氢气是可从自然界获取的储量最丰富的能源载体.氢能是清洁、高效的可再生能源, 为缓解传统化石燃料导致的能源与环境问题提供了可替代能源(Abdalla et al., 2018).在所有自然能源中, 氢能的能量密度最高(为120 ~142 MJ · kg-1), 且燃烧只产生水, 清洁无污染.在常温条件下氢气性质稳定, 能够适应不同的应用环境和储运条件, 具有巨大的研究和开发利用价值(Boyd et al., 2010).目前, 工业中90%以上的氢气通过化石燃料转化方法制取, 转化过程能耗大, 并且会对环境造成污染, 不能从根本上解决日趋严峻的能源问题(Abdalla et al., 2018).生物制氢是一种可持续、经济有效的制氢技术, 可以在温和的环境条件下, 利用废水和有机废弃物中的碳水化合物转化为氢气和其它副产品(Lee et al., 2010; Velazquez Abad et al., 2017).暗发酵法生物制氢技术可将有机废弃物的处理与产氢过程结合, 且无需光照、反应系统相对简单、操作能耗低、在常温常压条件下即可高效产氢, 与其它生物制氢技术相比, 具有突出的优势(Kothari et al., 2014).
发酵产氢细菌是暗发酵生物制氢的主要功能微生物, 广泛存于不同自然生态环境中, 其可以通过氢酶催化质子和电子生成H2.与光合产氢微生物相比, 发酵产氢细菌能够利用废水/废弃物中的复杂碳水化合物产生H2和小分子有机物, 具有较高的能量回收效率和较低的能量需求(Boyd et al., 2010; Prakash et al., 2018; Wrighton et al., 2014).暗发酵产氢反应器中的高效发酵产氢细菌主要包括梭菌属(Clostridium)、产乙醇杆菌属(Ethanoligenens)、肠杆菌属(Enterobacter)和芽孢杆菌属(Bacillus)等(Burow et al., 2012; Lee et al., 2011).早期研究证实pH、氧化还原电位(oxidation-reduction potential, ORP)和水力停留时间(hydraulic retention time, HRT)是影响生物制氢反应器菌群发酵类型的关键因素.不同发酵细菌的NADH/NAD+平衡导致了不同发酵产物积累, 从而形成不同的产酸发酵类型(Ren et al., 1997; Ren et al., 2007b; Ren et al., 2002).通过pH控制可以定向调控发酵类型的形成, 较高的pH(>5.5)有利于丁酸型和丙酸型发酵形成, 而较低的pH(4.0~4.5)更利于乙醇型发酵的形成(Ren et al., 1997; Ren et al., 2007b; Xing et al., 2008).群落分析和宏基因组学分析证实优势菌群和群落组装的差异是导致不同产酸发酵类型形成的主要生态学机制, Ethanoligenens和Clostridium是乙醇型发酵和丁酸型发酵的代表菌属(Ren et al., 2007b; Xing et al., 2008).
近年来, 为进一步提高乙醇型发酵产氢效能和稳定性, 反应器运行调控、功能产氢细菌代谢途径解析、以及耦合系统等方面取得了大量进展.为更好的利用乙醇型发酵实现能源回收, 本文系统地综述了乙醇型发酵的最新研究进展.
2 产乙醇杆菌主导的乙醇型产氢发酵(Ethanol-type fermentation for bio-hydrogen production dominated by Ethanoligenens)任南琪在1994年研究糖蜜废水厌氧生物处理实验过程中, 首次发现了乙醇型产酸发酵.当连续搅拌反应器(continuous stirred tank reactor, CSTR)运行条件控制在内部pH 4.5左右、氧化还原电位(ORP)<-200 mV, 发酵产物主要是H2、CO2、乙醇和乙酸(Ren et al., 1997).为证实产氢产乙醇途径, 林明从产氢CSTR中分离得到高效产氢新菌株B49 (Lin et al., 2003).随后, 邢德峰分离获得菌株YUAN-3、X-29、W-1, 这些菌种被命名为哈尔滨产乙醇杆菌(Ethanoligenens harbinense).代表性菌株YUAN-3具有自聚集生长特性, 在培养过程中能够形成直径2~10 mm的颗粒聚集体, 有利于反应器中厌氧颗粒污泥或生物膜的形成, 维持连续流生物制氢反应器内的生物量(Xing et al., 2006).乙醇型发酵的代表菌属为产乙醇杆菌属(Ethanoligenens), 末端代谢产物为乙醇、乙酸、H2和CO2, 这些代谢产物可以进一步作为中间体被其它微生物代谢利用, 或经回收后用于生产高价值的生物化学产品和高聚合物(Abdalla et al., 2018; Dahiya et al., 2019; Ren et al., 2011; Xing et al., 2006).与丁酸型发酵的代表菌属—梭菌属(Clostridium)相比, 产乙醇杆菌具有产氢速率高、耐酸性、产乙醇等特性, 发酵产物易于被产甲烷菌利用.此外, 产乙醇杆菌属还具备明显的自聚集和共聚集能力, 有利于形成颗粒聚集体和生物膜, 能够有效防止菌种流失, 因此,产乙醇杆菌主导的乙醇型产氢发酵成为重要的产氢发酵类型之一(Li et al., 2019a; Li et al., 2019b; Li et al., 2019e; Ren et al., 2007b; 任南琪等, 2004).
3 乙醇型发酵产氢反应器及运行调控(Design and operation optimization of ethanol-type fermentative hydrogen-producing reactors) 3.1 乙醇型发酵产氢反应器构型及运行参数优化近年来, 不同构型的厌氧反应器被应用于研究乙醇型发酵制氢(Ren et al., 2011).CSTR是最早被用于处理不同废水连续发酵制氢的系统之一(Ren et al., 2011).乙醇型发酵最初即是在以糖蜜(COD : N : P为800 : 5 : 1)为原料的CSTR中被发现, 反应器运行温度为(30 ± 0.5)℃, pH值保持在4.5左右, ORP为-250 mV, 有机负荷率(organic loading rate, OLR)高达80 ~ 90 kg · m3 · d-1(挥发性悬浮物(VSS)浓度为20 g · L-1).在此条件下, 反应器的代谢产物主要为乙醇、乙酸、H2和CO2, 有效避免了丙酸型发酵, 系统运行稳定, 有利于后续的产甲烷过程, 反应器氢分压高达50 kPa (Ren et al., 1997).在此基础上, 多项研究对CSTR乙醇型发酵产氢的运行参数进行调整和优化.研究表明, 在污泥接种量为15 g · L-1、反应器运行温度为(35 ± 1)℃, 系统pH在4.6~4.9之间, ORP为-450~-470 mV, OLR为40 kg · m3 · d-1、HRT为4 h等参数设置下可以实现乙醇型发酵连续流CSTR的高效稳定运行, 此时反应器的最大产氢速率为7.63 m3 · m-3 · d-1 (任南琪等, 2004).同时, 通过在CSTR中比较不同发酵类型的稳定性和产氢能力, 证明了乙醇型发酵是最优产酸发酵类型, 在OLR高达86.1 kg · m3 · d-1时仍保持稳定运行, 且产氢量最高可达14.99 L · d-1(Ren et al., 2007a).
与CSTR相比, 上流式厌氧污泥床(up-flow anaerobic sludge bed, UASB)反应器、厌氧折流式反应器(anaerobic baffled reactor, ABR)和膨胀颗粒污泥床(expanded granular sludge bed, EGSB)反应器等生物制氢反应器可以在较高的有机负荷率、较低的HRT下保持较高的生物量, 提高了底物转化率, 表现出更稳定的产氢性能(Jung et al., 2011; Ren et al., 2011; Ren et al., 2009).李建政等在有效容积为27.5 L的三室厌氧折流式反应器中利用稀释糖蜜进行乙醇型发酵产氢, 稳定运行状态下的产氢速率为32.51 L · d-1, 底物转化率为0.13 L · g-1(Li et al., 2007a).采用添加颗粒活性炭载体的EGSB反应器以糖蜜废水为底物进行乙醇型发酵制氢, 产氢速率最高可达0.71 L · L-1 · h-1, 氢气产率最高为3.47 mol-H2/mol-蔗糖(Guo et al., 2008a; Guo et al., 2008b).在以活性炭为载体的连续混合固定污泥反应器(continuous mixed immobilized sludge reactor, CMISR)中利用糖蜜废水为底物进行乙醇型发酵, 最大氢气产率为30.57 mmol-H2/mol-COD(Han et al., 2012).乙醇型发酵的新型厌氧下流式结构床反应器(anaerobic down-flow structured bed reactor, ADSBR)在运行OLR为3.8和6.2 g-蔗糖/g-VSS · d时的氢气产率可达2.0 mol-H2/mol-蔗糖, 氢气和乙醇的总能量转化率为23.40 kJ · h-1 · L-1(Anzola-Rojas Mdel et al., 2016).在厌氧序批式生物膜反应器(anaerobic sequencing batch biofilm reactor, AnSBBR)中利用干酪乳清和甘油共消化产氢, 乙醇型发酵的产氢速率和氢气产率分别达到129.0 mol · m-3 · d-1和5.4 mol-H2/kg-COD, 并可以完全抑制产甲烷(Lovato et al., 2017).而采用内循环(internal circulation, IC)反应器以糖蜜废水为底物进行乙醇型发酵的最大产氢率可达1.72 l/L(Li et al., 2019c).在添加固定化载体的纤维床生物反应器(fibrous bed bioreactor, FBB)中以酸预处理后的甘蔗汁为唯一碳源, 利用E. harbinense YUAN-3纯菌株分批补料发酵产氢, 最大产氢速率可达5.22 mmol · L-1 · h-1, 最高氢气产率为2.23 mol-H2/mol-己糖(Li et al., 2019d).不同构型生物反应器的应用, 增加了乙醇型发酵的底物来源和利用率, 并进一步提高了产氢效率.
除了比较不同生物反应器的性能, 发酵产氢反应器的运行参数优化被大量研究.研究表明, 水力停留时间(HRT)对乙醇型发酵系统稳定有显著影响, CSTR的HRT为5 h时获得了最高的产氢效率(12.27 mmol · L-1 · h-1)和能量(15.50 kJ · h-1 · L-1)(Wang et al., 2013).初始污泥负荷率(sludge loading rate, SLR)对乙醇型发酵的形成和产氢效率有显著影响, 较低的SLR(0.63 kg-COD/kg-MLVSS · d)有助于CSTR中乙醇型发酵的快速形成, 但较高的SLR(1.47 kg-COD/kg-MLVSS · d)可以获得更高的氢气产率(0.102 m3-H2/kg-MLVSS · d)(Li et al., 2013).不同HRT和不同接种源(猪污泥和家禽污泥)显著影响以干酪乳清为底物的乙醇型发酵厌氧流化床反应器(anaerobic fluidized bed reactors, AFBRs)的产氢能力, HRT为4 h时达到最大氢转化率(1.33 mol-H2/mol-乳糖)(Rosa et al., 2014).丁杰等将三维计算流体动力学(computational fluid dynamics, CFD)模拟与CSTR中的乙醇型发酵过程相结合, 发现不同类型和速度的叶轮产生不同的流动模式, 优化后的叶轮水动力性能在50~70 rev · min-1之间, 更适合乙醇型发酵产氢(Ding et al., 2010).除在上述实验规模的制氢反应器中研究乙醇型发酵外, 任南琪等还在以糖蜜为底物的连续流厌氧发酵反应器中进行了超过200 d的乙醇型发酵产氢中试研究(反应器有效容积为1.48 m3, 运行OLR为3.11~85.57 kg · m3 · d-1), 最大产氢速率为5.57 m3-H2/m3-反应器· d, 在OLR为35~55 kg · m3 · d-1范围内的最大氢气产率为26.13 mol-H2/kg-CODremoved(Ren et al., 2006).本课题组开发了连续流移动床生物膜反应器(moving bed biofilm reactors, MBBRs)和批次固定化耦合除氧产氢策略(SIDO), 加速了发酵产氢反应器的启动和高丰度产氢细菌生物膜的形成, 获得了高于非固定化反应器的产氢效率(Li et al., 2020e), 该方法也可推广用于乙醇型发酵反应器的快速启动和强化制氢.上述研究从反应器设计及运行角度出发, 通过改良反应器构型、优化运行参数从不同底物中获得了较高的氢气产量, 为乙醇型发酵的实际应用奠定了基础.然而, 此类研究仍面临诸多挑战, 利用廉价底物的大规模连续流发酵产氢系统快速启动和提高反应器生物量仍是亟待解决的问题.因此, 以生物膜或颗粒聚集体为基础的反应器设计和开发需要大力推进, 以快速富集并维持系统中更活跃有效的产氢功能菌, 实现稳定高效的乙醇型发酵产氢并逐步应用于大规模生产.
3.2 强化乙醇型发酵产氢的调控策略底物利用率和产氢效率低是利用复杂有机底物进行乙醇型发酵制氢面临的两个主要问题(Yang et al., 2017).对底物进行预处理和添加共发酵底物可以显著提高底物利用率, 促进乙醇型发酵的形成.利用热预处理后的污泥进行乙醇型发酵产氢, 在葡萄糖浓度为10.69 g · L-1时的最大氢气产率为1.9 mol-H2/mol-葡萄糖(Han et al., 2015).超声预处理后的活性污泥作为多种营养物质用于乙醇型发酵产氢, 可以显著提高E. harbinense B49的产氢效率, 在污泥浓度为7.75 g-VSS /L时, 产氢产率最高可达1.97 mol-H2/mol-葡萄糖;在不添加任何化学物质的情况下, E. harbinense B49可以利用超声预处理污泥作为营养物质, 高效地将废糖蜜转化为氢气(氢气产率为189.34 mL -H2/g-总糖)(Xie et al., 2016).在甘蔗糖蜜分批发酵中添加银杏叶共发酵可以通过改变E. harbinense从乙醇途径向乙酸酸途径的代谢通量分布, 增强了甘蔗糖蜜向H2的转化效率, 从而显著提高乙醇型发酵的氢气产率(1.58 mol-H2/mol-己糖), 与单一甘蔗糖蜜发酵相比氢气产率提高了28.03%(Li et al., 2020a).底物C/P和C/N比例对乙醇型发酵也有显著影响.在HRT为2 h、出水pH为3.7时的乙醇型发酵AFBR反应器中, C/P=700 : 1、C/N=200 : 1时的产氢速率和氢气产率为最高, 分别为0.70 L · L-1 · h-1和0.76 mol-H2/mol-葡萄糖(Carosia et al., 2017).
发酵体系中的金属离子是影响发酵产氢细菌生长代谢的关键因素之一.研究表明适宜浓度的铁、镍和镁等金属离子能够提高间歇培养E. harbinense的总产氢量, 促进效果依次为Fe2+>Ni2+>Mg2+(林明等, 2003).添加适当浓度(>50 mg · L-1)的Fe粉和Fe2+显著提高了间歇培养的乙醇型发酵混合菌群的产氢能力, 且在一定投加浓度范围内(<1500 mg · L-1)显著提高液相代谢产物中的乙醇比例(丁杰等, 2004).利用响应面法(response surface methodology, RSM)对E. harbinense B49的高效产氢培养基进行了优化, 间歇式反应器的操作条件为葡萄糖、Fe2+和Mg2+的浓度分别为14.5 g · L-1、180 mg · L-1和690 mg · L-1(初始pH为6.0, 实验温度为(35 ± 1)℃)时的氢气产率可达2.20 mol-H2/mol-葡萄糖(Guo et al., 2009).在EGSB反应器启动过程中加入L-半胱氨酸可以明显缩短反应器的启动时间, 与未添加L-半胱氨酸的反应器相比, 加入0.5 g · L-1 L-半胱氨酸显著提高了乙醇型发酵的产氢效率和底物利用率(最大氢气产率为1.93 mol-H2/mol-葡萄糖)(Guo et al., 2013).另有研究表明, 在小麦秸秆和活性污泥共消化生物制氢系统中加入Fe3O4, 可以显著富集乙醇型发酵Ethanoligenens, 通过改变发酵细菌种类和发酵类型减缓挥发性脂肪酸的积累, 缓解了体系内的酸性pH值, 从而提高厌氧消化和产氢效率(Zhao et al., 2018).而添加生物炭可以通过缓冲系统pH、降低氧化还原电位、释放矿物质营养素, 提供多孔的细胞固定化结构等多方面促进乙醇型发酵产氢(Li et al., 2020b).
末端代谢产物对发酵产氢细菌代谢过程可能存在反馈抑制作用.为了缓解乙酸的代谢抑制作用, 唐晶等采用双室双极性膜电渗析分离装置从乙醇型发酵的发酵液中去除乙酸盐, 提高了产氢效率(Tang et al., 2012).该产物分离制氢系统累计产氢量比非产物分离反应器提高了23%, 葡萄糖利用率提高了135%, 细胞生长提高27%, 氢气产率达到2.2 mol-H2/mol-葡萄糖(Tang et al., 2014).使用生物强化方式可以通过增强混合发酵中乙醇型发酵产氢细菌的菌群丰度, 促进乙醇型发酵的形成并提高产氢能力.投加E. harbinense B49可显著提高CSTR(OLR为12 kg-COD/m3 · d)的产氢能力并影响发酵产物组成, 有助于乙醇型发酵的快速建立(秦智 et al., 2007).将E. harbinense B49与Clostridium acetobutylicum X9共培养可迅速利用微晶纤维素单糖高效产氢, 且共培养的产氢效率显著高于单培养C. acetobutylicum X9的产氢效率(Wang et al., 2008).在以核桃壳为载体的连续流生物膜柱式反应器中定期或连续添加E. harbinense B49, 可以促进乙醇型发酵的形成并显著提高启动阶段的产氢能力(Guo et al., 2010).
4 乙醇型发酵产氢代谢的分子调控机制(Molecular regulation mechanism of ethanol-type fermentation) 4.1 乙醇型发酵产氢细菌的遗传进化[FeFe]-氢酶是产乙醇杆菌的产氢关键酶, 其与丁酸型发酵Clostridium的氢酶序列相似度为62.48% (Li et al., 2019e; Xing et al., 2008; Zhao et al., 2010)(图 1).[FeFe]-氢酶能够特异性地催化质子与电子结合产生H2, 释放发酵过程中多余的还原剂, 其催化活性中心为H-簇的单体蛋白, 包含一个[4Fe-4S]亚簇和一个2Fe亚簇(Greening et al., 2014; Mulder et al., 2010; Shafaat et al., 2013; Vignais et al., 2007).
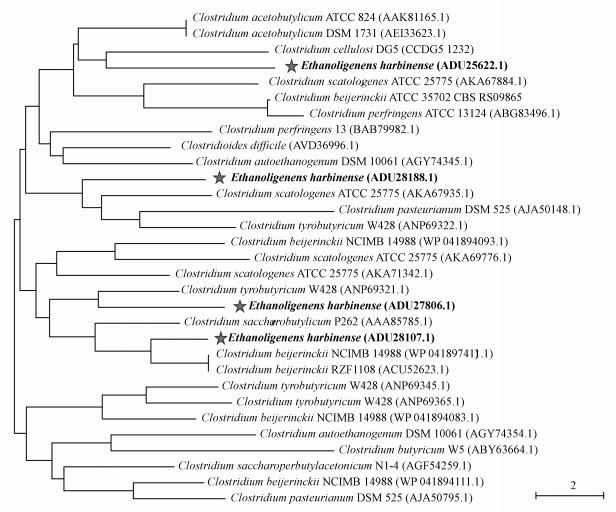 |
| 图 1 基于[FeFe]-氢酶(hyd)的哈尔滨产乙醇杆菌系统发育树(Li et al., 2019e) Fig. 1 The phylogenetic trees of E. harbinense based on [FeFe]-H2ase (Li et al., 2019e) |
我们课题组基于PacBio单分子实时测序技术构建了完整的E. harbinense基因组图谱和DNA甲基化修饰图谱(Li et al., 2019e).比较基因组学研究表明E. harbinense基因组中包含4个[FeFe]-氢酶基因, 3个[FeFe]-氢酶H-簇成熟酶编码基因(hydE、hydF和hydG), 2个编码氢离子跨膜转运活性蛋白编码基因(菌株W1除外)和1个编码钠/氢离子转化器编码基因.其中Ethha_2293(hyd1)和Ethha_2614(hyd2)基因各编码一个由大亚基(HydA)和小亚基(HydB)组成的二聚体氢酶, Ethha_0031(hyd3)和Ethha_2695(hyd4)各编码一个仅含大亚基(HydA)的单体氢酶(图 2)(Li et al., 2019e).hydE、hydF和hydG基因是核心H-簇成熟的关键基因, hydE和hydG基因编码的辅蛋白HydE和HydG能够与腺苷蛋氨酸(S-adenosyl-L-methionine, SAM)结合, 而hydF基因编码的HydF可作为GTPase与铁硫(Fe-S)结合以合成H-簇的2Fe单元(Leach et al., 2007; Lill, 2009; Mulder et al., 2010; Mulder et al., 2009; Posewitz et al., 2004).
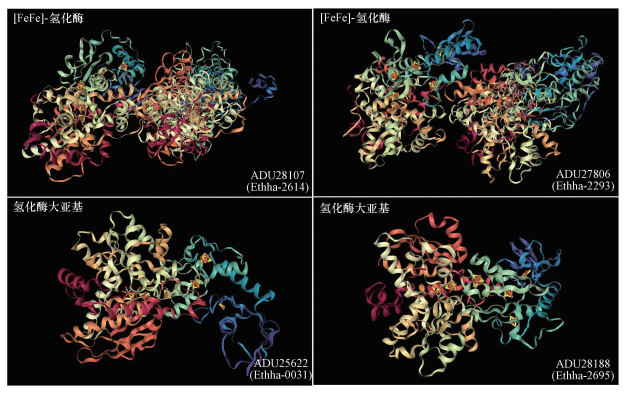 |
| 图 2 哈尔滨产乙醇杆菌[FeFe]-氢酶(Hyd)三维结构预测 Fig. 2 Three-Dimensional (3d) structure prediction of Hyd in E. harbinense |
除氢酶相关基因外, 在E. harbinense基因组上还注释到一系列铁氧化还原蛋白的编码基因, 包括4个4Fe-4S铁氧化还原蛋白, 1个[2Fe-2S]-铁氧化还原蛋白以及其它铁氧化还原蛋白(Li et al., 2019e).铁氧化还原蛋白是一种酸性胞质蛋白, 常出现在微生物产氢代谢过程中, 作为重要电子传递体与[FeFe]-氢酶协同作用(Huang et al., 2016; Peden et al., 2013).其中4Fe-4S铁氧化还原蛋白是[FeFe]-氢酶H-簇的核心组成部分, 而其它铁氧还原蛋白可能在电子传递中起重要作用(Buckel et al., 2013).此外, E. harbinense基因组中有7个编码乙醛脱氢酶/乙醇脱氢酶的基因和1个乙酸激酶的基因(Ethha_2004), 它们分别是乙醇、乙酸合成的关键酶.上述基因共同决定了E. harbinense的乙醇-H2共代谢特性, 在乙醇型发酵产氢细菌的能量代谢中起着重要的协同作用.
全基因组分析证实E. harbinense的产氢代谢途径主要为丙酮酸-铁氧化还原蛋白氧化还原酶(pyruvate ferredoxin oxidoreductase, PFOR)与[FeFe]-氢酶协同催化产H2-乙醇发酵代谢途径(图 3)(Li et al., 2019a).此外, E. harbinense还可能存在NADH+H+产氢途径, 在酸性pH环境下将细胞内过量的NADH产生更多的氢气(Ren et al., 2009).NADH+H+途径依赖于NADH:铁氧化还原蛋白氧化还原酶(NFOR)催化NADH转化为NAD+和电子, 再由位于内膜中的氢酶将电子与质子结合生成氢气(Kamalaskar et al., 2016).
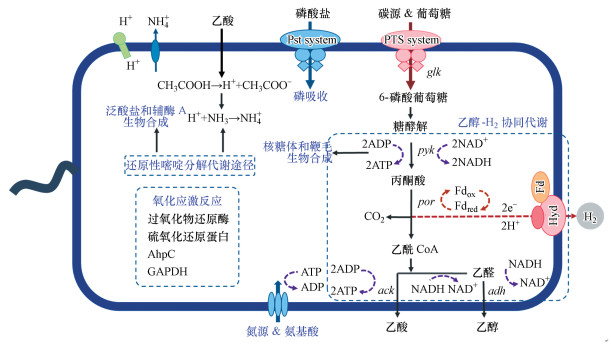 |
| 图 3 哈尔滨产乙醇杆菌对代谢产物抑制的分子响应机制(Li et al., 2019a; Li et al., 2019b; Li et al., 2019e) Fig. 3 Potential molecular response in E. harbinense against metabolite inhibition |
发酵产氢细菌的生长代谢受多种外界环境因素的影响.从本质上来说, 发酵产氢细菌主要通过调控基因表达、蛋白表达和蛋白翻译后修饰水平等以快速精准地调整自身的生化状态并对外界环境刺激做出反应.深入了解关键生态因子对乙醇型发酵的影响机制, 阐明乙醇型发酵产氢细菌的代谢调控网络, 有助于构建快速、稳定、高效的乙醇型生物制氢系统, 并制定高效产氢及代谢产物定向回收的精准调控策略.
pH作为重要的生态因子之一, 不仅对产氢发酵类型的形成和优势种群的富集具有重要影响, 而且对微生物的生长代谢也有重要的调节作用(Oh et al., 2003; Ren et al., 2015; Ryan et al., 2015; Shi et al., 2019).从细胞角度看, pH可以影响胞内酶的活性, 进而影响细菌的代谢途径.同时, pH可以影响细胞膜的结构, 改变酶对其底物的亲和力, 影响细菌对底物的吸收能力(Li et al., 2019a; Lund et al., 2014; Olson, 1993).乙醇型发酵能够耐受较低的pH值, 并可通过对pH波动自适应性调节来维持乙醇型发酵产氢的稳定性(Ren et al., 1997; Ren et al., 2007b; Wu et al., 2017; Xing et al., 2008).转录组学研究证实初始pH显著影响E. harbinense细胞生长和代谢产物生成相关基因表达, 碳和能量代谢、生物合成、细胞生长和繁殖、信号转导和耐受适应机制(包括磷酸转移酶系统、抗氧化系统和细菌趋化性等)等多个途径均在转录水平上受到调控.低初始pH显著影响E. harbinense的生长和增殖, 但没有显著抑制[FeFe]-氢酶和铁氧化还原蛋白的活性;高pH主要抑制E. harbinense产氢和产酸相关基因的表达(Li et al., 2020d).因此, 通过调节pH可以有效筛选和富集乙醇型发酵细菌, 实现发酵产氢反应器的快速定向启动, 并建立性能稳定的生物制氢系统.
李华华等通过定量蛋白质组学分析阐明了末端代谢产物乙醇、乙酸对乙醇型发酵产氢的反馈抑制机制和应激响应机制(Li et al., 2019a; Li et al., 2019b).乙醇累积会导致E. harbinense中产乙醇关键酶的双功能乙醛-CoA/乙醇脱氢酶(bifunctional acetaldehyde-CoA/alcohol dehydrogenase, ADHE)蛋白表达量显著增加, 从而导致乙醇产量的大幅提升, 同时由于对乙酰-CoA的竞争而抑制了乙酸的生成(图 3)(Li et al., 2019b).同时, 乙醇积累会导致E. harbinense中糖酵解相关酶类、内源性胍、脱硫铁氧化还原蛋白及歧化酶、谷胱甘肽过氧化物酶和组氨酸等多种蛋白表达量的明显增加, 共同抵御乙醇的代谢抑制, 同时提升E. harbinense对酸性胁迫条件的耐受性, 保护机体免受氧化胁迫危害(Li et al., 2019b).而产物乙酸的积累会引起系统pH值降低, 导致细胞内酸化现象产生, 使糖酵解、磷酸转移酶系统、无机磷酸盐特异性转运系统(phosphate specific transporter system, Pst system)等相关酶活性受抑制, 进而影响E. harbinense的生长代谢(图 3)(Li et al., 2019a).同时, 乙酸胁迫下细胞会通过调节蛋白表达量来启动相应防御机制, 包括氧化应激反应以及甘油醛-3-磷酸脱氢酶(GAPDH)等介导的DNA及蛋白质结构修复机制等, 从而抵抗酸化对细胞内结构造成的破坏(Li et al., 2019a).
此外, 添加不同浓度的Mg2+、Fe2+以及半胱氨酸能够引起E. harbinense YUAN-3的产氢产乙醇代谢途径关键酶(如[FeFe]-氢酶、乙醇脱氢酶、乙酸激酶等)编码基因的表达变化, 提高酶的表达量和催化活性, 进而提高产氢效率(Zhao et al., 2017; Zhao et al., 2019).但添加金属离子等促进因子的方式其作用机理较为复杂, 尚需开展相关研究对其分子机制进行系统深入探究.
除上述分子调控机制外, 产乙醇杆菌的自凝集机制及与其它细菌形成的颗粒聚集体或生物膜中的电子传递机制也亟待研究.微生物群落中种间电子传递(interspecies electron transfer, IET)在有机物厌氧生物处理过程中起着关键的作用, 对微生物的自聚集或共聚集过程至关重要.Summers等在电活性地杆菌G. metallireducens和G. sulfurreducens共培养体系中通过对共聚集颗粒的16S rRNA基因测序和基因组测序揭示了种间直接电子转移机制, 并证明共聚集颗粒中的G. sulfurreducens在多次传代后基因组发生单一位点的突变, 且该突变可增强参与细胞外电子转移的细胞色素C的产生并加速聚集体的形成(Summers et al., 2010);Gunter Wegener等证实了甲烷氧化自聚集颗粒中硫酸盐还原细菌和嗜热甲烷厌氧氧化菌的直接电子传递机制(Wegener et al., 2015).目前, 产乙醇杆菌自聚集机制及种间电子传递机制仍不够清楚.对乙醇型发酵体系内电子转移机制的研究将有助于利用微生物种间互作方式来提高产氢效率.
5 基于乙醇型发酵的耦合梯级产氢(Cascade hydrogen production based on coupling ethanol-type fermentation with other systems)乙醇型发酵的液相产物乙醇、乙酸等可作为底物合成中长链脂肪酸、生物燃料、聚羟基链烷酸酯(PHA)等高价值化学品的原料(Agler et al., 2012; Bakonyi et al., 2018; Yin et al., 2017), 因此乙醇型发酵近年来得到了广泛关注和研究.已知暗发酵产氢反应的理论最大氢气产率(4 mol-H2/mol-葡萄糖)仅为葡萄糖完全转化为氢气的25%, 而其它小分子代谢产物没有被进一步转化为氢气, 中温条件下H2转化率通常不高于2 mol-H2/mol-葡萄糖(Ren et al., 2009).此外, 加快厌氧发酵系统的启动和提高系统氢气转化率仍然是大规模发酵产氢系统需要解决的问题(Li et al., 2020b; Zhang et al., 2017).因此, 深入研究乙醇型产氢菌生长代谢的分子调控机制, 开发通过调控乙醇型发酵代谢途径来强化制氢的新方法将成为乙醇型发酵产氢规模化应用的关键.
5.1 乙醇型发酵与光发酵耦合复杂碳水化合物可以先被发酵产氢细菌代谢利用, 而产生的有机酸可进一步作为光合细菌产氢的底物, 可以获得更高的氢气产率(Dasa et al., 2001; Ren et al., 2011).目前通过光-暗发酵混合系统获得的最大产氢量为7.1 mol-H2/mol-葡萄糖(Asada et al., 2006), 影响H2产率的主要参数为温度和pH值(暗发酵pH值4.5~6.5, 光发酵pH值7以上).乙醇型发酵产氢E. harbinense B49的代谢产物可作为底物被固定化的红假单胞菌Rhodopseudomonas faecalis RLD-53利用, 总产氢产率可达6.32 mol-H2/mol-葡萄糖, 显著提高了单底物(葡萄糖)的氢气产率(Lu et al., 2009b).而E. harbinese B49利用超声预处理污泥作为营养物质代谢产生的乙酸和液体培养基中剩余的营养物质, 可以通过光发酵R. faecalis RLD-53进一步转化为氢, 氢气产率为271.36 mL-H2/g-总糖(Xie et al., 2016).这两株细菌共培养可以在葡萄糖浓度6 g · L-1、磷酸盐缓冲液浓度50 mmol · L-1、初始pH 7.5的条件下可获得最佳的产氢效率(最大氢气产率和最大产氢速率分别为3.10 mol-H2/mol-葡萄糖和17.2 mmol-H2/L · h), 同时能够保持共培养体系的稳定(Xie et al., 2010).刘冰峰等开发了耦合暗-光发酵一体化反应器, 在E. harbinese B49和R. faecalis RLD-53用膜分隔培养同时生产H2和乙醇.E. harbinese B49代谢生成的乙酸可以自由通过膜被R. faecalis RLD-53作为碳源利用, 在磷酸盐缓冲液浓度为20 mmol · L-1、葡萄糖浓度为8 g · L-1、暗发酵菌与光发酵菌接种比为1:20的条件下, 可获得最大产氢量为4.96 mol-H2/mol-葡萄糖(Liu et al., 2015)(图 4).乙醇型发酵和藻类复配培养系统也可以在产氢的同时处理有机废水, 并实现微藻脂质合成.乙醇型发酵和藻类复配系统的氢气产率可达203.3 mL/g · COD, 脂质含量为33.8%、总产能20.51 kJ · L-1 (Ren et al., 2018).
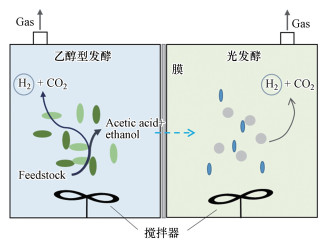 |
| 图 4 暗-光发酵耦合反应器示意图(Liu et al., 2015) Fig. 4 The schematic of the integrated dark and photo-fermentative reactor(Liu et al., 2015) |
光-暗发酵混合系统作为一种有效策略, 对提高氢气产量和生物能源回收效率等方面具有很大的优势, 但由于两种发酵细菌的生长速率不一致, 且暗发酵代谢终产物的积累导致pH值迅速下降, 不利于光合细菌的生长和产氢, 因此在同一发酵系统中两种细菌的协同作用比较困难, 需要设计更佳的反应器构型并优化系统操作条件, 同时共培养互作机制和代谢调控机制也有待进一步研究.
5.2 乙醇型发酵与电化学系统耦合乙醇型发酵可与包括如微生物电解池(microbial electrolysis cells, MECs)和电发酵(electro-Fermentation, EF)系统在内的微生物电化学技术(microbiological electrochemical technology, MET)相结合以提高暗发酵效率(图 5).微生物电解池法是利用具有电化学活性的细菌在专门设计的微生物电解池中以废水中的有机物作为底物制取氢气的新型生物制氢技术(Lu et al., 2016).乙醇型发酵液富含易于被MECs中微生物利用的乙醇和乙酸, 可作为MECs的底物, 发酵法-MECs耦合技术已经能够利用有机废物实现85%(10.2 mol-H2/mol-葡萄糖)的氢气产率(Liu et al., 2010; Lu et al., 2009a).利用单室MEC与乙醇型发酵系统耦合的总氢气回收率为96%, 产氢速率为2.11 m3 · m-3 · d-1, 电能效率为287%, 大幅度提高了乙醇型发酵的产氢量.因此乙醇型发酵-MECs耦合系统具有潜在的适用性(Bakonyi et al., 2018; Parameswaran et al., 2009).研究表明, MECs耦合发酵法制氢技术可以减少发酵细菌的末端代谢产物抑制, 从而进一步提高氢气转化效率(Hua et al., 2019; Lu et al., 2009a).在发酵法-MECs耦合系统中微生物的3种互养互作机制分别为发酵细菌与电活性细菌之间的互作机制, 发酵细菌、电活性细菌和产乙酸菌互作机制, 以及发酵细菌和产甲烷菌的互作机制(Lu & Ren, 2016; Lu et al., 2012).
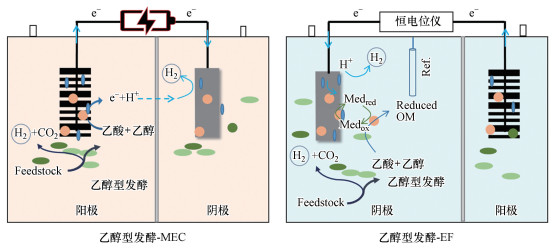 |
| 图 5 乙醇型发酵与电化学耦合系统示意图 Fig. 5 The schematic of the integrated ethanol-type fermentation MET |
乙醇型发酵的pH通常在4~5之间(Li et al., 2007b), 而形成电活性生物膜通常需要中性或弱碱性的pH值(pH 6~9)(Chatterjee et al., 2019; Patil et al., 2011).因此, 维持发酵法-MECs耦合系统的性能稳定较为困难, 需要采用稀释发酵液、添加缓冲溶液以及富集耐酸的电活性细菌等方法, 并保持合理的循环时间和溶液电导率以增加底物的可利用性.在没有分离器的单室MECs系统中, 随时间推移常常伴有甲烷生成, 从而造成氢气产率的降低;对MECs中不同微生物种群互作机制的理解尚不深入, 不能有效通过控制微生物群落组成和代谢活性来进行针对性的系统优化和操作条件优化以提高产氢效率等(Lo et al., 2009; Lu & Ren, 2016).因此, 运用分子生物学手段研究乙醇型发酵产氢细菌和MECs中其他微生物的互作机制、以及胞外电子传递机制, 将为发酵法-MECs耦合系统中有机废物转化为H2的过程提供新的思路, 使通过控制微生物群落强化MECs产氢成为可能.
此外, 电发酵作为一种通过调控微生物氧化还原电位和电子流向来调控微生物代谢途径的手段, 可实现发酵产物的定向调控, 提高发酵目标产物的纯度及浓度(Choi et al., 2014; Hirose et al., 2018; Jiang et al., 2018; Moscoviz et al., 2016; Schievano et al., 2016).因此探究电发酵系统中乙醇型产氢菌代谢机制, 以及研究在电发酵系统中不同电势、添加不同种电子中介物、不同底物等条件下对乙醇型发酵微生物产氢代谢机制的影响具有重要意义, 可以为通过电发酵系统定向调节微生物代谢方向, 从而提高资源能源回收率提供理论依据和指导.
除上述耦合系统外, 乙醇型发酵还可与厌氧消化耦合, 将乙醇型发酵液与活性污泥混合物厌氧共消化, 共消化系统的产甲烷量和有机物去除率显著高于单独活性污泥消化系统, 甲烷产量达到260 mL/g-COD(Li et al., 2020c).此外, 乙醇型发酵的代谢产物(乙酸、乙醇和H2)可用作二次转化的底物, 生产高价值化学物质, 例如中长链脂肪酸、聚羟基链烷酸酯(PHA)和有机燃料等(Schievano et al., 2016), 而CO2则可以用生物或非生物方法进行捕捉和溶解, 进而联合微生物电合成系统(MES)将CO2还原为多碳化合物或甲烷(Jiang et al., 2019).
6 展望(Perspective)乙醇型发酵可以同步产氢产乙醇, 与丁酸型发酵相比, 可以在较低pH环境产氢, 由于具有较强的自聚集生长能力易于形成细菌颗粒和生物膜, 在废水废弃物的生物处理与能源回收方面具有良好的应用前景.为提高乙醇型发酵的产氢效率和系统稳定性, 有必要在优化反应器构型和运行参数的基础上, 从分子生物学角度深入解析乙醇型发酵产氢微生物的代谢调控机制和其与发酵系统中其它微生物互作机制, 为构建稳定高效的乙醇型发酵生物制氢系统、制定高效产氢及代谢产物定向回收的精准调控策略提供理论依据.同时, 乙醇型发酵与其它系统耦合梯级产氢的新方法也亟待开发, 以充分利用微生物种间互作和电化学系统等定向调控微生物代谢方向, 获得更高的氢气产率和转化率, 为推动乙醇型发酵的大规模工业应用奠定基础.
Abdalla A M, Hossain S, Nisfindy O B, et al. 2018. Hydrogen production, storage, transportation and key challenges with applications:a review[J]. Energy Conversion and Management, 165: 602-627. DOI:10.1016/j.enconman.2018.03.088 |
Agler M T, Spirito C M, Usack J G, et al. 2012. Chain elongation with reactor microbiomes:upgrading dilute ethanol to medium-chain carboxylates[J]. Energ Environ Sci, 5(8): 8189-8192. DOI:10.1039/c2ee22101b |
Anzola-Rojas Mdel P, Zaiat M, De Wever H. 2016. Improvement of hydrogen production via ethanol-type fermentation in an anaerobic down-flow structured bed reactor[J]. Bioresource Technology, 202: 42-49. DOI:10.1016/j.biortech.2015.11.084 |
Asada Y, Tokumoto M, Aihara Y, et al. 2006. Hydrogen production by co-cultures of Lactobacillus and a photosynthetic bacterium, Rhodobacter sphaeroides RV[J]. International Journal of Hydrogen Energy, 31(11): 1509-1513. DOI:10.1016/j.ijhydene.2006.06.017 |
Bakonyi P, Kumar G, Kook L, et al. 2018. Microbial electrohydrogenesis linked to dark fermentation as integrated application for enhanced biohydrogen production:a review on process characteristics, experiences and lessons[J]. Bioresour. Technol., 251: 381-389. DOI:10.1016/j.biortech.2017.12.064 |
Boyd E S, Hamilton T L, Spear J R, et al. 2010. [FeFe]-hydrogenase in Yellowstone National Park:evidence for dispersal limitation and phylogenetic niche conservatism[J]. The ISME Journal, 4(12): 1485-95. DOI:10.1038/ismej.2010.76 |
Buckel W, Thauer R K. 2013. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation[J]. Biochimica et Biophysica Acta, 1827(2): 94-113. DOI:10.1016/j.bbabio.2012.07.002 |
Burow L C, Woebken D, Bebout B M, et al. 2012. Hydrogen production in photosynthetic microbial mats in the Elkhorn Slough estuary, Monterey Bay[J]. The ISME Journal, 6(4): 863-74. DOI:10.1038/ismej.2011.142 |
Carosia M F, dos Reis C M, Sakamoto I K, et al. 2017. Influence of C/P and C/N ratios and microbial characterization in hydrogen and ethanol production in an anaerobic fluidized bed reactor[J]. International Journal of Hydrogen Energy, 42(15): 9600-9610. DOI:10.1016/j.ijhydene.2017.01.127 |
Chatterjee P, Dessi P, Kokko M, et al. 2019. Selective enrichment of biocatalysts for bioelectrochemical systems:A critical review[J]. Renewable & Sustainable Energy Reviews, 109: 10-23. |
Choi O, Kim T, Woo H M, et al. 2014. Electricity-driven metabolic shift through direct electron uptake by electroactive heterotroph Clostridium pasteurianum[J]. Scientific Reports, 4: 6961. |
Dahiya S, Mohan S V. 2019. Selective control of volatile fatty acids production from food waste by regulating biosystem buffering:a comprehensive study[J]. Chemical Engineering Journal, 357: 787-801. DOI:10.1016/j.cej.2018.08.138 |
Dasa D, Veziroglu N. 2001. Hydrogen production by biological processes a survey of literature[J]. International Journal of Hydrogen Energy, 26: 13-28. DOI:10.1016/S0360-3199(00)00058-6 |
Ding J, Wang X, Zhou X F, et al. 2010. CFD optimization of continuous stirred-tank (CSTR) reactor for biohydrogen production[J]. Bioresource Technology, 101(18): 7005-7013. DOI:10.1016/j.biortech.2010.03.146 |
Greening C, Cook G M. 2014. Integration of hydrogenase expression and hydrogen sensing in bacterial cell physiology[J]. Curr Opin Microbiol, 18: 30-8. DOI:10.1016/j.mib.2014.02.001 |
Guo W Q, Ding J, Cao G L, et al. 2013. Accelerated startup of hydrogen production expanded granular sludge bed with L-Cysteine supplementation[J]. Energy, 60: 94-98. DOI:10.1016/j.energy.2013.08.025 |
Guo W Q, Ren N Q, Wang X J, et al. 2010. Accelerated startup of biological hydrogen production process by addition of Ethanoligenens harbinense B49 in a biofilm-based column reactor[J]. International Journal of Hydrogen Energy, 35(24): 13407-13412. DOI:10.1016/j.ijhydene.2009.11.115 |
Guo W Q, Ren N Q, Wang X J, et al. 2008a. Biohydrogen production from ethanol-type fermentation of molasses in an expanded granular sludge bed (EGSB) reactor[J]. International Journal of Hydrogen Energy, 33(19): 4981-4988. DOI:10.1016/j.ijhydene.2008.05.033 |
Guo W, Ren N, Chen Z, et al. 2008b. Simultaneous biohydrogen production and starch wastewater treatment in an acidogenic expanded granular sludge bed reactor by mixed culture for long-term operation[J]. International Journal of Hydrogen Energy, 33(24): 7397-7404. DOI:10.1016/j.ijhydene.2008.09.039 |
Guo W Q, Ren N Q, Wang X J, et al. 2009. Optimization of culture conditions for hydrogen production by Ethanoligenens harbinense B49 using response surface methodology[J]. Bioresource Technology, 100(3): 1192-1196. DOI:10.1016/j.biortech.2008.07.070 |
Han W, Wang B, Zhou Y, et al. 2012. Fermentative hydrogen production from molasses wastewater in a continuous mixed immobilized sludge reactor[J]. Bioresource Technology, 110: 219-223. DOI:10.1016/j.biortech.2012.01.057 |
Han W, Wang X, Ye L, et al. 2015. Fermentative hydrogen production using wheat flour hydrolysate by mixed culture[J]. International Journal of Hydrogen Energy, 40(13): 4474-4480. DOI:10.1016/j.ijhydene.2015.02.016 |
Hirose A, Kasai T, Aoki M, et al. 2018. Electrochemically active bacteria sense electrode potentials for regulating catabolic pathways[J]. Nature Communications, 9(1): 1083. DOI:10.1038/s41467-018-03416-4 |
Hua T, Li S, Li F, et al. 2019. Microbial electrolysis cell as an emerging versatile technology:a review on its potential application, advance and challenge[J]. Journal of Chemical Technology & Biotechnology, 94(6): 1697-1711. |
Huang H, Hu L, Yu W, et al. 2016. Heterologous overproduction of 2[4Fe4S]- and[2Fe2S]-type clostridial ferredoxins and[2Fe2S]-type agrobacterial ferredoxin[J]. Protein Expression and Purification, 121: 1-8. DOI:10.1016/j.pep.2015.12.019 |
Jiang Y, Lu L, Wang H, et al. 2018. Electrochemical control of redox potential arrests methanogenesis and regulates products in mixed culture electro-fermentation[J]. ACS Sustainable Chemistry & Engineering, 6(7): 8650-8658. |
Jiang Y, May H D, Lu L, et al. 2019. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation[J]. Water Res, 149: 42-55. DOI:10.1016/j.watres.2018.10.092 |
Jung K W, Kim D H, Kim S H, et al. 2011. Bioreactor design for continuous dark fermentative hydrogen production[J]. Bioresource Technology, 102: 8612-8620. DOI:10.1016/j.biortech.2011.03.056 |
Kamalaskar L, Kapse N, Pore S, et al. 2016. Genome sequence and gene expression studies reveal novel hydrogenases mediated hydrogen production by Clostridium biohydrogenum sp. nov., MCM B-509T[J]. International Journal of Hydrogen Energy, 41(28): 11990-11999. DOI:10.1016/j.ijhydene.2016.05.101 |
Kothari R, Pandey A K, Kumar S, et al. 2014. Different aspects of dry anaerobic digestion for bio-energy:an overview[J]. Renewable and Sustainable Energy Reviews, 39: 174-195. DOI:10.1016/j.rser.2014.07.011 |
Leach M R, Zamble D B. 2007. Metallocenter assembly of the hydrogenase enzymes[J]. Current Opinion in Chemical Biology, 11(2): 159-65. DOI:10.1016/j.cbpa.2007.01.011 |
Lee D J, Show K Y, Su A. 2011. Dark fermentation on biohydrogen production:pure culture[J]. Bioresource Technology, 102(18): 8393-8402. DOI:10.1016/j.biortech.2011.03.041 |
Lee H S, Vermaas W F, Rittmann B E. 2010. Biological hydrogen production:prospects and challenges[J]. Trends in Biotechnology, 28(5): 262-71. DOI:10.1016/j.tibtech.2010.01.007 |
Li H, Mei X, Liu B, et al. 2019a. Insights on acetate-ethanol fermentation by hydrogen-producing Ethanoligenens under acetic acid accumulation based on quantitative proteomics[J]. Environment International, 129: 1-9. DOI:10.1016/j.envint.2019.05.013 |
Li H, Mei X, Liu B, et al. 2019b. Quantitative proteomic analysis reveals the ethanologenic metabolism regulation of Ethanoligenens harbinense by exogenous ethanol addition[J]. Biotechnology for Biofuels, 12: 166. DOI:10.1186/s13068-019-1511-y |
Li J, Ai B, Ren N. 2013. Effect of initial sludge loading rate on the formation of ethanol type fermentation for hydrogen production in a continuous stirred-tank reactor[J]. Environmental Progress & Sustainable Energy, 32(4): 1271-1279. |
Li J, Li B, Zhu G, et al. 2007a. Hydrogen production from diluted molasses by anaerobic hydrogen producing bacteria in an anaerobic baffled reactor (ABR)[J]. International Journal of Hydrogen Energy, 32(15): 3274-3283. DOI:10.1016/j.ijhydene.2007.04.023 |
Li Q, Lv Y, Ding R, et al. 2019c. Hydrogen production efficiency and microbial community of ethanol-type fermentation[J]. Journal of Renewable and Sustainable Energy, 11(1): 013105. |
Li W, Cheng C, Cao G, et al. 2020a. Enhanced biohydrogen production from sugarcane molasses by adding Ginkgo biloba leaves[J]. Bioresource Technology, 298: 122523. DOI:10.1016/j.biortech.2019.122523 |
Li W, Cheng C, Cao G, et al. 2019d. Potential of hydrogen production from sugarcane juice by Ethanoligenens harbinense YUAN-3[J]. Journal of Cleaner Production, 237: 117552. DOI:10.1016/j.jclepro.2019.07.027 |
Li W, He L, Cheng C, et al. 2020b. Effects of biochar on ethanol-type and butyrate-type fermentative hydrogen productions[J]. Bioresource Technology, 306: 123088. DOI:10.1016/j.biortech.2020.123088 |
Li Y, Qiren N, Chen Y, et al. 2007b. Ecological mechanism of fermentative hydrogen production by bacteria[J]. International Journal of Hydrogen Energy, 32(6): 755-760. DOI:10.1016/j.ijhydene.2006.08.004 |
Li Y, Tang Y, Xiong P, et al. 2020c. High-efficiency methanogenesis via kitchen wastes served as ethanol source to establish direct interspecies electron transfer during anaerobic Co-digestion with waste activated sludge[J]. Water Res, 176: 115763. DOI:10.1016/j.watres.2020.115763 |
Li Z, Liu B, Cui H, et al. 2019e. The complete genome sequence of Ethanoligenens harbinense reveals the metabolic pathway of acetate-ethanol fermentation:a novel understanding of the principles of anaerobic biotechnology[J]. Environment International, 131: 105053. DOI:10.1016/j.envint.2019.105053 |
Li Z, Lou Y, Ding J, et al. 2020d. Metabolic regulation of ethanol-type fermentation of anaerobic acidogenesis at different pH based on transcriptome analysis of Ethanoligenens harbinense[J]. Biotechnology for Biofuels, 13(1): 101. |
Li Z, Wang J, Feng K, et al. 2020e. Rapid recruitment of hydrogen-producing biofilms for hydrogen production in a moving bed biofilm reactor by a sequential immobilization and deoxygenization approach[J]. Bioresource Technology, 317: 123979. DOI:10.1016/j.biortech.2020.123979 |
Lill R. 2009. Function and biogenesis of iron-sulphur proteins[J]. Nature, 460(7257): 831-8. DOI:10.1038/nature08301 |
Lin M, Ren N, Wang A. 2003. Cooperation of mixed culturing bacteria in the hydrogen production by fermentation[J]. Environmental Science, 24(2): 54-59. |
Liu B F, Xie G J, Wang R Q, et al. 2015. Simultaneous hydrogen and ethanol production from cascade utilization of mono-substrate in integrated dark and photo-fermentative reactor[J]. Biotechnology for Biofuels, 8: 8. DOI:10.1186/s13068-014-0191-x |
Liu H, Hu H, Chignell J, et al. 2010. Microbial electrolysis:novel technology for hydrogen production from biomass[J]. Biofuels, 1(1): 129-142. |
Lo Y C, Lee K S, Lin P J, et al. 2009. Bioreactors configured with distributors and carriers enhance the performance of continuous dark hydrogen fermentation[J]. Bioresource Technology, 100(19): 4381-4387. DOI:10.1016/j.biortech.2009.04.024 |
Lovato G, Lazaro C Z, Zaiat M, et al. 2017. Biohydrogen production by co-digesting whey and glycerin in an AnSBBR:performance optimization, metabolic pathway kinetic modeling and phylogenetic characterization[J]. Biochemical Engineering Journal, 128: 93-105. DOI:10.1016/j.bej.2017.09.011 |
Lu L, Ren N, Xing D, et al. 2009a. Hydrogen production with effluent from an ethanol-H2-coproducing fermentation reactor using a single-chamber microbial electrolysis cell[J]. Biosensors & Bioelectronics, 24(10): 3055-3060. |
Lu L, Ren Z J. 2016. Microbial electrolysis cells for waste biorefinery:a state of the art review[J]. Bioresource Technology, 215: 254-264. DOI:10.1016/j.biortech.2016.03.034 |
Lu L, Xing D, Ren N, et al. 2012. Syntrophic interactions drive the hydrogen production from glucose at low temperature in microbial electrolysis cells[J]. Bioresource Technology, 124: 68-76. DOI:10.1016/j.biortech.2012.08.040 |
Lu Y, Zhao H, Zhang C, et al. 2009b. Perturbation of formate pathway for hydrogen production by expressions of formate hydrogen lyase and its transcriptional activator in wild Enterobacter aerogenes and its mutants[J]. International Journal of Hydrogen Energy, 34(12): 5072-5079. DOI:10.1016/j.ijhydene.2009.04.025 |
Lund P, Tramonti A, De Biase D. 2014. Coping with low pH:molecular strategies in neutralophilic bacteria[J]. FEMS Microbiology Reviews, 38(6): 1091-1125. DOI:10.1111/1574-6976.12076 |
Moscoviz R, Toledo-Alarcon J, Trably E, et al. 2016. Electro-fermentation:how to drive fermentation using electrochemical systems[J]. Trends in Biotechnology, 34(11): 856-865. DOI:10.1016/j.tibtech.2016.04.009 |
Mulder D W, Boyd E S, Sarma R, et al. 2010. Stepwise[FeFe]-hydrogenase H-cluster assembly revealed in the structure of HydAΔEFG[J]. Nature, 465(7295): 248-51. DOI:10.1038/nature08993 |
Mulder D W, Ortillo D O, Gardenghi D J, et al. 2009. Activation of HydAΔEFG requires a preformed[4Fe-4S] cluster[J]. Biochemistry, 48(26): 6240-8. DOI:10.1021/bi9000563 |
Oh S-E, Van Ginkel S, Logan B E. 2003. The relative effectiveness of pH control and heat treatment for enhancing biohydrogen gas production[J]. Environmental Science & Technology, 37(22): 5186-5190. |
Olson E R. 1993. Influence of pH on bacterial gene expression[J]. Molecular Microbiology, 8(1): 5-14. |
Parameswaran P, Torres C I, Lee H-S, et al. 2009. Syntrophic interactions among anode respiring bacteria (ARB) and non-ARB in a biofilm anode:electron balances[J]. Biotechnology and Bioengineering, 103(3): 513-523. DOI:10.1002/bit.22267 |
Patil S A, Harnisch F, Koch C, et al. 2011. Electroactive mixed culture derived biofilms in microbial bioelectrochemical systems:the role of pH on biofilm formation, performance and composition[J]. Bioresour Technol, 102(20): 9683-90. DOI:10.1016/j.biortech.2011.07.087 |
Peden E A, Boehm M, Mulder D W, et al. 2013. Identification of global ferredoxin interaction networks in Chlamydomonas reinhardtii[J]. J Biol Chem, 288(49): 35192-209. DOI:10.1074/jbc.M113.483727 |
Posewitz M C, King P W, Smolinski S L, et al. 2004. Discovery of two novel radical S-adenosylmethionine proteins required for the assembly of an active[Fe] hydrogenase[J]. Journal of Biological Chemistry, 279(24): 25711-20. DOI:10.1074/jbc.M403206200 |
Prakash J, Sharma R, Ray S, et al. 2018. Wastewater:a potential bioenergy resource[J]. Indian Journal Microbiology, 58(2): 127-137. DOI:10.1007/s12088-017-0703-z |
Ren H Y, Kong F, Ma J, et al. 2018. Continuous energy recovery and nutrients removal from molasses wastewater by synergistic system of dark fermentation and algal culture under various fermentation types[J]. Bioresource Technology, 252: 110-117. DOI:10.1016/j.biortech.2017.12.092 |
Ren J, Sang Y, Ni J, et al. 2015. Acetylation regulates survival of Salmonella enterica serovar Typhimurium under acid stress[J]. Applied and Environmental Microbiology, 81(17): 5675-5682. DOI:10.1128/AEM.01009-15 |
Ren N, Chua H, Chan S, et al. 2007a. Assessing optimal fermentation type for bio-hydrogen production in continuous-flow acidogenic reactors[J]. Bioresource Technology, 98(9): 1774-1780. DOI:10.1016/j.biortech.2006.07.026 |
Ren N, Guo W, Liu B, et al. 2011. Biological hydrogen production by dark fermentation:challenges and prospects towards scaled-up production[J]. Current Opinion in Biotechnology, 22: 365-370. DOI:10.1016/j.copbio.2011.04.022 |
Ren N, Guo W, Liu B, et al. 2009. Biological hydrogen production from organic wastewater by dark fermentation in China:overview and prospects[J]. Frontiers of Environmental Science & Engineering in China, 3(4): 375-379. |
Ren N, Li J, Li B, et al. 2006. Biohydrogen production from molasses by anaerobic fermentation with a pilot-scale bioreactor system[J]. International Journal of Hydrogen Energy, 31(15): 2147-2157. DOI:10.1016/j.ijhydene.2006.02.011 |
Ren N, Wang B, Huang J C. 1997. Ethanol-type fermentation from carbohydrate in high rate acidogenic reactor[J]. Biotechnology and Bioengineering, 54(5): 428-433. DOI:10.1002/(SICI)1097-0290(19970605)54:5<428::AID-BIT3>3.0.CO;2-G |
Ren N, Xing D, Rittmann B E, et al. 2007b. Microbial community structure of ethanol type fermentation in bio-hydrogen production[J]. Environmental Microbiology, 9(5): 1112-1125. DOI:10.1111/j.1462-2920.2006.01234.x |
Ren N, Zhao D, Chen X, et al. 2002. Mechanism and controlling strategy of the production and accumulation of propionic acid for anaerobic wastewater treatment[J]. Science in China, 45(3): 319-327. DOI:10.1360/02yb9041 |
Rosa P R, Santos S C, Sakamoto I K, et al. 2014. Hydrogen production from cheese whey with ethanol-type fermentation:effect of hydraulic retention time on the microbial community composition[J]. Bioresource Technology, 161: 10-19. DOI:10.1016/j.biortech.2014.03.020 |
Ryan D, Pati N B, Ojha U K, et al. 2015. Global transcriptome and mutagenic analyses of the acid tolerance response of Salmonella enterica serovar Typhimurium[J]. Applied and Environmental Microbiology, 81: 8054-8065. DOI:10.1128/AEM.02172-15 |
Schievano A, Pepe Sciarria T, Vanbroekhoven K, et al. 2016. Electro-fermentation-merging electrochemistry with fermentation in industrial applications[J]. Trends in Biotechnology, 34(11): 866-878. DOI:10.1016/j.tibtech.2016.04.007 |
Shafaat H S, Rudiger O, Ogata H, et al. 2013. [NiFe] hydrogenases:a common active site for hydrogen metabolism under diverse conditions[J]. Biochimica et Biophysica Acta, 1827(8): 986-1002. |
Shi E, Li J, Zhang M. 2019. Application of IWA Anaerobic Digestion Model No. 1 to simulate butyric acid, propionic acid, mixed acid, and ethanol type fermentative systems using a variable acidogenic stoichiometric approach[J]. Water Research, 161: 242-250. DOI:10.1016/j.watres.2019.05.094 |
Summers Z M, Fogarty H E, Leang C, et al. 2010. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria[J]. Science, 330(6009): 1413-1415. DOI:10.1126/science.1196526 |
Tang J, Jia S, Qu S, et al. 2014. An integrated biological hydrogen production process based on ethanol-type fermentation and bipolar membrane electrodialysis[J]. International Journal of Hydrogen Energy, 39(25): 13375-13380. DOI:10.1016/j.ijhydene.2014.04.085 |
Tang J, Yuan Y, Guo W-Q, et al. 2012. Inhibitory effects of acetate and ethanol on biohydrogen production of Ethanoligenens harbinese B49[J]. International Journal of Hydrogen Energy, 37: 741-747. DOI:10.1016/j.ijhydene.2011.04.067 |
Velazquez Abad A, Dodds P E. 2017. Production of hydrogen[J]. Encyclopedia of Sustainable Technologies, 3: 293-304. |
Vignais P M, Billoud B. 2007. Occurrence, classification, and biological function of hydrogenases:an overview[J]. Chemical Reviews, 107(10): 4206-72. DOI:10.1021/cr050196r |
Wang A, Ren N, Shi Y, et al. 2008. Bioaugmented hydrogen production from microcrystalline cellulose using co-culture-Clostridium acetobutylicum X9 and Ethanoigenens harbinense B49[J]. International Journal of Hydrogen Energy, 33(2): 912-917. DOI:10.1016/j.ijhydene.2007.10.017 |
Wang B, Li Y, Ren N. 2013. Biohydrogen from molasses with ethanol-type fermentation:effect of hydraulic retention time[J]. International Journal of Hydrogen Energy, 38(11): 4361-4367. DOI:10.1016/j.ijhydene.2013.01.120 |
Wegener G, Krukenberg V, Riedel D, et al. 2015. Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria[J]. Nature, 526(7574): 587-90. DOI:10.1038/nature15733 |
Wrighton K C, Castelle C J, Wilkins M J, et al. 2014. Metabolic interdependencies between phylogenetically novel fermenters and respiratory organisms in an unconfined aquifer[J]. The ISME Journal, 8(7): 1452-63. DOI:10.1038/ismej.2013.249 |
Wu Y, Wang C, Zheng M, et al. 2017. Effect of pH on ethanol-type acidogenic fermentation of fruit and vegetable waste[J]. Waste Management, 60: 158-163. DOI:10.1016/j.wasman.2016.09.033 |
Xie G J, Liu B F, Ren N Q, et al. 2010. Control strategies for hydrogen production through co-culture of Ethanoligenens harbinense B49 and immobilized Rhodopseudomonas faecalis RLD-53[J]. International Journal of Hydrogen Energy, 35(5): 1929-1935. DOI:10.1016/j.ijhydene.2009.12.138 |
Xie G J, Liu B F, Wang Q, et al. 2016. Ultrasonic waste activated sludge disintegration for recovering multiple nutrients for biofuel production[J]. Water Research, 93: 56-64. DOI:10.1016/j.watres.2016.02.012 |
Xing D, Ren N, Li Q, et al. 2006. Ethanoligenens harbinense gen. nov., sp. nov., isolated from molasses wastewater[J]. International Journal of Systematic and Evolutionary Microbiology, 56: 755-760. DOI:10.1099/ijs.0.63926-0 |
Xing D, Ren N, Rittmann B E. 2008. Genetic diversity of hydrogen-producing bacteria in an acidophilic ethanol-H2-coproducing system, analyzed using the[Fe]-hydrogenase gene[J]. Applied and Environmental Microbiology, 74(4): 1232-1239. DOI:10.1128/AEM.01946-07 |
Yang G, Wang J. 2017. Fermentative hydrogen production from sewage sludge[J]. Critical Reviews in Environmental Science and Technology, 47(14): 1219-1281. DOI:10.1080/10643389.2017.1348107 |
Yin Y N, Zhang Y F, Karakashev D B, et al. 2017. Biological caproate production by Clostridium kluyveri from ethanol and acetate as carbon sources[J]. Bioresource Technology, 241: 638-644. DOI:10.1016/j.biortech.2017.05.184 |
Zhang S, Liu M, Chen Y, et al. 2017. Achieving ethanol-type fermentation for hydrogen production in a granular sludge system by aeration[J]. Bioresource Technology, 224: 349-357. DOI:10.1016/j.biortech.2016.11.096 |
Zhao X, Xing D, Qi N, et al. 2017. Deeply mechanism analysis of hydrogen production enhancement of Ethanoligenens harbinense by Fe2+ and Mg2+:monitoring at growth and transcription levels[J]. International Journal of Hydrogen Energy, 42(31): 19695-19700. DOI:10.1016/j.ijhydene.2017.06.038 |
Zhao X, Xing D, Zhang L, et al. 2010. Characterization and overexpression of a[FeFe]-hydrogenase gene of a novel hydrogen-producing bacterium Ethanoligenens harbinense[J]. International Journal of Hydrogen Energy, 35(18): 9598-9602. DOI:10.1016/j.ijhydene.2010.06.098 |
Zhao X, Ye S, Qi N, et al. 2019. Mechanisms of enhanced bio-H2 production in Ethanoligenens harbinense by L-cysteine supplementation:analyses at growth and gene transcription levels[J]. Fuel, 252: 143-147. DOI:10.1016/j.fuel.2019.04.037 |
Zhao Z, Li Y, Quan X, et al. 2018. Improving the co-digestion performance of waste activated sludge and wheat straw through ratio optimization and ferroferric oxide supplementation[J]. Bioresource Technology, 267: 591-598. DOI:10.1016/j.biortech.2018.07.052 |
丁杰, 任南琪, 刘敏, 等. 2004. Fe和Fe2+对混合细菌产氢发酵的影响[J]. 环境科学, 25(4): 48-53. |
林明, 任南琪, 王爱杰, 等. 2003. 几种金属离子对高效产氢细菌产氢能力的促进作用[J]. 哈尔滨工业大学学报, 35(2): 20-24. |
秦智, 任南琪, 李建政. 2007. 发酵生物制氢反应器的产氢菌生物强化作用研究[J]. 环境科学, 28(12): 2843-2848. |
任南琪, 宫曼丽, 邢德峰. 2004. 连续流生物制氢反应器乙醇型发酵的运行特性[J]. 环境科学, 25(6): 113-116. |
 2020, Vol. 40
2020, Vol. 40


