文章信息
- Li Keyou, Tang Derui, Li Lin, Zhu Hailan, Zhao Zhong, Hou Lin
- 李科友, 唐德瑞, 李林, 朱海兰, 赵忠, 侯琳
- Adventitious Bud Induction and Plantlet Regeneration in vitro from Mature Zygotic Embryos of Ponderosa Pine
- 离体条件下西黄松成熟合子胚不定芽的诱导及植株再生
- Scientia Silvae Sinicae, 2008, 44(5): 38-45.
- 林业科学, 2008, 44(5): 38-45.
-
文章历史
Received on: Mar., 12, 2007
-
作者相关文章
2. 桂林电子科技大学应用科技学院 桂林 541004
2. Applied Science and Technology College, Guilin University of Electronic Technology Guilin 541004
The most promising systems for the mass propagation of forest trees are the methods based on in vitro culture and plant regeneration. Besides conventional methods, there is an urgent need to develop effective protocols for plantlet regeneration in conifers by tissue culture, not only to supplement the nursery-derived seedling transplantation, but also for clonal propagation of elite genotypes of specific plant species and production of transgenic plants (Mathur et al., 1999; Tang et al., 2003). Plantlet regeneration in conifers occurs by somatic embryogenesis and organogenesis. Over the past 20 years, tremendous progress has been in the area of somatic embryogenesis in conifers. Somatic embryogenesis has many advantages for plant clonal propagation and genetic transformation, however, it has disadvantages too. Organogenesis is another tissue culture pathway that allows for in vitro regeneration of conifers. Adventitious organogenesis usually occurs on original explants or on primary cultures derived from original explants. Plantlets regeneration from a large number of conifers species have been successfully propagated via organogenesis using various explants. Almost all of the organogenesis systems developed for Pinus species have used embryonic tissues as starting material for plantlet regeneration.
Ponderosa Pine (Pinus ponderosa) is one of the most widespread conifers in North America, occurring across a variety of climate and edaphic conditions (Tuskan et al., 1990). Full seed production is unpredictable and delayed by time requirement for a tree to reach full reproductive capacity (Ellis et al., 1984). There have been reports on inducing bud and shoot formation from Ponderosa Pine by organogenesis. For instance, Ellis and Bilderback (1984; 1989) obtained buds from excised Ponderosa Pine embryos cultured on various media and plant growth regulators combination. Tuskan et al. (1990) reported influence of plant growth regulators, basal media and carbohydrate levels on in vitro development of Ponderosa Pine cotyledon explants. Lin et al. (1991) also reported that axillary buds were induced from immature shoot explant taken from terminal buds of branches from 29- and 34-year-old Ponderosa Pine. To the authors' knowledge, there is little information available in literature about Ponderosa Pine in vitro rooting; Until recently, there is some lack of knowledge about Ponderosa Pine in vitro rooting. The main purpose of the present study was to develop a method for the induction of adventitious buds from mature zygotic embryos of P. ponderosa, to stimulate the development of these buds into shoots, and to induce these shoots to produce root, then to establish large-scale propagation. The method presented here will be most useful for future Ponderosa Pine clonal propagation and genetic transformation programs.
1 Materials and methods 1.1 Plant materialThe Ponderosa Pine seeds were imported from Oregon, USA. Seeds were stored plastic bags at 4℃ until used as the source of embryos for all experiment and soaked in water for 2 or 3 days for full inflation. The well-marinated seeds were sterilized with 0.2% KMnO4 for 10 min, and their seed coat removed. The zygotic embryos were treated by 75%(v/v) ethanol for 20 s, then 0.1% HgCl2 for 10 min, and rinsed five times in sterile distilled water. Finally, the endosperm was striped with little dissection blade to get mature zygotic embryo, which be placed vertically on the adventitious bud induction medium in flasks under sterile condition.
1.2 Experimental methods 1.2.1 Adventitious bud inductionThree factors including basal media, 6-benzyladenine (6-BA) and naphthaleneacetic acid (NAA) were considered as to induce buds from mature zygotic embryo of Ponderosa Pine. Basal media in this study included MS(Murashige & Skoog, 1962), SH (Schenk & Hildebrandt, 1972), GD (Gresshoff & Doy, 1974) and N6 (Lissa, 1982). 6-BA and NAA were added in the bud induction medium at four levels of 0.00, 1.97, 9.85 and 19.70 μmol·L-1, and of 0.00, 1.44, 2.88 and 14.42 μmol·L-1 respectively. The factors and levels were designed by orthogonal design experiment according to L16(45) with total 16 treatments. For each treatment, 40 mature zygotic embryos were cultured and each experiment replicated three times. After 40 days of incubation adventitious buds and rates were assessed for induction.
1.2.2 Buds proliferation and elongationAfter the induction of buds, multiple bud (0.8~1.0 cm) originating from the cotyledons of mature zygotic embryos were separated before they were subjected to proliferation and elongation. They were respectively subcultured onto hormone-free 1/2GD and 1/2SH basal media(half-strength) with 1%, 2%, 3%, 4% and 5% sucrose for proliferation and with activated charcoal (AC) 0, 1, 2, 3 g·L-1 for proliferation and elongation. For each treatment, 30~40 buds were cultured and each experiment replicated three times. Proliferation rate and elongation of adventitious bud were counted and measured after 40 days.
1.2.3 Influence of shoot height on the root inductive ratesPonderosa Pine shoots were divided into 6 groups: ≤1.0 cm, 1.0~2.0 cm, 2.0~2.5 cm, 2.5~3.0 cm, 3.0~4.0 cm, ≥4.0 cm. Those shoots were respectively cultured on 1/2GD with 1.97μ mol·L-1 6-BA and 14.42 μmol·L-1 NAA.
1.2.4 Influence of medium on Ponderosa Pine roots inductive ratesOne hundred and sixty shoots with 2 cm longer in height were classified into four groups and cultured on GD, SH, 1/2GD and 1/2SH media with 1.97 μmol·L-1 6-BA, 14.42 μmol·L-1 NAA and 0.1% AC respectively to induce adventitious roots.
1.2.5 Influence of phytohormones on the root inductive ratesThirty six adventitious shoots with 2.5~3.0 cm long were respectively cultured on 1/2GD and 1/2SH with 0.00, 2.88, 14.42 and 28.84 μmol·L-1 of NAA and 0.00 and 4.17 μmol·L-1 of GA3(gibberellic acid) respectively supplemented 1% sucrose and 0.1% AC. The shoot induction media were used randomly in the rooting experiments. Adventitious shoots were placed vertically on the media and were transferred at monthly intervals.
Above experiments were replicated at least twice and rooting data were taken after 60 days.
1.2.6 Media preparation culture conditionAll the media except special note were supplemented with 2% sucrose and 0.6% agar. The pH of all the media was adjusted to 5.8 with 1 mol·L-1 KOH and 1 mol·L-1 HCl prior to autoclaving at 121℃ for 20 min. Induction, proliferation and elongation, and rooting of adventitious shoots occurred at 25~27 ℃ in the light provided by cool white fluorescent tubes (82 μmol·m-2s-1, 16-h photoperiod except rooting of adventitious shoots 14-h photoperiod).
1.2.7 Data analysisUsing ANOVA, the data were subjected to the analysis of variance test (F test) and Duncan's multiple range test was used to indicate significant differences (α=0.05). This provided test for main effects but not for interactions of the factors. Data of inducing rate (%) were transformed by sin-1 
Inducing rate of adventitious buds=(number of mature zygotic embryos producing adventitious bud/total number of mature zygotic embryos inoculated)×100%.
Adventitious buds proliferation rate=adventitious buds total/producing-bud mature zygotic embryos.
Root induction rate=(rooting shoots/inoculation shoots total)×100%.
2 Results 2.1 Adventitious bud inductionMature zygotic embryos on phytohormones-free medium developed as normal seedlings without the production of callus or adventitious buds. When mature zygotic embryos were cultured on bud induction medium for 3 d, the cotyledons were opened. They turned green on the 7th day, while the radicles dilated and turned red (the radicle did not grow and the root cap became necrotic). After 14 d, the cotyledons were splayed (Plate Ⅰ-A). After 20~30 d, asynchronous adventitious buds developed on the apex of the cotyledons and in the region around the base of the cotyledons. Many of buds clustered on the expanded apical dome, most likely because central buds elongated consecutively on the apical dome(Plate Ⅰ-B, C, D). Partly adventitious budsformed directly from cotyledons on the 28th~35thday (Plate Ⅰ-E).

|
PlateⅠ A. Mature zygotic embiyos 14 days in culture on GD basal medium with 19.70 μmol-1 6-BA; B ~ E. Adventitious buds differentiated from mature: zygotic embryos; F~G. Adventitious buds on elongation medium; H~K. Shoots with adventitious root on rooting medium; L. Adventitious root on donation medium. |
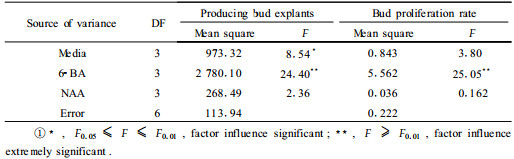
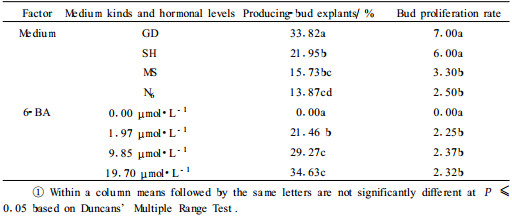
After 40 days, analysis of variance showed that basal medium and 6-BA significantly influenced adventitious induction but NAA did not (Tab. 1), and that cytokinin alone sufficient to bring about bud formation, which has been confirmed with several other coniferous species (Thorpe, 1980; Biondi et al., 1982; Abdullah et al., 1985). Among the different basal media used in this study, it was obvious that use of GD (0~65.8%) and SH (0~40.0%) basal media resulted in the greater frequency of adventitious bud formation than that use of MS (0~30.0%) and N6 (0~20.0%) basal media, which have the higher nitrogen content and were the most toxic of the four media. The highest inductive rate of 65.8% adventitious buds of mature zygotic embryos occurred on GD+ 19.70 μmol·L-1 6-BA. Most of the mature zygotic embryos cultured on MS and N6 showed an initial enlargement and dedifferentiation, but finally turned brown and died. This finding is consistent with that reported for mature zygotic embryo explants of P. wallichiana (Mathur et al., 1999) and P. massoniana (Zhang et al., 2006).
|
|
Single factor was analyzed and the results showed that there was no buds on the all media without 6-BA. The inductive rate of adventitious bud increased with 6-BA concentration increasing. When the auxin NAA was present in the media in combination with 6-BA, the inductive action of cytokinin was partially antagonized by the auxin, and a lower number of buds was obtained. NAA also affected the development of buds when it was included in the induction media. On GD and SH, proliferation rate of adventitious bud was also higher than that on MS and N6. The highest mean number of shoots was produced on GD, although not significantly better than SH. The mean number of adventitious bud was 7.00 and 6.00 buds on GD and SH respectively, the most proliferation was 10.There were not significant differences at concentration for 6-BA except at 0.00 μmol·L-1(Tab. 2).
|
|
For bud proliferation, we used 1/2GD and 1/2SH free plant growth regulators with different sucrose concentrations. The data were transferred into regression line. The results indicated that proliferation rate of adventitious bud decreased with the increasing sucrose concentration on 1/2GD and 1/2SH. Proliferation rate of adventitious bud on 1/2GD was more changeable than that on 1/2SH. When the sucrose concentration was higher, bud proliferation rate remarkably reduced. On 1/2GD medium, proliferation rate of adventitious bud(y) and sucrose concentration(x) regression equation is y=-0.001 4x2+ 0.029 7 x + 5.7, with the correlation coefficient being up to 0.987 6.The optimum sucrose concentration calculated was 10.6 g·L-1. On 1/2SH medium, proliferation rate of adventitious bud (y) and sucrose concentration (x) regression equation is y=-0.001 6x2 + 0.656x + 4.84, with the correlation coefficient being up to 0.988 9.The optimum sucrose concentration calculated was 20.5 g·L-1. In the proliferation medium sucrose is essential as carbon source and to keep osmotic pressure but too high and too low sucrose concentration would inhibit buds proliferation rate. When other factors were suitable, sucrose concentration decreased properly was beneficial to bud proliferation. Thus the best condition for bud proliferation from mature zygotic embryos was on 1/2GD with 1% sucrose or 1/2SH with 2% sucrose. Both mean number of shoots was 5.8 and 5.5 respectively.
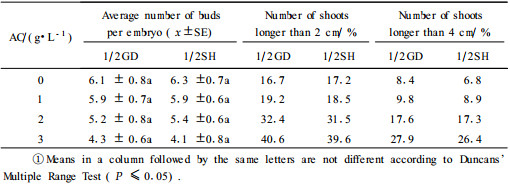
2.2.2 Influence of activated charcoal(AC)on Ponderosa Pine buds elongationTo encourage shoot formation, culture medium with reduced strength and lower concentration of plant growth regulators in time was used. For bud proliferation and elongation, we used 1/2GD and 1/2SH free plant growth regulators with different AC concentrations. Adventitious buds and shoots were counted after 40 days (Tab. 3). The results showed that media with AC did not significantly affect adventitious bud proliferation. Adventitious bud proliferation rate on 1/2GD and 1/2SH is reduced with AC increasing. As AC was 3.0 g·L-1, adventitious bud proliferation rate significantly decreased. However, AC was beneficial to adventitious bud growth, especially for bud elongation. At presence of AC, adventitious bud elongation was remarkable on 1/2GD and 1/2SH (Plate Ⅰ-F, G). It is necessary to add AC on the media for promoting shoots. The density growth of adventitious buds were cut and then transfrerred on subculture medium with AC. For bud propagation and elongation, 1/2GD was used respectively with 1% and 3% AC in this study.
|
|
An important step in vitro micropropagation is the formation of plantlets. In vitro, rooting of conifers is more difficult than that of angiosperms and has usually been done by using agar-solidified medium as the substrate, which ensures uniform distribution of auxins and nutrients, and good contact between shoots and substrate that results in more synchronous rooting. Major factors that influence root production are basal medium, sucrose, phytohormones, shoot quality, etc. Rooting is a high energy consumption process that requires a continuous supply of free sugar from the medium. For various pine species, optimal sucrose concentration ranges from 0.5% to 2.0% and rarely reached higher (Mohammed et al., 1988), hence, 1% sucrose was supplemented into the following media. Here the basal medium, shoots height and phytohormones affecting rooting formation were studied.
2.3.1 Influence of medium on Ponderosa Pine root inductive ratesDifferent media significantly affected the adventitious root induction. No roots was induced on GD and SH. Adventitious roots were induced from shoots on 1/2GD(12.5%) and 1/2SH(10.0%), and on 1/2GD inductive rate was higher than that on 1/2SH. Rooting was observed with 1.97 μmol·L-1 6-BA and 14.42 μmol·L-1 NAA(Plate Ⅰ-H, I, J, K).
2.3.2 Influence of shoot height on the root inductive ratesShoots were classified into six groups by length and were cultured on 1/2GD with 0.1% AC, 1.97 μmol·L-1 6-BA and 14.42 μmol·L-1 NAA to induce adventitious root. The rooting rate of cultured shoots were counted after 60 days. The data showed that adventitious root responded to different groups of shoots. No rooting was obtained when shoots were smaller than 2 cm. The rooting rate of cultured shoots were 6.3%, 9.4%, and 10.0% under the groups of 2.0~2.5 cm, 2.5~3.0 cm, and 3.0~4.0 cm respectively. The highest rooting rate (15.0%) was observed when shoots were longer than 4 cm. When shoots were longer than 2 cm, rooting rate increased with increasing of shoot height. Thus, shoots that are as high as possible should be used for rooting. But during the experiments, most shoots in height were 2.0~3.0 cm, therefore, it is very important to improve shoot length for rooting. Root induction treatments were relatively effective if the shoot length for prolonging culture varying from 2.5 to 4 cm was applied.
2.3.3 Influence of phytohormones on the root inductive ratesAuxin, including the type, concentration and mode of application, is the most important factor affecting rooting. Shoots elongated 2.5~3.0 cm in height were subjected to a rooting study. Different concentration of NAA and GA3 were investigated for their effects on 1/2GD and 1/2SH media with 1% sucrose and 0.1% AC. Results indicated that rooting was not obtained when NAA was absent. NAA was the most effective auxin for inducing roots in conifers. Shoot rooting rate was up to 16.7% and 8.3% on 1/2GD with 0.1% AC, 28.84 μmol·L-1 NAA and 4.17 μmol·L-1 GA3, and on 1/2SH with 0.1% AC, 14.42 μmol·L-1 NAA and 4.17 μmol·L-1 GA3 respectively. Thus GA3 were able to promote rooting from shoots of Ponderosa Pine. GA3 inhibited rooting in many pine species but stimulated rooting at very low concentration (Huang et al., 1994). AC promoted root growth (Plate Ⅰ-L).
3 DiscussionPlant regeneration protocols through adventitious organogenesis have been developed in some of conifers including pine species (Gladfelter et al., 1987; Tang et al., 2004). For conifer propagation, it is known that the most critical factors for induction of buds are explants and cytokinin treatment. The best results have obtained using embroynic explants, and 6-BA has been shown very effective in promoting bud differentiation in most of the species (Pedro et al., 1987). The results of the present investigation showed that embroynic explants of Ponderosa Pine were capable of producing multiple shoots in vitro and, subsequently, roots. Plantlet formation could be divided into three distinct phases: 1) Initiation of buds on mature zygotic embryos, 2) proliferation and elongation of buds, and 3) rooting of shoots. As shown here and in agreement with other studies, each stage has its own nutritional, phytohormonal and physical requirements during culture (Mathur et al., 1999).
The process of bud induction was strongly influenced by the basal medium and the type and concentration of phytormones. The result showed that GD and SH were suitable for adventitious bud induction. Similar results were also reported previously (Ellis et al., 1984; 1989; Tuskan et al., 1990). The highest mean number of shoots was produced on GD in agreement with the conclusion drawn by Tuskan et al. (1990). GD was also successfully used for other pine species for adventitious bud induction (Sommer et al., 1975; Sul et al., 1998; Dragana et al., 1999).
Shoot initiation in most conifers is the presence of a cytikinin such 6-BA, either alone or combined with one or more auxin (Amerson et al., 1985). In our study, Adventitious bud formation was induced on cultured zygotic embryos upon exogenous application of 6-BA. The excisable buds were initiated or formed directly from the cotyledons. Similar results have been obtained in P. elliottii (Pedro et al., 1987) and P. brutia (Abdullah et al., 1985). Our results also indicated that bud production only occurs in medium supplemented with 6-BA in accordance with the conclusion from Ellis et al. (1984). The results indicate cytokinin alone is sufficient to bring about bud formation, and this has been confirmed with several other conifer species (Thorpe, 1980; Biondi et al., 1982; Abdullah et al., 1985). 6-BA was also the most effective cytokinin for adventitious bud induction in P. sylvestris (Sul et al., 1998) and P. pinaster (Rancillac, 1991).Incorporation of NAA in the medium had a deleterious effect on bud formation and encouraged callus formation in agreement with Lin et al. (1991). Similar effect of NAA was earlier reported in P. strobusi (Kalia et al., 2007). On the contrary, Kaul (1987) reported a positive role of NAA on morphogenetic responses in P. roxburghii (Kaul, 1987). The variable response of different species to auxin-supplemented media may be due to different endogenous levels of auxin.The inhibition of bud formation may be due to action of auxin accumulated at the basal end of the explants (Marks et al., 1994).
The development of adventitious buds was asynchronous. There were often many small adventitious, initial buds surrounding the larger shoots. For bud propagation and elongation, we used 1/2GD free plant growth regulators with 3% AC as the bud subculture medium in the study. Similar observations have previously been reported in P. roxburghii (Parasharami et al., 2003) and P. brutia (Abdullah et al., 1987). Charcoal has been reported to adsorb the metabolites inhibiting morphogenesis thus supporting better growth (Fridborg et al., 1978). Shoot formation was enhanced by AC. Patel et al. (1986) and Jang et al. (1991) also reported a beneficial effect of AC on the formation of shoots.
High rooting rates were obtained by determining the basal medium, the best stage of shoot development and shoot quality, the type and concentration of phytormones and the incorporation of AC in medium ect.(Mohammed et al., 1988; Jang et al., 1991). For rooting, nutrients are usually reduced to half-strength of those used for shoot production (Mohammed et al., 1988). Most commonly, a single root developed from each adventitious shoot (Tang, 2001; Zhang et al., 2006). In our study, 1/2GD and 1/2SH were used for root production. Similar results were also reported previously in other conifers (Mohammed et al., 1988). When shoots were longer than 2 cm, rooting rate increased with increasing of shoot height. The highest rooting rate (15.0%) was observed when shoots were longer than 4 cm. So it was important to promote shoot height for rooting. Similar results have been obtained in Douglas Fir and Radiata Pine, where 4 cm for Fir and 2.4~2.7 cm for pine were best for rooting (Mohammed et al., 1988). In our study, roots were not obtained without NAA, indicating NAA was the most effective auxin for rooting in Ponderosa Pine. Shoot rooting rate was up to 16.7% and 8.3% on 1/2GD with 0.1% AC, 28.84 μmol·L-1 NAA and 4.17 μmol·L-1 GA3. Combination of NAA and GA3 have been more effective for Ponderosa Pine. Rooting is a high energy consumption process that requires a continuous supply of free sugar from the medium. For various pine species, the optimal sucrose concentration ranges from 0.5% to 2.0% and rarely reached higher (Mohammed et al., 1988). In this study, 1% sucrose was supplemented into rooting media. Addition of AC seemed to be beneficial for rooting either by inhibiting light at the shoot base or by adsorbing rooting inhibitors, as suggested by Dumas and Monteuuis (1995).
In conclusion, our study demonstrated that adventitious buds were initiated from cultured mature zygotic embryo explants of Ponderosa Pine on culture media supplemented with 6-BA. The highest inductive rate of 65.8% adventitious buds of embryos occurred on GD+ 19.70 μmol·L-1 6-BA. The adventitious buds were initiated or formed directly from the cotyledons and not from other parts of the mature zygotic embryos. For bud propagation and elongation, 1/2GD was used with 3% AC. Elongated adventitious shoots, 2.5~3.0 cm in height, were subjected to a rooting study. Shoot rooting rate was up to 16.7% on 1/2GD with 28.84 μmol·L-1 NAA, 4.17 μmol·L-1 GA3 and 0.1% AC. To our knowledge, this is the first report in which plantlets were regenerated from Ponderosa Pine mature zygotic embryos cultures. The plant regeneration protocol in this study may also facilitated future research in genetic transformation and in vitro propagation of superior Ponderosa Pine. More research is needed to improve rooting rate.
Abdullah A A, Yeoman M M, Grace J. 1985. In vitro adventitious shoot formation from embryonic and cotyledonary tissue of Pinus brutia Ten. Plant Cell, Tissue and Organ Culture, 5: 35-44. |
Abdullah A A, Yeoman M M, Grace J. 1987. Micropropagation of mature Calabrian pine (Pinus brutia Ten.) from fascicular buds. Tree Physiol, 3: 123-136. DOI:10.1093/treephys/3.2.123 |
Amerson H V, Frampton L J, Mckeand S E, et al. 1985.Loblolly pine tissue culture: laboratory, greenhouse, and field study //Henke R R, Hughes K W, Constantin M J, et al. Tissue culture in forestry and agriculyure. New York: Plenum Press, 117-137.
|
Biondi S, Thorpe T A.1982.Clonal propagation of forest tree species//Rao A N. Proceedings COSTED Symposium on tissue and cell culture of economically important plants. Singapore: National University of Singapore, 197-204.
|
Dragana Stojicić, Snežana Budimir, LjuBinka Ćulafić. 1999. Micropropagation of Pinus heldreichii. Plant Cell, Tissue and Organ Culture, 59: 147-150. DOI:10.1023/A:1006373218772 |
Dumas E, Monteuuis O. 1995. In vitro rooting of micropropagated shoot from juvenile and mature Pinus pinaster explant:influence of activated charcoal. Plant Cell, Tissue and Organ Culture, 40: 231-235. |
Ellis D D, Bilderback D E. 1989. Temporal competence of embryonic Pinus ponderosa cotyledons to form multiple buds in vitro. Amer J Bot, 76: 348-355. DOI:10.1002/ajb2.1989.76.issue-3 |
Ellis D D, Bilderback D E. 1984. Multiple bud formation by cultured embryos of Pinus ponderosa. J Plant Physiol, 115: 201-204. DOI:10.1016/S0176-1617(84)80120-0 |
Fridborg G, Pederson M, Landstrom L, et al. 1978. The effect of activated charcoal on tissue cultures: adsorption of metabolites inhibiting morphogenesis. Physiol Plant, 43: 104-106. DOI:10.1111/ppl.1978.43.issue-2 |
Gladfelter H J, Phillip G C. 1987. De novo shoot organogenesis of Pinus eldarica Med. in vitro 1: Reproducible from long-term callus cultures. Plant Cell Rep, 6: 163-166. DOI:10.1007/BF00268468 |
Huang Jianqiu(黄健秋), Wei Zhiming(卫志明).1994.Tissue and protoplast culture of Pinus species. Chinese Bulletin of Botany (植物学通报), 11(1): 34-42.
|
Jang J C, Tainter F H. 1991. Micropropagation of shortleaf, Virginia and loblolly×shortleaf pine hybrids via organogenesis. Plant Cell, Tissue and Organ Culture, 25: 61-67. DOI:10.1007/BF00033914 |
Kalia R K, Arya S, Kalia S, et al. 2007. Plantlet regeneration from fascicular of seeding shoot apices of Pinus roxburghii Sarg. Boilogia Plantarum, 51(4): 653-659. DOI:10.1007/s10535-007-0138-1 |
Ka ul. 1987. Plant regeneration from cotyledon-hypocotyl explants of Pinus strobus L. Plant Cell Rep, 6: 5-7. DOI:10.1007/BF00269726 |
Lin Y Q, Wager M R, Heidmann L J. 1991. In vitro for mation of axillary buds by immature shoots of Pinus ponderosa. Plant Cell, Tissue and Organ Culture, 26(3): 161-166. |
Lissa K S.1982.Ultrastructure of callus culture from Betula pendula and Picea abies.Prec.5th Intl Cong. plant tissue and cell culture, 173-174.
|
Marks T R, Simpson S E. 1994. Factors affecting shoot development in apically dominant Acer cultivars in vitro. J hort Sci, 69: 543-551. DOI:10.1080/14620316.1994.11516486 |
Mathur G, Nadgauda R. 1999. In vitro plantlet regeneration from mature zygotic embryos of Pinus wallichiana A.B.Jacks. Plant Cell Rep, 19: 74-80. DOI:10.1007/s002990050713 |
Mohammed G H, Vidaver W E. 1988. Root production and plantlet development in tissue-cultured conifers. Plant Cell, Tissue and Organ Culture, 14: 137-160. DOI:10.1007/BF00043405 |
Parasharami V A, Poonawala I S, Nadgauda R S. 2003. Bud break and plantlet regeneration in vitro from mature trees of Pinus roxburghii Sarg. Curr Sci, 84: 203-208. |
Patel K R, Kim H R, Thorpe T A. 1986. Plantlet formation in pitch pine(Pinus rigida Mill.) by tissue culture methods. For Ecol Manag, 15: 147-160. DOI:10.1016/0378-1127(86)90143-X |
Pedro Pérez-Bermúdez, Sommer H E. 1987. Factors affecting adventitious bud induction in Pinus elliottii(Engelm.) embryos cultured in vitro. Plant Cell, Tissue and Organ Culture, 11: 25-35. DOI:10.1007/BF00036573 |
Rancillac M.1991.Maritime pine (Pinus pinaster Sol.)//Bajaj Y P S. Biotechnology in Agriculture and Forestry: Vol. 16, Tree Ш. Berlin: Spring-Verlag, 317-338.
|
Sommer H E, Brown C L, Kormdik P P. 1975. Differentiation of plantlets in long-leaf pine(Pinus palustris Mill.) tissue cultured in vitro. Bot Gaz, 136: 196-200. DOI:10.1086/336802 |
Sul I W, Korban S S. 1998. Effect of media, carbon sources and cytokinins on shoot organogenesis in the Christmas tree Scots pine (Pinussylves tris L.). J Hortic Sci Biotechnol, 73: 822-827. DOI:10.1080/14620316.1998.11511054 |
Tang Wei. 2001. In vitro regeneration of loblolly pine and random amplified polymorphic DNA analyses of regenerated plantlets. Plant Cell Rep, 20: 163-168. DOI:10.1007/s002990000297 |
Tang Wei, Newton R J. 2003. Genetic transformation of conifers and its application in forest biotechnology. Plant Cell Rep, 22: 1-15. DOI:10.1007/s00299-003-0670-1 |
Tang Wei, Latoya C H, Vilay O, et al. 2004. The effect of different plant growth regulators on adventitious shoot formation from Virginia pine(Pinus virginiana) zygotic embryo explants. Plant Cell, Tissue and Organ Culture, 78: 237-240. DOI:10.1023/B:TICU.0000025658.73970.57 |
Thorpe T A.1980.Organogenesis in vitro, structural, physiological and biochemical aspects//Vasil. International review of cytology. Suppl 11A.London: Academic Press, 71-111.
|
Tuskan G A, Sargent W A, Rensem T. 1990. Influence of plant growth regulators, basal media and carbohydrate levels on the in vitro development of Pinus ponderosa cotyledon explants. Plant Cell, Tissue and Organ Culture, 20(1): 47-52. DOI:10.1007/BF00034756 |
Zhang Yu, Wei Zhiming, Xi Mengli, et al. 2006. Direct organogenesis and plantlet regereration from mature zygotic embryos of mass on pine (Pinus massoniana L.). Plant Cell, Tissue and Organ Culture, 84: 119-123. DOI:10.1007/s11240-005-9004-z |
 2008, Vol. 44
2008, Vol. 44