation of large, thick, white cotyledons. When sucrose concentrations increased, the maturation and germination of somatic embryos were enhanced compared to the control(3% sucrose)(Tab. 2; P < 0.05 LSD test). However the rate of abnormal embryos increased markedly at the same time, especially with 6% sucrose. Increasing sucrose concentration from 5% to 6% showed no effect in either embryo maturation or germination.
In order to determine effects of ABA on somatic embryo maturation, ABA was added at different concentrations in MS medium containing 3% sucrose. The results showed that ABA favored somatic embryo maturation. The color of mature somatic embryos was light yellow-green or yellow green. However, adding ABA on maturation media did not promote somatic embryo germination markedly compared with high sucrose concentration e.g., 4%~6%(Tab. 2; P < 0.05 LSD test). In the experiment to test AC in maturation medium, the rate of embryo germination was not increased(Tab. 2). Over all, the best result was achieved with 5% sucrose. In this treatment, 63.5% embryos matured and 33.8% mature embryos germinated.
To promote germination, selected mature somatic embryos were cultured on medium containing PGRs. Epicotyl and/or radicle formation was observed in germinating somatic embryos. The epicotyl and radicle induction rate showed no difference in the three PGR germination treatments(P < 0.05 LSD test)(Tab. 3). However adding BA to MS medium resulted in somatic embryo conversion effectively. The best result was obtained with BA and IBA combination treatment(9.4% conversion). The chilling treatment was ineffective for epicotyl formation and plantlet conversion(Tab. 3).
|
|
Based on our analysis, we developed a protocol for somatic embryo proliferation, maturation, and germination. PEMs from induction medium were grown on proliferation medium consisted of MS basal medium with 1 mg·L-1 BA, 0.25 mg·L-1 NAA and 3% sucrose for 6 weeks, until reaching the cotyledonary stage(Plate Ⅰ-7). Individual cotyledonary embryos were then grown on the maturation medium(MS basal medium with 5% sucrose and 0.5% PVP) for 4 weeks until shoot and radicles were developed(Plate Ⅰ-8). Embryos were then germinated on the germination medium(1/2 MS basal medium with 0.5 mg·L-1 BA and 0.25 mg·L-1 IBA). After 1~2 weeks, the epicotyl developed(Plate Ⅰ-9). New leaves were induced within 3 weeks(Plate Ⅰ-10). After additional 2 weeks on germination medium, the plantlets(Plate Ⅰ-11) were transplanted into pots(Plate Ⅰ-12). The plantlets survived when they were transferred to the field after hardening treatment.
3 Discussion and conclusionsIn the present study, proliferation of somatic embryos of Q. variabilis occurred in two ways: 1) multiplication of somatic embryos from the explant through primary somatic embryogenesis; and 2) proliferation of secondary somatic embryos from primary somatic embryos through repetitive embryogenesis. Anomalous morphological somatic embryos were also observed. Similar anomalies were reported in Q. suber(Puigderrajols et al., 1996).
Once calli with embryogenically potential cells was determined, it was effective to induce embryos with BA or with a combination of BA and NAA at low level. This also confirmed that different PGRs and concentrations were required at various stages during somatic embryogenesis(Pasternak et al., 2002). The capacity of Q. variabilis somatic embryos for secondary embryogenesis was relatively low. The best treatment was the one including 1 mg·L-1 BA. Comparing with other Quercus species, Q. robur embryogenic cultures of zygotic origin have been maintained by repetitive embryogenesis on BA-containing media(Chalupa, 1995), but Q. suber secondary embryogenic lines were maintained on medium without PGRs(Fernández-Guijarro et al., 1995).
Various sucrose concentrations demonstrated significant influence on embryo maturation. The 3% sucrose was used for most media. Effects of sucrose at high concentration in maturation medium may result from high osmolarity, which has been used to prevent precocious embryo germination and enhance embryo maturation in other plant species(Janick et al., 1993; Pliego-Alfaro et al., 1996). In this experiment, the best result of somatic embryo maturation was obtained with 5% sucrose. Our results also agreed with Konan et al.(1994), who observed that 6% sucrose was the optimal concentration for somatic embryo development after an induction treatment using 2% sucrose in cassava (Manihot esculenta). Xing et al.(1999) improved somatic embryo conversion in American chestnut by raising sucrose levels from 2% at development stage to 6% at maturation stage. However, sucrose at high levels are not always beneficial for somatic embryo maturation. Here the 6% sucrose produced more abnormity embryos. A similar effect for controlling secondary embryogenesis was reported for high sucrose concentration in hole oak cultures(Mauri et al., 2003).
Abscisic acid is well known to play an important role in regulating embryo maturation storing carbohydrate reserves as well as surviving desiccation(Attree et al., 1993). Konan et al.(1994) found that 0.5 mg·L-1 ABA improved embryo initiation and development in the cultured mature cotyledons of cassava. In the present experiment, although ABA enhanced somatic embryos maturation, embryo germination was not improved. Vieitez(1995) reported that 0.1~2 mg·L-1 ABA had no effect on embryo maturation in hybrid chestnuts. Medium supplementation with AC was used to normalize development and germination of Q. variabilis somatic embryos, but the results presented here indicated no beneficial effects on embryo maturation. Sánchez et al.(2003) also observed that AC had no positive effect on germination and plant development from chestnut embryos.
Corredoira et al.(2003) indicated that the incorporation of 0.1 mg·L-1 BA was more effective than PGR-free medium in chestnut somatic embryos germination. In media supplemented with0.5 mg·L-1 BA, Catharanthus roseus somatic embryo germinated well(Junaid et al., 2006). In the present experiment the similar result was also observed.
Generally, the cold treatment is to break the dormancy, promote germination and synchronize shoot and root development(Merkle et al., 1995). Manzanera et al.(1993) reported that in Q. suber the epicotyl dormancy of somatic embryos could be broken by a chilling treatment. The application of a 2-month cold treatment at 4 ℃ to somatic embryos matured on medium with 3% maltose was necessary for embryos conversion in chestnut(Corredoira et al., 2003). But in the present experiment, chilling treatments were ineffective for inducing epicotyl and radicle formation, as well as plant conversion.
In conclusion, the protocol for the proliferation, maturation and germination of Q. variabilis somatic embryos was developed. However the low frequency for somatic embryos to convert into viable plantlets was still problematic. Further experiments are required to increase quality of mature embryo, in order to convert Q. variablies embryos to plants.
Attree S M, Fowke L C. 1993. Embryogeny of gymnosperms: advances in synthetic seed technology of conifers. Plant Cell Tiss Org Cult, 35: 1-35. DOI:10.1007/BF00043936 |
Bueno M A, Gómez A, Manzanera J A. 2000. Somatic and gematic embryogenesis in Quercus suber L. //Gupta P K, Jain S M, Newton R J. Somatic embryogenesis in woody plants: vol 6. Kluwer, Dordrecht, The Netherlands, 479-507.
|
Chalupa V. 1990. Plant regeneration by somatic embryogenesis from cultured immature embryos of oak (Quercus robur L.) and linden(Tilia cordata Mill.). Plant Cell Rep, 9: 398-401. |
Chalupa V. 1995. Somatic embryogenesis in oak (Quercus spp)//Jain S M, Gupta P K, Newton R J. Somatic embryogenesis in woody plants: vol 2 Angiosperms. Kluwer, Dordrecht, The Netherlands, 67-87.
|
Corredoira E, Ballester A, Vieitez A M. 2003. Proliferation, maturation and germination of Castanea sativa Mill. somatic embryos originated from leaf explants. Annals of Botany, 92: 129-136. DOI:10.1093/aob/mcg107 |
Cuenca B, San-José M C, Martínez M T, et al. 1999. Somatic embryogenesis from stem and leaf explant of Quercus robur L. Plant Cell Rep, 18: 538-543. DOI:10.1007/s002990050618 |
Etienne H, Montoro P, Michaux-Ferrière N, et al. 1993. Effects of desiccasion, medium osmolarity and abscisic acid on the maturation of Hevea brasiliensis somatic embryos. Journal of Experimental Botany, 44: 1613-1619. DOI:10.1093/jxb/44.10.1613 |
Fernández-Guijarro B, Celestino C, Toribio M. 1995. Influence of external factors on secondary embryogenesis and germination in somatic embryos from leaves of Quercus suber. Plant Cell Tiss Org Cult, 41: 99-106. DOI:10.1007/BF00051578 |
Gingas V M, Lineberger R D. 1989. Asexual embryogenesis and plant regeneration in Quercus. Plant Cell Tiss Org Cult, 17: 191-203. DOI:10.1007/BF00046867 |
Janick J, Kim Y H, Kitto S, et al. 1993. Desiccated synthetic seeds//Redenbaugh K. Synseeds: applications of synthetic seeds to crop improvement. CRC Press, Boca Rato, 409-425.
|
Jörgensen J. 1993. Embryogenesis in Quercus petraea and Fagus sylvatica. J Plant Physiol, 132: 638-640. |
Junaid A, Mujib A, Bhat M A, et al. 2006. Somatic embryo proliferation, maturation and germination in Catharanthus roseus. Plant Cell Tiss Org Cult, 84: 325-332. DOI:10.1007/s11240-005-9041-7 |
Kim Y W, Youn Y, Noh E R, et al. 1997. Somatic embryogenesis and plant regeneration from immature embryos of five families of Quercus acutissima. Plant Cell Rep, 16: 869-873. DOI:10.1007/s002990050336 |
Kim Y W, Youn Y, Noh E R. 1995. Somatic embryogenesis and germination from immature embryos of Quercus variabilis. Research Report of the Forest Genetics Research Institute, 31: 147-152. |
Konan N K, Sangwan R S, Sangwan B S. 1994. Somatic embryogenesis from cultured mature cotyledons of cassava (Manihot esculenta Crantz). Plant Cell Tiss Org Cult, 37: 91-102. DOI:10.1007/BF00043602 |
Manzanera J A, Astorga R, Bueno M A. 1993. Somatic embryo induction and germination in Quercus suber L. Silv Genet, 42: 90-93. |
Mauri P V, Manzanera J A. 2003. Induction, maturation and germination of holm oak(Quercus ilex L.) somatic embryos. Plant Cell Tiss Org Cult, 74: 229-235. DOI:10.1023/A:1024072913021 |
Merkle S A, Parrot W A, Filnn B S. 1995. Morphogenic aspects of somatic embryogenesis//Thorpe T A. In vitro embryogenesis in plants. Dordrecht: Kluwer Acdemic Publishers, 155-203.
|
Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant, 15: 473-497. DOI:10.1111/ppl.1962.15.issue-3 |
Pasternak T P, Prinsen E, Ayaydin F, et al. 2002. The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast derived cells of alfalfa. Plant Physiol, 129: 1807-1819. DOI:10.1104/pp.000810 |
Pliego-Alfaro F, Monsalud M J R, Litz R E, et al. 1996. Effect of abscisic acid, osmolarity and partial desiccation on the development of recalcitrant mango somatic embryos. Plant Cell Tiss Org Cult, 44: 63-70. DOI:10.1007/BF00045914 |
Puigderrajols P, Fernández-Guijarro B, Toribio M, et al. 1996. Origin and early development of secondary embryos in Quercus suber L. Int J Plant Sci, 157: 674-684. DOI:10.1086/297389 |
Robichaud R L, Lessard V C, Merkle S A. 2004. Treatments affecting maturation and germination of American chestnut somatic embryos. J Plant Physiol, 161: 957-969. DOI:10.1016/j.jplph.2004.03.003 |
Sánchez M C, Martínez M T, Valladares S, et al. 2003. Maturation and germination of oak somatic embryos originated from leaf and stem explants: RAPD markers for genetic analysis of regenerants. J Plant Physiol, 160: 699-707. DOI:10.1078/0176-1617-00754 |
SAS Institute Inc. 1985. SAS User's Guide: Statistics. Version 5 Edition. Cary, NC: SAS Institute Inc.: 956.
|
Vieitez F J. 1995. Somatic embryogenesis in chestnut//Jain S M, Gupta P K, Newton R J. Somatic embryogenesis in woody plants Vol. 2: Angiosperms. Dordrecht : Kluwer Academic Publishers, 375-407.
|
Xing Z Z, Powell W A, Maynard C A. 1999. Development and germination of American chestnut somatic embryos. Plant Cell Tiss Org Cult, 57: 47-55. DOI:10.1023/A:1006360730898 |
Zhang Huanling(张焕玲), Zhang Cunxu(张存旭). 2005a. Embryogenic callus induction and proliferation lines construction of Quercus variabilis. J Northwest Forestry University(西北林学院学报), 20: 74-77. http://en.cnki.com.cn/Article_en/CJFDTotal-XBLX20050100I.htm
|
Zhang Cunxu(张存旭), Yao Zengyu(姚增玉), Zhao Zhong(赵忠). 2005b. Factors influencing the induction of somatic embryogenesis in Quercus variabilis. Scientia Silvae Sinicae(林业科学), 41: 174-177. http://www.linyekexue.net/EN/Y2005/V41/I2/174
|
 2008, Vol. 44
2008, Vol. 44