2. 城市水资源与水环境国家重点实验室, 哈尔滨 150090
2. State Key Laboratory of Urban Water Resources Centre, Harbin 150090
电-Fenton基本原理涉及溶解氧或氧气在阴极表面通过2电子氧还原反应(oxygen reduction reaction, ORR)原位生成H2O2(式(1)), 与溶液中的Fe2+发生经典Fenton反应产生强氧化性羟基自由基(· OH)(式(3)), 继而对目标污染物进行无选择性氧化降解.因反应3产生的Fe3+能在阴极附近电还原为Fe2+(式(2)), 减少了电-Fenton体系Fe2+投加量及产生的铁泥量(Brillas et al., 2009; 周蕾等, 2013), 以上是电-Fenton体系较传统Fenton具备的优势.
H2O2原位积累量决定着电-Fenton中· OH产生速率及总浓度, 但电-Fenton技术却面临着阴极H2O2积累量不足的难题.所以近些年电-Fenton技术主要围绕阴极的设计和制备展开, 即电极材料设计、修饰强化2电子ORR的电子转移(电化学过程).然而, 2电子ORR电合成H2O2不仅由电子转移控制, 还受限于氧液相传质过程.目前电-Fenton体系缺乏从2电子ORR电化学合成H2O2全过程角度去提高阴极H2O2积累量的研究.所以本综述拟在剖析2电子ORR电化学合成H2O2原理基础之上, 从强化氧的液相传质、提高2电子ORR反应活性/选择性、减少H2O2无效分解的全过程角度对电-Fenton阴极H2O2积累进行归纳与总结, 在此基础之上阐明电-Fenton发展趋势.

|
(1) |

|
(2) |
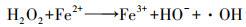
|
(3) |
如图 1所示, 2电子ORR电化学合成H2O2的前提是氧气(O2, gas)溶解于电解液形成溶解氧(O2, bulk), O2, bulk从溶液本体, 依次通过“阴极/溶液”界面薄液层所形成的扩散层、双电层, 最终到达阴极表面活性位点.前驱体氧完成液相传质后, 以吸附氧(O2, ad)形式吸附于阴极活性位点, 接下来2电子ORR电化学合成H2O2由电子传递过程控制, 其分别决定着2电子ORR的反应活性及选择性.据最新ORR分子轨道理论表明(Xia et al., 2020; Gao et al., 2020), 吸附于阴极的O2, ad得到一个电子形成过渡态HOO*(式(5)), 若O2, ad在阴极活性位点以端基形式吸附(图 3a), HOO*中O—O键保持完整性, 则遵循2电子ORR电化学合成H2O2路径(式(6)), 否则O2, ad以侧基形式吸附(图 3b), O—O键断裂转变为4电子ORR产H2O反应(式(7)~(8)).因此, 设计有利于O2, ad以端基形式吸附的阴极, 控制其与中间过渡态HOO*适中的结合强度, 对调控阴极2电子ORR反应活性、选择性至关重要.同时, 原位产生的H2O2不可避免会发生如下分解(Brillas et al., 2009; Zhou et al., 2019a):① H2O2在阴极界面进一步电化学还原转变为H2O(式(9));②小部分H2O2会在从双电层到溶液本体的液相传质过程中发生自分解释放O2(式(10));③若采用无隔膜电解池, 因阳极氧化造成的H2O2的消耗(式(11)).考虑到式(9)~(11)所涉及H2O2分解均无· OH生成, 其分解视为无效分解.因此, 目前电-Fenton体系中测定的H2O2为其积累量(式(12)), 即原位H2O2产生量减H2O2无效耗损量(Liang et al., 2018).综上分析可知, 提高原位2电子ORR电化学合成H2O2的量, 同时减少/抑制其无效分解是电-Fenton体系提高阴极H2O2积累量的有效途径.综合2电子ORR电化学合成H2O2全过程可知, 当阴极局部质子充足时, 2电子ORR电化学合成H2O2不仅受到前驱体O2, bulk传质过程控制, 同时也受到阴极界面电子传递过程控制(2电子ORR反应活性、选择性).所以为了强化2电子ORR电化学合成H2O2, 需协同强化前驱体氧传质及2电子ORR反应活性、选择性.所以本文从3方面综述提高电-Fenton体系阴极H2O2积累量:①2电子ORR过程中前驱体氧液相传质强化;② 2电子ORR反应活性、选择性的强化;③减少/抑制H2O2无效分解.
 |
| 图 1 阴极2电子ORR电化学积累H2O2过程 Fig. 1 Mechanism of H2O2 accumulation via two-electron oxygen reduction reaction |
 |
| 图 2 氧气在阴极活性位点的不同吸附方式(Jiang et al., 2018) Fig. 2 The different style of oxygen adsorption on active sites on cathode |
 |
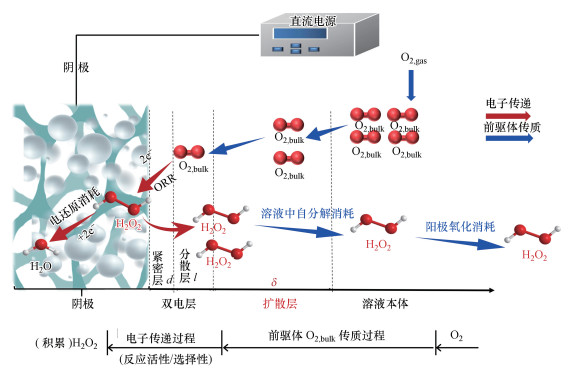
| 图 3 电-Fenton阴极发展史总结 Fig. 3 A summary of cathode used in the electro-Fenton process |

|
(4) |

|
(5) |

|
(6) |
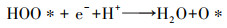
|
(7) |
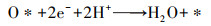
|
(8) |
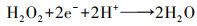
|
(9) |
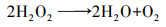
|
(10) |
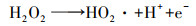
|
(11) |
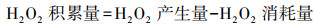
|
(12) |
2电子ORR电化学合成H2O2由氧的液相传质步骤和阴极界面电子传递步骤组成.其中液相传质包括:电迁移、对流和扩散.如图 1所示, 根据氧的液相传质作用区域可将“阴极/溶液”薄液层划分为:双电层(紧密层和分散层)、扩散层及溶液本体(对流区).双电层内物质浓度分布仅受双电层内电场强度控制(电极电势控制), 不受其他传质过程影响, 且一般而言, 双电层厚度(≈100 Å)仅为扩散层厚度(≈106 Å)的0.1%, 所以双电层内氧的液相传质可忽略不计(徐艳辉, 2015).而对流区距离阴极表面较远, 该区域氧的浓度与溶液本体浓度保持一致, 此时氧的液相传质由对流传质主导.在扩散层中, 溶液对流速度小且氧分子保持电中性, 致使氧的液相传质由扩散作用承担.前驱体氧在对流层与扩散层的液相传质是连续(串联)进行, 而扩散传质速度远远低于对流传质速度, 此时氧在扩散层中扩散传质成为氧液相传质的限速步骤, 所以2电子ORR反应的前驱体氧的液相传质主要由氧在扩散层中的扩散速度决定(图 1).
根据Fick定律可知, 前驱体氧在扩散层中扩散传质驱动力为浓度差(CO2b-CO2*/δN), 此时由扩散传质控制的2电子ORR反应的扩散电流密度满足式(13).根据公式可知, 强化氧在扩散层中的扩散传质有以下途径:提高其初始氧浓度(CO2b)、减小电极/溶液扩散层厚度(δN)及增加其扩散系数(DO2)等.
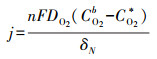
|
(13) |
式中, j为扩散电流密度(A · m-2), n为电子转移数目, F为法拉第常数(96485 C · mol-1), DO2为氧气扩散系数(cm2 · s-1), CO2*和CO2b分别为氧在电极界面和溶液本体中的浓度(mol · m-3), δN为扩散层厚度(cm).
2.2.1 提高初始氧浓度依据相似相容原理, O2为非极性分子(偶极矩=0 D), 水为强极性分子(偶极矩=1.85 D), 所以氧气在水中溶解度有限, 仅为8.25 mg · L-1 (25 ℃, 101 kPa) (姚鹏城等, 2019).而常压下, 空气中O2, gas高达310 mg · L-1, 比水溶液中O2, bulk高37倍, 且氧气在空气中的扩散系数(2.1×10-5 m2 · s-1)远高于水溶液(1.9×10-9 m2 · s-1) (Zhou et al., 2018a), 基于此, 学者们设计了直接利用空气或者氧气诱发阴极2电子ORR反应, 使氧气无需溶解而直接利用气体扩散电极(Gas diffusion electrode, GDEs) (Brillas et al., 2009).GDEs疏水界面层的存在, 可以在形成的气、固、液三相界面直接利用空气中O2, gas及O2, bulk, 从而极大提高了初始氧浓度, 因此, GDEs电极被认为是电-Fenton体系中H2O2产量最高的阴极之一(Brillas et al., 2009).借鉴GDEs利用空气中O2, gas原理, 周伟巧妙提出了“漂浮”阴极, 充分利用空气中O2, gas, 促使电-Fenton阴极原位H2O2产量对比浸没式阴极提高了4.3倍(Zhou et al., 2018b).同样秉承提高初始氧浓度的思路, 周明华课题组设计了新型的“旋转阴极”, 同时利用O2, bulk与空气中O2, gas, 在电流密度为50 A · m-2, 旋转速度为10 r · min-1, pH=3时, 1 h内H2O2积累量高达116 mg · L-1, 远高于不采用该设计阴极H2O2积累量(37 mg · L-1) (Yu et al., 2014; Yu et al., 2015).同时, 周明华课题组在2016年提出了“穿透式”阴极的设计, 对比普通的浸没式阴极, 发现对染料的降解率可以提高40%以上(Ma et al., 2016; Lang et al., 2020a), 该设计主要得益于空气中氧气的充分利用, 以及水流穿过电极/电解质界面时形成的扰动.
除了采用如上方法利用空气中O2, gas提高2电子ORR初始氧浓度外, 学者们也提出了多种策略增加电解液中O2, bulk浓度.Scialdone根据亨利定律(式(14))提高氧分压达到增加O2, bulk浓度的目的, 当压力从100 kPa增加到600 kPa时, 石墨阴极电化学合成H2O2产量从1.2 mmol · L-1增加到7 mmol · L-1 (Scialdone et al., 2015).增加氧分压提高H2O2量同样适用于碳毡阴极(Pérez et al., 2018a).Pérez设计的加压射流鼓风电-Fenton反应器, 利用氧分压原理将O2, bulk浓度提高至21.9 mg · L-1, 增加了O2, bulk的过饱和浓度, 继而强化阴极电合成H2O2(Pérez et al., 2017; Pérez et al., 2018b).而在进行2电子ORR反应时, 若O2, bulk以细小气泡形式被利用时, 则更有利于H2O2的电合成(Sheng et al., 2014; Oloman et al., 1975).如Pérez利用文丘里原理设计的射流曝气反应器促进了O2, bulk以更小的气泡形式释放, 从而使O2, bulk达到过饱和浓度, 该装置在电流密度分别为20、30、50 mA · cm-2时, H2O2积累量分别为134、156、204 mg · L-1 (Pérez et al., 2019).这是由于O2, bulk小气泡尤其是微气泡时(直径<50 μm), 有助于提高O2, bulk的过饱和浓度及在水中的停留时间(Pérez et al., 2019).Scialdone等设计了微流体电-Fenton反应器(阴阳极间距为10~100 μm), 利用阳极析氧反应(式(15))产生分散、小尺寸、接近饱和的O2, bulk, 因阴阳极距离微小, O2, bulk直接被阴极利用, 使石墨阴极H2O2产量提高一个数量级至6 mmol · L-1 (Scialdone et al., 2013).
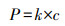
|
(14) |
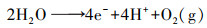
|
(15) |
式中, P为气体的分压, k为亨利常数, c为溶于溶剂内的体积摩尔浓度.
2.2.2 提高氧扩散系数及减小扩散层厚度氧扩散系数(DCO2)与温度(T)、粘度系数(η)及氧分子的半径(r)等参数相关(式(16)).目前电-Fenton体系通过改变氧扩散系数、减小扩散层厚度来提高阴极电合成H2O2的研究比较有限.赵国华利用微波热效应使“阴极/溶液”的双电层、扩散层局部过热(溶液本体的温度保持不变), 提高氧扩散系数, 强化氧在扩散中的液相传质, 最终使阴极电合成H2O2的产量提高了近10倍(Compton et al., 1998; 王宇晶, 2011; Wang et al., 2012).最新研究表明流通式电-Fenton反应器相较于传统电-Fenton反应器, 能强化对流传质, 减小了扩散层厚度, 制备的修饰石墨毡阴极电化学合成H2O2能耗较低(3.08 kWh · kg-1) (Ren et al., 2019a; Li et al., 2020).周伟改变了传统的电-Fenton供电模式, 以脉冲电流模式供电, 结果显示当采用“2 s开+2 s关”供电模式时, 多孔阴极材料H2O2最终积累量提高了61.6%, 这在于脉冲电流抑制了多孔电极对原位合成H2O2的还原分解作用(Deng et al., 2018).除能减少部分H2O2在阴极孔道内的还原消耗外, 推测当体系处于断电状态时, “阴极/溶液”界面存在的扩散层瞬间消失, 溶液本体O2, bulk利用间隙时间补充了扩散层中被消耗的O2, bulk;当处于供电状态时, 扩散层中的高浓度O2, bulk有利于2电子ORR反应.
阴极自身结构也会影响氧的液相传质.刘鸿等采用新的电极动力学拟合方程引入了阴极结构对氧传质的影响系数(kO2), 定量分析了阴极结构对氧液相传质的影响, 通过动力学方程推导出多孔阴极对液相氧传质呈正影响(kO20>0), 即多孔电极可调控扩散层, 强化前驱体氧传质, 其中介孔优于微孔, 佐证了前期学者观点并更好地解释了电极结构对氧液相传质的影响(Deng et al., 2018).

|
(16) |
式中, k为Boltzmann常数, T为温度(K)、η为粘度系数(Pa · s), r为氧分子的半径(Å).
2.3 电子ORR反应活性、选择性的强化4电子ORR反应是燃料电池、金属-空气电池、电解水等领域的研究重点, 但近年来, 2电子ORR电合成H2O2因反应原料(水、空气)清洁易得而在分散式H2O2的合成领域引发诸多关注(赵苹苹等, 2019).当前驱体氧传质强化后, O2, bulk以吸附态吸附于阴极活性位点, 此时ORR电化学合成H2O2受电子转移控制.而2电子ORR反应涉及多步质子耦合电子转移, 动力学反应缓慢, 且ORR更倾向于4电子反应, 导致2电子ORR选择性低, 所以需要将兼具高活性和高选择性的H2O2电合成催化剂添加至阴极以加速其动力学过程、降低过电位, 提高电流效率(Ma et al., 2019).
王兴总结了近些年高效2电子ORR电合成H2O2的催化剂(Jiang et al., 2018).根据Sabatier原理, 利用第一性原理密度泛函模拟的实验结果, 归纳总结了多种ORR催化剂与中间产物(HOO*)之间的结合能, 通过“火山图”关系确定催化剂的2电子ORR反应活性和选择性, 理论上来说, 处于“火山图”顶点位置的催化剂2电子ORR反应活性、选择性最好.其中Pd、Pd-Hg、Pt-Hg、Pt-Ni、Pd-Au等催化剂更接近火山顶点, “火山图”理论预测和实验结果表明上述催化剂对2电子ORR的过电位低、选择性高(≈95%) (Gao et al., 2020).而据最新研究报道, 具有明确定义活性中心的单原子催化剂(SAC)因其活性位点具备的单个、分散的特点, 有利于氧的端基吸附(图 2a)从而保证HOO*中O—O键的完整, 在2电子ORR反应中具备高活性和高选择性, 如过渡金属单原子催化剂(Mn、Fe、Co、Ni、Cu) (Gao et al., 2020).其中基于金属-氮-碳(M-N-C, M为过渡金属)的单原子分散催化剂, 其活性中心是利用仿生学技术模拟生物卟啉中心的金属-氮配位结构, 被认为是最有希望替代贵金属的催化剂, 如Co-SAC (Gao et al., 2020)、Fe-C-N (Jiang et al., 2019)、Co-C-N (Jung et al., 2020)等.
以上研究成果借助密度泛函的量子化学计算揭示了2电子ORR电合成H2O2的基元反应能垒及2电子ORR催化剂的微观结构、反应机理, 为电-Fenton阴极在分子或原子尺度的理论设计提供指导.目前, 2电子ORR催化剂的构筑材料多为纳米尺寸, 如何从微观跨越至宏观、构筑出稳定高效的2电子ORR阴极值得深入探究.
电-Fenton研究中宏观阴极材料的发展进程如图 3所示, 自1939年Berl学者以活性碳为阴极电化学合成H2O2以来(Berl et al., 1939), 碳基阴极在电化学研究中得到广泛应用, 最具代表性的是20世纪70年代Oloman采用石墨颗粒制备流化床阴极于碱性条件下电合成H2O2(Oloman et al., 1975).电-Fenton雏形始于1971年Tomat和Vecchi学者利用Hg池阴极电还原产Fenton试剂(H2O2、Fe2+), 产生的· OH氧化降解苯酚(Tomat et al., 1971), 然而他们并未命名该体系.在此基础上的后期研究主要围绕电化学还原产Fenton试剂、强化· OH产生而展开.如1986年Sudoh以石墨板阴极产H2O2与投加的Fe2+发生经典Fenton反应, 生成· OH降解苯酚(Sudoh et al., 1986).因石墨、Hg阴极氧液相传质缓慢及Hg阴极潜在毒性等问题, 1993年Hsiao以三维结构的网状玻璃碳(泡沫碳)为阴极替代石墨和Hg阴极进行电化学合成H2O2(Tzedakis et al., 1989; Hsiao et al., 1993).1996年Brillas课题组Casado将三维结构气体扩散阴极(石墨-PTFE阴极, GDEs)应用于电-Fenton阴极电合成H2O2研究并率先命名电-Fenton体系(Brillas et al., 1996), 三维结构碳阴极也因其巨大的比表面积和良好的氧传质效率、相较于传统石墨阴极可提高约200倍H2O2积累量等特点, 为学者们所熟知(Kornienko et al., 2003; Brillas et al., 2009).Oturan课题组于2000年将三维结构、比表面积大的碳毡阴极应用于电-Fenton体系, 实现了4-硝基苯酚的降解研究(Oturan et al., 2000; 2001).
回顾电-Fenton阴极发展史可知(图 3), 2003—2004年电-Fenton阴极研究主要集中在碳毡和GDEs, 而活性炭毡(ACF) (Xia et al., 2015a)、碳布(carbon cloth)、多壁碳纳米管(MWNT)、碳海绵(carbon sponge)、BDD、介孔碳等主要在2004—2010年后期研究中被用来作为电-Fenton阴极.以上传统碳材料性质稳定、电导率高、价格低廉、对H2O2催化分解作用小, 但其阴极H2O2积累能力有待进一步提升.近年来在土壤修复、碳固存、生物燃料等领域受到重视的生物质转化生物炭, 也可制成生物质基碳阴极应用于电-Fenton体系.高书燕团队综述了生物质基碳阴极在电-Fenton中的应用, 在此不再赘述(韩佳佳等, 2019).
随着材料学科的发展, 2009年后的电-Fenton阴极研究迎来了传统碳材料表面修饰的热潮.其中对阴极修饰可归纳为3方面(图 4):①其他碳活性组分的引入, 比如石墨烯、碳纳米管、炭黑、乙炔黑、生物炭等;②阴极表界面异质原子杂化修饰, 比如含O、N、S、F、P、B等非金属元素;③金属及金属氧化物等活性组分的负载修饰.
 |
| 图 4 电-Fenton阴极的修饰 Fig. 4 The fabrication of cathode in the electro-Fenton process |
① 其他碳活性组分引入.为了强化传统碳阴极电合成H2O2的量, 越来越多的“外援”碳活性成分被引入传统碳阴极, 如图 4所示.
石墨烯具有良好的导电性、导热性、巨大的比表面积, 在纳米器件、储氢等领域被广泛应用(Guinea et al., 2009; Dreyer et al., 2010), 而作为活性组分引入传统碳阴极后, 其ORR电子转移数更偏向2电子ORR电合成H2O2 (Zhang et al., 2018a).其对ORR强化机制可归纳为:石墨烯优异的导电性提高了传统阴极导电能力;其次石墨烯表面丰富的含氧官能团(—COOH、—OH、O)调控了阴极界面亲/疏水性(Garcia-Rodriguez et al., 2018).自Khataee学者用碳纳米管(CNTs)修饰石墨阴极获得约7倍H2O2产量提升后, CNTs对碳毡、碳纸等阴极修饰也见诸报端(Khataee et al., 2011; Djafarzadeh et al., 2014).孙杰利用有序介孔碳修饰活性碳制备ACF@OMC阴极, 介孔碳的引入提高了活性碳导电能力, 同时也强化了前驱体氧的扩散吸附及还原作用(Ren et al., 2016).Sheng等(2014)以乙炔黑修饰气体扩散电极, 促使阴极表界面亲/疏水性得以平衡, 实现阴极H2O2产量高达54.2 mg · cm-3 · h-1.同样Li利用炭黑修饰GEDs, H2O2高达275.5 mmol · L-1, 是传统碳阴极的8倍(Luo et al., 2015).
② 异质原子掺杂引入.碳基阴极中碳的电子结构可通过异质原子的修饰进行调整, 调整后C原子sp2杂化电子结构平衡被打破, 引起碳原子电荷离域, 促使共价带降低、费米能级电子云密度增加, 最后与异质原子相连的碳原子间的π电子活化成为ORR活性位点(Xia et al., 2019a).异质原子的掺杂, 即将O、N、S、P、F、B等元素替代了碳骨架中的C, 即利用异质元素与C之间电负性差异有效地调控了C的电负性(Yu et al., 2010).
1) O元素.O异质原子的掺杂主要通过电化学氧化、强氧化剂(NaOH或KOH、HNO3、H2SO4、H2O2等)氧化、等离子体氧化等手段在阴极表界面形成含氧官能团(OGs) (Zhou et al., 2019b).
Zhou等(2013)采用电化学氧化-循环伏安法对石墨毡阴极进行含氧官能团的修饰, 经由处理的石墨毡阴极表面含氧官能团C=O、COOH分别增加了0.084%、33.57%, 显著提高了阴极H2O2积累量.据报道含氧官能团(如醌官能团)是2电子ORR反应的活性位点, 同时形成的酸性含氧官能团如—COOH、—COH、—COO、—R—OH等能改善阴极界面的亲水性, 有利于溶解氧传质至电极活性位点、以吸附氧形式吸附于电极上, 进而强化2电子ORR的发生.而该课题组采用KOH碱液辅助高温手段(Wang et al., 2015)、水合肼(Zhou et al., 2013; 2014)活化石墨毡, 同样提高了电化学活性表面积及含氧官能团种类及含量.Xu等也曾报道过电化学氧化的方法修饰碳纤维(Xia et al., 2015a; Xue et al., 2017a).除此之外, 等离子体氧化法也能进行电-Fenton阴极O掺杂的修饰研究(Khataee et al., 2017b).Pan利用高温乙酸活化碳毡进而增加其孔径、比表面积及ORR活性位点sp3-C、缺陷及含氧官能团(Pan et al., 2018).虽然通过不同氧化手段对阴极进行异质O原子掺杂易操作、成本低且能增加阴极2电子ORR活性位点及选择性, 但是杂化后的OGs阴极在电还原过程中会被还原而逐渐失去活性位点(Zhou et al., 2019c).因此有学者利用电氧化法对失活的阴极再次进行电氧化, 从而实现阴极含氧掺杂的循环再生(Zhou et al., 2019c; Yang et al., 2019a).
2) N元素.N异质原子杂化在燃料电池、电解水制氢、二氧化碳电还原等领域备受关注, 同时N异质原子杂化被认为是最有效提高2电子ORR反应活性、选择性的方法之一.Dai学者于2009年在Science报道N修饰的CNTs形成的与吡啶N相连的C原子为ORR活性位点后, N杂原子修饰在电-Fenton阴极研究成为焦点(Gong et al., 2009).N、C元素位于元素周期表相近位置且原子半径相似, 但因不同的电子云排布, N的掺杂引起碳结构中最小晶格不匹配, 同时N的高电负性及强的吸电子的能力会导致与其相邻的C原子通过分子内部的电荷转移而表现为正电荷, 所以N的引入会引起C晶格的电子离域及电性改变(Zhou et al., 2019d).
孙杰以双氰胺为氮源修饰活性炭纤维阴极, 制备氮掺杂的有序介孔碳阴极证明了氮元素的引入降低了阴极ORR过电位、提高了阴极的孔径及比表面积(Peng et al., 2014).同时该课题组以硝酸铵为电解质, 石墨为工作阳极, 利用阳极氧化原位制备含N的石墨阴极, 经氧化后的石墨阴极因N杂原子引入, 具有更大的比表面积、更强的亲水性、更高的ORR活性, 促使2电子ORR电流效率由57.96%提高至83.76% (Qiu et al., 2018).徐海波利用富含N的聚丙烯腈基碳纤维刷(N含量2%~6%)作为电-Fenton阴极, 其H2O2积累量高达90.4 mg · L-1 · h-1(Xia et al., 2015b).而以苯胺电氧化聚合成导电、含N的聚苯胺修饰石墨毡阴极可以强化石墨毡阴极的2电子ORR电合成H2O2, 以其作为阴极在电-Fenton体系中进行邻苯二甲酸二甲酯降解, 一级反应速率常数被提高至0.0753 min-1(Xia et al., 2015b).Christian分别利用N、S元素修饰介孔碳阴极, 发现经由N、S修饰的介孔碳阴极对2电子ORR选择性高达85%, 其中N修饰的介孔碳阴极效果优于S元素(Perazzolo et al., 2016).Panagiotis以氨水作为氮源, 利用水热合成法制备了N杂化的石墨烯CNTs阴极, 其2电子ORR的起始过电位低于文献报道(起始电位约为-0.2 V) (Liu et al., 2016).Alireza利用N2、O2、Ar2等离子体处理石墨电极后, 阴极表面出现了三维立体的纳米结构可以改变电极的亲疏水性(Khataee et al., 2017b).Zhou等采用水合肼、乙醇水热法制备的N掺杂石墨毡阴极, 在应用于电-Fenton体系对左氧氟沙星降解的过程中, 4 h后的TOC去除率高达92% (Liu et al., 2017; Xue et al., 2017).聚吡咯作为一种导电性优异的含N聚合物, 被Jalal等掺杂CNTs修饰石墨阴极, 当CNTs质量分数为2.5%时, 修饰后的电极在最适电压-0.55 V vs. SCE下的H2O2积累量为5.6 mg · L-1(Babaei-Sati et al., 2017).周明华以硝酸铵为氮源修饰石墨烯阴极, 当硝酸铵/石墨烯的质量比为1 : 1时, 获得的阴极ORR催化效果最佳, 其N(石墨型氮、吡啶型氮、嘧啶型氮)含量为0.66% (Yang et al., 2019; Yang et al., 2018).同时N修饰的石墨烯不仅能够提高2电子ORR, 同时也能促进H2O2在无Fe2+催化剂时转化为· OH, 从而构建无铁催化剂的电-Fenton体系.He采用2 mol · L-1的HNO3处理石墨电极引入更多的缺陷位点(据报道缺陷也是ORR反应的活性位点(Zhou et al., 2019a))及含N/O的官能团, 促使H2O2产量在3 mA · cm-2的电流密度下提高了3倍(He et al., 2019).Ko等(2018)首次采用不同温度(700、800、900、1000 ℃)热解离子液体制备N-负载的碳阴极材料.结果显示热解温度为900 ℃时, 2电子ORR的活性位点(吡啶型氮含量为4.29%、石墨型氮7.30%)含量最佳.Zhu等(2018)利用泡沫状三聚氰胺海绵作为起始基底阴极材料, 利用一步高温热解法制备富含N的泡沫碳阴极, 结果显示在热解温度升高为900 ℃时, 更多的N转变为石墨氮, 所制备的阴极H2O2产量为87.19 μmol · L-1.
3) F元素.F作为电负性最大的元素, 将其引入碳阴极后形成的C—F键极性强于CDN键, 更易引起C电荷极化、改变费米能级, 从而改变“电极/溶液”薄液层中的活性物质传质(Liu et al., 2012b).电-Fenton阴极F元素掺杂工作最早由全夑课题组开展, 他们以金属有机框架或废弃生物质(绿萝)为前驱体辅以HF合成F杂化的多级孔碳阴极通过引入约3.41%共价CF2, 3调整了碳的电子结构、改变中间产物HOO*的吸附能, 提高2电子ORR选择性, 促进电合成H2O2 (Zhao et al., 2018a; 2018b).实验结果表明, F修饰后的阴极在电势为-0.2~-0.6 V时, H2O2产率为22.4~64.5 mmol · L-1, 其中2电子ORR电合成H2O2选择性高达97.5%左右.
4) S、P、B等元素.根据电负性理论, 如S、P、B等其他非金属元素的掺杂也能够调控C的电负性, 这些掺杂在涉及4电子ORR的燃料电池等领域被诸多报道, 而在电-Fenton阴极中报道有限.P原子的半径更大、给电子能力强, 理论上对2电子ORR活性影响更大, 而大半径P的引入可能会破坏C骨架, 导致P的掺杂量偏低.Yan采用H3PO4(85%)水热合成法修饰碳纳米管, 制备的P-CNTs GDE阴极电合成H2O2的电流效率高达88.5% (Xia et al., 2019b).为了进一步提高2电子ORR反应活性、选择性, 学者们针对两种或多种异质元素共掺杂进行了探究, 如N-P (Li et al., 2018)、B-N (Chen et al., 2018)、N-S (Perazzolo et al., 2016)等.
③ 金属或金属氧化物引入.碳基阴极界面上利用金属、金属氧化物、金属酞菁等进行修饰也是电-Fenton阴极研究重点.其中包括CeO2、Nb2O5、Ta2O5、WO2.72、CexA1-xO2 (A=Zr、Cu、Ni)、MnO2、CoS2、Nb2O5、V2O5、CoSxPy等(Ye et al., 2019; Zhou et al., 2019b).Zhang等(2018b)利用Co-负载的MoO2和氨腈为前驱物修饰碳毡制备Co-N-负载MoO2碳毡阴极, 其ORR起始还原电位为-0.157 V vs. Ag/AgCl, 旋转圆环测试技术(RRDE)结果显示该阴极圆环电流密度高达35.5 μA, H2O2产量>20%.但由于电-Fenton体系内的pH值一般控制在3左右, 金属或金属氧化物的溶出及潜在危害值得警惕.
2.3.2 金属基阴极电-Fenton体系阴极除了碳基阴极及以其为基底制备的修饰电极外, 金属基阴极的应用也较为普遍.2013年April首次以Ti金属氧化物作为阴极, 考察其2电子ORR产H2O2的能力, 在其研究过程中阴极直接利用阳极析氧反应产生的氧气从而减少体系外部供氧能耗, 但较于碳基阴极, Ti阴极H2O2积累能力十分有限(< 1 mg · L-1), 推测为Ti金属本身会促进溶液本体中H2O2自分解, 不利于其积累(Zhang et al., 2018).不锈钢因价格低廉、稳定性高等特点, 也被作为电-Fenton阴极进行科学研究, 如张辉等利用不锈钢阴极考察其对氯贝酸降解能力(Lin et al., 2014).Brillas等也曾利用不锈钢阴极在电-Fenton体系中降解染料(Cheng et al., 2017; Aguilar et al., 2017).Pt金属被用作电-Fenton阴极也有报道(Huang et al., 2017).
泡沫镍(Ni-F)电极因良好的导电性和机械性能、易切割和低成本等特点, 近年来被应用于电-Fenton体系的研究工作中.Ni-F作为电-Fenton阴极或者颗粒电极的研究最早始于2010年张礼知课题组(Fan et al., 2010; Liu et al., 2012a).研究发现Ni-F阴极不仅能够通过2电子ORR积累H2O2, Ni0还能够活化分子氧产生H2O2, 从而使电-Fenton体系H2O2积累有了两条选择途径(式(17)、(18)).Marta课题组利用Ni-F阴极电-Fenton体系处理酿酒废水, 结果证明了Ni-F阴极产H2O2过程涉及O2 · -中间产物(Iglesias et al., 2015).为了利用Ni-F的三维骨架结构, Panagiotis将碳包覆于Ni-F上制备C@Ni-F阴极, 其电合成H2O2的能力是原始Ni-F的4倍(Song et al., 2015).Tang利用CNTs修饰Ni-F, 在100 mA电流下电解2 h, H2O2可达到307 mg · L-1则进一步提高了Ni-F阴极的H2O2积累能力(Tang et al., 2015).Elvira利用壳聚糖的包覆作用, 将铁负载于Ni-F上制备出适合连续流模式下运行的电-Fenton阴极材料使得体系能耗低至15.75 kWh · kg-1(Bocos et al., 2016).但Ni-F阴极在电-Fenton体系中因严格的pH值而溶出的镍, 不仅造成潜在环境风险, 还会导致三维骨架的坍塌.随后Deng等提出了基于非常规多聚磷酸钠电解质的电-Fenton, 将电-Fenton工作pH范围拓宽至近中性, 提高了泡沫镍阴极实际应用的可能性(Deng et al., 2019a).而为了进一步提高Ni-F阴极H2O2的积累量, Deng等将废弃芦苇转化为生物炭(Deng et al., 2019b)、富含N的多孔碳(Deng et al., 2019c; Chen et al., 2019a)修饰Ni-F阴极, 使2电子ORR电合成H2O2分别提高了3.24倍、13.8倍.
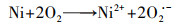
|
(17) |
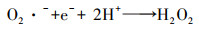
|
(18) |
阴极电合成H2O2引发的无效分解如阴极还原、阳极氧化及溶液自分解是无法避免的(式(9)~(11)), 所以减少甚至抑制H2O2的无效分解同样是提高电-Fenton阴极H2O2积累量的重要途径.目前电-Fenton技术针对弱化/抑制H2O2无效分解的研究报道有限.在抑制阳极氧化方面主要是在电-Fenton体系中利用隔膜将阴阳反应池隔离开来, 限制阴极电合成的H2O2到达阳极室而被氧化分解, 一定程度上能够抑制H2O2的阳极消耗(Brillas et al., 2009).周伟博士提出的脉冲电流供电模式在不供电周期内使原位合成的H2O2从多孔阴极内部扩散至溶液本体, 避免了电还原对H2O2消耗, 研究显示实验结果表明脉冲供电模式使多孔阴极H2O2积累量提高61.6% (Zhou et al., 2018b).Yuta研究发现相较于微孔, 设计有序介孔(3.4~4.0 nm)可在传质过程中减少H2O2与阴极孔道的接触, 从而利于H2O2从阴极内部扩散至溶液本体, 进一步减少H2O2电还原消耗(Park et al., 2014).借鉴H2O2漂白稳定剂的思路, Deng等提出的基于多聚磷酸钠的非常规电解质, 利用多聚磷酸钠(偏磷酸钠、三聚磷酸钠及四聚磷酸钠等)对H2O2的稳定作用, 即多聚磷酸钠能够缓解H2O2在溶液本体的自分解, 提高电-Fenton阴极电化学积累H2O2量, 还能够将工作pH范围扩展至近中性并强化电-Fenton体系氧化效能(Deng et al., 2019b; 2019d).
3 电-Fenton技术在环境中的应用(The application of electro-Fenton in environment)经由电-Fenton技术产生的· OH, 因无选择性、高氧化性(E(· OH/H2O)=2.8 V vs SHE)能够氧化绝大多数有机物质至完全矿化(式(19)).电-Fenton技术在水污染、土壤、大气、消毒、矿物等环境污染治理方面均有应用, 其研究进展总结为:

|
(19) |
电-Fenton技术所产生的活性氧(· OH、H2O2)能与水体中大多数的有机污染物发生链式反应, 快速将污染物降解/矿化.如表 1所示, 电-Fenton技术近年来被广泛应用于抗生素(Proguett et al., 2020)、农药/除虫剂(Diaw et al., 2020a)、染料(Labiadh et al., 2019)及其他酚类物质(Deng et al., 2020)的降解研究中, 尤以抗生素和农药/除虫剂为研究热点.同时, 电-Fenton还被应用于畜牧业废水(Kuang et al., 2019)、叶酸废水(Zheng et al., 2019)、洗衣废水(Ghanbari et al., 2019)、炼油废水(Monteil et al., 2019)、制药废水(Olvera-Vargas et al., 2021)等实际废水处理中.目前, · OH降解有机污染物主要通过如下的3种方式:①脱氢作用或从H2O中脱除氢原子;②羟基化或亲电加成到不饱和键上;③电子转移或氧化还原反应.Buxton曾详细报道过· OH与烷类、醇类的一级反应速率常数在106~108 M-1 · s-1, 与芳香化合物的绝对反应速率常数高达108~1010 M-1 · s-1 (Buxton et al., 1988).
| 表 1 电-Fenton技术处理有机污染物的分类 Table 1 Classification of organics treated in the electro-Fenton process |
2020年席卷全球的新型冠状病毒使水质消毒研究再次成为热点.常用的消毒工艺有加氯消毒、紫外线消毒、二氧化氯消毒和臭氧消毒等(赵旭等, 2020).而电-Fenton技术与常规消毒方法的不同之处在于Fenton反应产生的活性氧化物可有效灭活病原菌, 同时不产生卤化消毒副产物.其消毒原理是活性氧物质能够破坏病毒的遗传物质, 如影响衣壳蛋白的蛋白交联作用、使其丧失宿主连接及基因注入能力进而无法感染宿主, 最终导致病毒的完整性被破坏无法保护核酸, 活性氧物质还可以进一步氧化核酸形成5-氯胞啶, 促使病毒丢失复制功能(Suquet et al., 2010).
Chen等采用颗粒活性炭(GAC)作为阴极, 通过电-Fenton工艺产生H2O2和· OH用于水消毒.研究发现108 CFU · mL-1大肠杆菌经300 min后, 无论何种菌种均可灭活, 此外, 电-Fenton过程造成的溶液酸化能抵抗水中高浓度的缓冲碳酸盐(Chen et al., 2019b).Hamidi等用电-Fenton工艺处理低浓度大肠菌群(200 MPN/100 m · L-1)的帕劳博隆垃圾填埋场渗滤液和含有高浓度大肠菌群(>24×104 MPN/100 m · L-1)的垃圾渗滤液, 去除率分别高达100%和99.9% (Aziz et al., 2013).周明华等课题组选择细菌总数作为电-Fenton工艺的评价指标, 结果表明电-Fenton消毒率可达98.65%, 细菌总数从(4.86×104±2065)个· mL-1急剧下降到687~2624个· mL-1, 而过滤吸附法对细菌总数的去除率仅为3.23%, 证明电-Fenton消毒是造成细菌总数显著较低的主要原因(Ren et al., 2019b).Carmina等采用电-Fenton对生乳品废水进行连续处理, 分别对异养菌、乳酸菌、大肠杆菌和肠球菌的灭活效果进行评价, 结果表明电-Fenton体系中的细菌基本失活, 而电絮凝(EC)中形成的絮体去除细菌的效果较差.并且与EC相比, 电-Fenton有效避免了含活性细菌的有害污泥的形成(Bruguera-Casamada et al., 2019).Kourdali采用电-Fenton工艺对大肠杆菌进行灭活, 实验发现高电流强度对大肠杆菌细胞有较好的灭活和破坏作用, 加入NaCl或Na2SO4均可提高消毒效果, 这是由于电解过程中产生大量原位氧化剂, 氯化物(Cl-)和硫酸盐(SO42-)可以通过与其他强氧化剂的联合电化学反应, 从而提高电化学过程的消毒效能.此外, 相较于EPC和EC, 电-Fenton工艺在灭活大肠杆菌和降低能耗等方面具体更好的效果(Kourdali et al., 2018).
3.3 电-Fenton技术在土壤污染治理中的应用近年来, 土壤问题已经成为全球共同关注的重点环境问题, 我国作为全球土壤污染最严重的国家之一, 土壤污染治理工作刻不容缓.除常规土壤污染治理措施和手段外, 电化学法也成为极具前景的方法之一, 其中电-Fenton法近来也有诸多成果产出.肖柏林等分别通过阳极法和阴极法对石油烃污染土壤进行修复研究.阴极为原位产H2O2结合外加Fe2+;阳极法用铁为阳极、不锈钢板为阴极时, 土壤TPH降解过程符合二级动力学拟合, 运行6 h后的TPH去除率可达80%以上(肖柏林, 2019).徐文迪等将电-Fenton与生物泥浆法联合修复芘污染土壤, 72 h内去除率高达92%, 相较于两方法单独使用的去除率提高到50%以上.由于联合修复缩短了电芬顿处理时间, Pyr在· OH的作用下迅速开环, 生成小分子和低环的中间产物的过程提高了污染物的生物可利用性, 降解菌适应期明显缩短, 因而提高生物降解效率(徐文迪等, 2019).Liu等同样利用联合修复手段进行多环芳烃含量高达3605 mg · kg-1的焦化土壤的修复工作, 运行40 d后的PAHs去除率为95.2%.以实验室规模开发的电-Fenton-生物浆联用方法避免了单一使用EF造成的大量细胞死亡的情况, 可使细菌活性和泥浆中的细菌迅速恢复, 联合方法的效果得到了明显提升(Liu et al., 2020c).Carboneras等发现地下水洗涤而获得的土壤洗涤废水中含有主要成分为二氯吡啶酸的商业除草剂, 在利用电芬顿(EF)进行降解试验的过程中发现, 地下水因其较高的矿化度可作为具有持续性的支持电解质, 二氯吡啶酸在自由基的作用下被氧化, 伴随着羧酸产量的提升, 溶液的生物降解性能也得到了强化(Carboneras Contreras et al., 2019).Liu等在2020年进行的土壤淋洗同电化学高级氧化结合起来进行的受柴油污染土地的修复工作.采用顺序土壤洗涤和电化学高级氧化工艺(EAOP)修复被柴油污染的合成土壤, 通过进行EO和EF的柴油去除效率和荧光成分转化等结果的对比, EF的去除效果最多较EO约提升了10%, 证明了EF在治理土壤污染研究工作中的较好潜力(Liu et al., 2020b).
3.4 电化学法在大气污染防治中的应用中国是煤炭消费和进口的第一大国.作为产煤、用煤大国, 我国的煤机装备制造及使用量位居世界第一.煤机装备是煤炭科技转化为生产力的重要载体, 更是煤炭工业发展不可或缺的支柱.电化学脱硫技术是煤炭燃烧前脱硫技术研究的方向之一, 进而实现烟气净化避免含硫废气的产生.该方面的试验研究始于20世纪60年代.近年来, 随着对煤炭脱硫技术的不断深入研究, 煤炭的脱硫技术取得了理论和应用的较大进展, 从而也大大推动了煤炭电化学脱硫技术的发展.20世纪60年代, 人们对煤的电解还原脱硫进行了研究, 但是进展不大, 至70年代末, 对煤炭的电化学脱硫进行了开发性的研究, 不但克服了传统电化学脱硫高温、高压的缺点, 还可以联产氢气, 大大降低了生产成本(罗万江等, 2009).煤的电化学脱硫是在水、煤粉和添加剂的水煤浆环境下进行的, 借助煤在电解槽阳极发生电化学氧化反应, 将煤中的黄铁矿及有机硫化物氧化成可溶性的硫化物, 从而达到净化煤的目的(朱红等, 2003).根据电解液的不同, 可以分为碱性和酸性电化学法.其中在碱性条件中, 阳极表面因OH-失去电荷而形成的具有高活性的物质如· OH、· O、O2-等发挥着强氧化剂的作用氧化无机硫和有机硫, 形成可溶性的硫酸盐和多硫化合物达到脱硫目的, 此时脱硫主要由电化学反应中阳极反应完成;酸性条件下的脱硫涉及到了催化剂的参与, 是在电化学的作用下间接电解而将煤中的硫氧化脱去.国内孙康(2007)研究了在氢氧化钠体系中脱除煤中硫分的适宜条件;除煤炭工业外, 我国其他能源行业的低硫“清洁燃料”生产也受到了广泛关注.Lu等展开电解液循环利用对铝土矿浆电解脱硫的影响进行研究, 铝土矿中的大多数硫最终被氧化成SO42-并进入电解质(LÜ et al., 2016).我国于2005年开始对于汽油质量标准的硫含量进行了约40%的下调, 从800 μg · g-1下调至500 μg · g-1, 其中北京因奥运会的举办则被要求限制在150 μg · g-1, 因此针对车用燃油开展深度脱硫势在必行.王文波等(2005)针对汽油电化学-化学氧化耦合脱硫进行了研究, 以自制电极置于Ce(NO3)3、HNO3及去离子水混合制成的复合电解液通以直流电进行汽油脱硫的耦合处理, 脱硫率达到了55%.
4 结论(Conclusions)本文针对电-Fenton技术中存在的阴极氧气传质效率低、反应活性/选择性有限, 而存在的电生H2O2浓度低的科学问题, 从强化2电子ORR前驱体氧传质策略、提高2电子ORR电合成H2O2的反应活性/选择性方法、减小/抑制H2O2无效分解等方面, 系统归纳和总结了强化阴极H2O2产量的方法和策略.同时将电-Fenton技术在水处理、土壤防控、大气污染、消毒等领域的应用进行整理和归纳.最后对电-Fenton的发展趋势进行了阐述.
5 展望(Outlook)目前, 电-Fenton技术研究的重点是阴极材料的调控和制备, 即主要是通过调控电子转移过程从而强化2电子ORR电合成H2O2.通过前文可知, 2电子ORR电合成H2O2为氧的液相传质和电子转移两步骤共同控制.所以秉承全过程调控的思路来调控阴极积累H2O2的策略更为有效.
氧的液相传质强化涉及传质学、流体力学、电化学等交叉学科, 因此可借鉴其他学科的手段从微观层面解析氧气溶解、液相传质原理等进行强化.如氧在水溶液中溶解度低主要是由于非极性氧分子与极性水分子间的色散作用所引起.因此, 当氧分子极化率增加时, 甚至改变氧分子极性, 溶解度会相应提高.借鉴该原理, 可施加各种力场, 如磁场、微波等, 使O2, gas发生极化, 增大O2, gas与H2O之间的相互作用力, 实现液相传质的强化(吴海珍等, 2018).
目前电-Fenton阴极研究的主流思路是制备-实验论证-推测机制, 而理想的研究策略是在充分认识了2电子ORR电合成H2O2过程及借助第一性原理密度泛函量子化学计算揭示2电子ORR催化剂的微观结构、反应机理基础上, 在分子或原子尺度进行阴极的理论设计, 并指导基于该催化剂的阴极的可控制备与定向合成, 即利用化学计算模拟最合适的结构, 指导实验可控合成, 最后实验论证.
电-Fenton技术中阴极表界面2电子ORR电合成H2O2、Fe3+/Fe2+电还原是决定体系中· OH产生速率及浓度的关键.目前关注焦点为阴极电合成H2O2, 对于同在阴极且还原电势不同于ORR的Fe3+/Fe2+反应未引起足够重视.值得注意的是, 当2电子ORR电合成H2O2的量越高时, “阴极/溶液”微区域消耗更多H+, 而溶液本体H+传质有限, 将会在“阴极/溶液”微区域积累大量OH-, Fe3+/Fe2+水解而沉积于阴极表面将导致活性位点被覆盖, 不利于ORR及Fe3+/Fe2+电还原反应(Deng et al., 2020b).所以, 平衡2电子ORR电合成H2O2与Fe3+/Fe2+之间的反应, 而不是仅关注于阴极电合成H2O2是提高电-Fenton体系氧化效能的核心.
Aguilar Z G, Brillas E, Salazar M, et al. 2017. Evidence of Fenton-like reaction with active chlorine during the electrocatalytic oxidation of Acid Yellow 36 azo dye with Ir-Sn-Sb oxide anode in the presence of iron ion[J]. Applied Catalysis B:Environmental, 206: 44-52. DOI:10.1016/j.apcatb.2017.01.006 |
Aveiro L R, Da Silva A G M, Candido E G, et al. 2020. MnO2/Vulcan-based gas diffusion electrode for mineralization of diazo dye in simulated effluent[J]. Electrocatalysis, 11(3): 268-274. DOI:10.1007/s12678-020-00583-1 |
Aziz H A, Othman O M, Abu Amr S S. 2013. The performance of Electro-Fenton oxidation in the removal of coliform bacteria from landfill leachate[J]. Waste Management, 33(2): 396-400. DOI:10.1016/j.wasman.2012.10.016 |
Babaei-Sati R, Basiri Parsa J. 2017. Electrogeneration of H2O2 using graphite cathode modified with electrochemically synthesized polypyrrole/MWCNT nanocomposite for electro-Fenton process[J]. Journal of Industrial and Engineering Chemistry, 52: 270-276. DOI:10.1016/j.jiec.2017.03.056 |
Ben Hafaiedh N, Fourcade F, Bellakhal N, et al. 2020. Iron oxide nanoparticles as heterogeneous electro-Fenton catalysts for the removal of AR18 azo dye[J]. Environmental Technology, 41(16): 2146-2153. DOI:10.1080/09593330.2018.1557258 |
Berl E. 1939. A new cathodic process for the production of H2O2[J]. Transactions of the Electrochemical Society, 76(1): 359-370. DOI:10.1149/1.3500291 |
Bocos E, Pérez-álvarez D, Pazos M, et al. 2016. Coated nickel foam electrode for the implementation of continuous electro-Fenton treatment[J]. Journal of Chemical Technology & Biotechnology, 91(3): 685-692. |
Brillas E, Mur E, Casado J. 1996. Iron(Ⅱ) catalysis of the mineralization of aniline using a carbon-PTFE O2-fed cathode[J]. Journal of the Electrochemical Society, 143(3): 49-53. |
Brillas E, Sirés I, Oturan M A. 2009. Electro-Fenton process and related electrochemical technologies based on Fenton's reaction chemistry[J]. Chemical Reviews, 109(12): 6570-6631. DOI:10.1021/cr900136g |
Bruguera-Casamada C, Araujo R M, Brillas E, et al. 2019. Advantages of electro-Fenton over electrocoagulation for disinfection of dairy wastewater[J]. Chemical Engineering Journal, 376: 119975-119999. DOI:10.1016/j.cej.2018.09.136 |
Buxton G V, Greenstock C L, Helman W P, et al. 1988. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O- in aqueous solution[J]. Journal of Physical and Chemical Reference Data, 17(2): 513-886. DOI:10.1063/1.555805 |
Campos S, Salazar R, Arancibia-Miranda N, et al. 2020. Nafcillin degradation by heterogeneous electro-Fenton process using Fe, Cu and Fe/Cu nanoparticles[J]. Chemosphere, 247: 125813-125834. DOI:10.1016/j.chemosphere.2020.125813 |
Cao P, Quan X, Zhao K, et al. 2020. Selective electrochemical H2O2 generation and activation on a bifunctional catalyst for heterogeneous electro-Fenton catalysis[J]. Journal of Hazardous Materials, 382: 121102-121124. DOI:10.1016/j.jhazmat.2019.121102 |
Carboneras Contreras M B, Fourcade F, Assadi A, et al. 2019. Electro Fenton removal of clopyralid in soil washing effluents[J]. Chemosphere, 237: 112468-124447. |
Chen Y, Yang L, Paul Chen J, et al. 2019a. Electrospun spongy zero-valent iron as excellent electro-Fenton catalyst for enhanced sulfathiazole removal by a combination of adsorption and electro-catalytic oxidation[J]. Journal of Hazardous Materials, 371: 576-585. DOI:10.1016/j.jhazmat.2019.03.043 |
Chen L, Pinto A, Alshawabkeh A N. 2019b. Activated carbon as a cathode for water disinfection through the electro-Fenton process[J]. Catalysts (Basel, Switzerland), 9(7): 601-618. |
Chen S, Chen Z, Siahrostami S, et al. 2018. Designing boron nitride islands in carbon materials for efficient electrochemical synthesis of hydrogen peroxide[J]. Journal of the American Chemical Society, 140(25): 7851-7859. DOI:10.1021/jacs.8b02798 |
Chen W, Liang J. 2009. Electrochemical destruction of dinitrotoluene isomers and 2, 4, 6-trinitrotoluene in spent acid from toluene nitration process[J]. Journal of Hazardous Materials, 161(2): 1017-1023. |
Chen Y, Wang M, Tian M, et al. 2017. An innovative electro-fenton degradation system self-powered by triboelectric nanogenerator using biomass-derived carbon materials as cathode catalyst[J]. Nano Energy, 42: 314-321. DOI:10.1016/j.nanoen.2017.10.060 |
Cheng T, Huang C, Huang Y, et al. 2017. Kinetic study and optimization of electro-Fenton process for dissolution and mineralization of ion exchange resins[J]. Chemical Engineering Journal, 308: 954-962. DOI:10.1016/j.cej.2016.09.142 |
Chou S, Huang Y, Lee S, et al. 1999. Treatment of high strength hexamine-containing wastewater by electro-Fenton method[J]. Water Research, 33(3): 751-759. |
Compton R G, Coles B A, Marken F. 1998. Microwave activation of electrochemical processes at microelectrodes[J]. Chemical Communications, (23): 2595-2596. DOI:10.1039/a806511j |
Cruz-González K, Torres-López O, García-León A, et al. 2010. Determination of optimum operating parameters for Acid Yellow 36 decolorization by electro-Fenton process using BDD cathode[J]. Chemical Engineering Journal, 160(1): 199-206. |
de Matos D B, Barbosa M P R, Leite O M, et al. 2020. Characterization of a tubular electrochemical reactor for the degradation of the commercial diuron herbicide[J]. Environmental Technology, 41(10): 1307-1321. DOI:10.1080/09593330.2018.1531941 |
de Oliveira Lopes E, Dalponte Dallabona I, Weinschutz R, et al. 2020. Fe/polymer-based photocatalyst synthesized by sono-sorption method applied to wastewater treatment[J]. Journal of Photochemistry and Photobiology A:Chemistry, 396: 112545-112567. DOI:10.1016/j.jphotochem.2020.112545 |
Deng F, Garcia-Rodriguez O, Olvera-Vargas H, et al. 2018. Iron-foam as a heterogeneous catalyst in the presence of tripolyphosphate electrolyte for improving electro-Fenton oxidation capability[J]. Electrochimica Acta, 272: 176-183. DOI:10.1016/j.electacta.2018.03.160 |
Deng F, Olvera-Vargas H, Garcia-Rodriguez O, et al. 2020a. Unconventional electro-Fenton process operating at a wide pH range with Ni foam cathode and tripolyphosphate electrolyte[J]. Journal of Hazardous Materials, 396: 122641-122655. DOI:10.1016/j.jhazmat.2020.122641 |
Deng F, Li S, Cao Y, et al. 2020b. A dual-cathode pulsed current electro-Fenton system:Improvement for H2O2 accumulation and Fe3+ reduction[J]. Journal of Power Sources, 466: 228342-228354. DOI:10.1016/j.jpowsour.2020.228342 |
Deng F, Qiu S, Zhu Y, et al. 2019a. Tripolyphosphate-assisted electro-Fenton process for coking wastewater treatment at neutral pH[J]. Environmental Science and Pollution Research, 26(12): 11928-11939. DOI:10.1007/s11356-019-04548-w |
Deng F, Li S, Zhou M, et al. 2019b. A biochar modified nickel-foam cathode with iron-foam catalyst in electro-Fenton for sulfamerazine degradation[J]. Applied Catalysis Environmental, 256: 117796-117810. DOI:10.1016/j.apcatb.2019.117796 |
Deng F, Qiu S, Olvera-Vargas H, et al. 2019c. Electrocatalytic sulfathiazole degradation by a novel nickel-foam cathode coated with nitrogen-doped porous carbon[J]. Electrochimica Acta, 297: 21-30. DOI:10.1016/j.electacta.2018.11.180 |
Deng F, Olvera-Vargas H, Garcia-Rodriguez O, et al. 2019d. Waste-wood-derived biochar cathode and its application in electro-Fenton for sulfathiazole treatment at alkaline pH with pyrophosphate electrolyte[J]. Journal of Hazardous Materials, 377: 249-258. DOI:10.1016/j.jhazmat.2019.05.077 |
Diaw P A, Oturan N, Gaye Seye M D, et al. 2020. Removal of the herbicide monolinuron from waters by the electro-Fenton treatment[J]. Journal of Electroanalytical Chemistry, 864: 114087-114099. DOI:10.1016/j.jelechem.2020.114087 |
Djafarzadeh N, Safarpour M, Khataee A. 2014. Electrochemical degradation of three reactive dyes using carbon paper cathode modified with carbon nanotubes and their simultaneous determination by partial least square method[J]. Korean Journal of Chemical Engineering, 31(5): 785-793. DOI:10.1007/s11814-013-0267-5 |
Dreyer D R, Park S, Bielawski C W, et al. 2010. The chemistry of graphene oxide[J]. Chemical Society Reviews, 39(1): 228-240. |
Droguett C, Salazar R, Brillas E, et al. 2020. Treatment of antibiotic cephalexin by heterogeneous electrochemical Fenton-based processes using chalcopyrite as sustainable catalyst[J]. Science of the Total Environment, 740: 140154-140167. DOI:10.1016/j.scitotenv.2020.140154 |
Fan Y, Ai Z, Zhang L. 2010. Design of an electro-Fenton system with a novel sandwich film cathode for wastewater treatment[J]. Journal of Hazardous Materials, 176(1): 678-684. |
Fayazi M, Ghanei-Motlagh M. 2020. Electrochemical mineralization of methylene blue dye using electro-Fenton oxidation catalyzed by a novel sepiolite/pyrite nanocomposite[J]. International Journal of Environmental Science and Technology. |
Forti J C, Loretti G H, Tadayozzi Y S, et al. 2020. A phytotoxicity assessment of the efficiency 2, 4-D degradation by different oxidative processes[J]. Journal of Environmental Management, 266: 110588. DOI:10.1016/j.jenvman.2020.110588 |
Fu J, Zhang X, Lei L. 2007. Fe-modified multi-walled carbon nanotube electrode for production of hydrogen peroxide[J]. Acta Physico-Chimica Sinica, 23(8): 1157-1162. DOI:10.1016/S1872-1508(07)60060-6 |
Ganzenko O, Trellu C, Oturan N, et al. 2020. Electro-Fenton treatment of a complex pharmaceutical mixture:Mineralization efficiency and biodegradability enhancement[J]. Chemosphere, 253: 126659. DOI:10.1016/j.chemosphere.2020.126659 |
Gao J, Yang H B, Huang X, et al. 2020. Enabling direct H2O2 production in acidic media through rational design of transition metal single atom catalyst[J]. Chem, 2: 658-674. |
Garcia-Rodriguez O, Lee Y Y, Olvera-Vargas H, et al. 2018. Mineralization of electronic wastewater by electro-Fenton with an enhanced graphene-based gas diffusion cathode[J]. Electrochimica Acta, 276: 12-20. DOI:10.1016/j.electacta.2018.04.076 |
Ghanbari F, Martínez-Huitle C A. 2019. Electrochemical advanced oxidation processes coupled with peroxymonosulfate for the treatment of real washing machine effluent:A comparative study[J]. Journal of Electroanalytical Chemistry, 847: 113182-113199. DOI:10.1016/j.jelechem.2019.05.064 |
Ghanbarlou H, Nasernejad B, Nikbakht Fini M, et al. 2020. Synthesis of an iron-graphene based particle electrode for pesticide removal in three-dimensional heterogeneous electro-Fenton water treatment system[J]. Chemical Engineering Journal, 395: 125025-125040. DOI:10.1016/j.cej.2020.125025 |
Ghasemi M, Khataee A, Gholami P, et al. 2019. Template-free microspheres decorated with Cu-Fe-NLDH for catalytic removal of gentamicin in heterogeneous electro-Fenton process[J]. Journal of Environmental Management, 248: 109236-109246. DOI:10.1016/j.jenvman.2019.07.007 |
Ghasemi M, Khataee A, Gholami P, et al. 2020. In-situ electro-generation and activation of hydrogen peroxide using a CuFeNLDH-CNTs modified graphite cathode for degradation of cefazolin[J]. Journal of Environmental Management, 267: 110629-110645. DOI:10.1016/j.jenvman.2020.110629 |
Gholizadeh A M, Zarei M, Ebratkhahan M, et al. 2020. Removal of Phenazopyridine from wastewater by merging biological and electrochemical methods via Azolla filiculoides and electro-Fenton process[J]. Journal of Environmental Management, 254: 109802-109820. DOI:10.1016/j.jenvman.2019.109802 |
Gong K, Du F, Xia Z, et al. 2009. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction[J]. Science, 323(5915): 760-764. DOI:10.1126/science.1168049 |
Görmez F, Görmez Ö, Gözmen B, et al. 2019. Degradation of chloramphenicol and metronidazole by electro-Fenton process using graphene oxide-Fe3O4 as heterogeneous catalyst[J]. Journal of Environmental Chemical Engineering, 7(2): 102990-103011. DOI:10.1016/j.jece.2019.102990 |
Gozzi F, Sirés I, de Oliveira S C, et al. 2018. Influence of chelation on the Fenton-based electrochemical degradation of herbicide tebuthiuron[J]. Chemosphere, 199: 709-717. DOI:10.1016/j.chemosphere.2018.02.060 |
Guelfi D R V, Ye Z, Gozzi F, et al. 2019a. Ensuring the overall combustion of herbicide metribuzin by electrochemical advanced oxidation processes. Study of operation variables, kinetics and degradation routes[J]. Separation and Purification Technology, 211: 637-645. DOI:10.1016/j.seppur.2018.10.029 |
Guelfi D R V, Brillas E, Gozzi F, et al. 2019b. Influence of electrolysis conditions on the treatment of herbicide bentazon using artificial UVA radiation and sunlight. Identification of oxidation products[J]. Journal of Environmental Management, 231: 213-221. |
Guinea F, Peres N M R, Novoselov K S, et al. 2009. The electronic properties of graphene[J]. Reviews of Modern Physics, 81(1): 109-162. |
Hassan M, Ashraf G A, Zhang B, et al. 2020. Energy-efficient degradation of antibiotics in microbial electro-Fenton system catalysed by M-type strontium hexaferrite nanoparticles[J]. Chemical Engineering Journal, 380: 122483-122499. |
He H, Jiang B, Yuan J, et al. 2019. Cost-effective electrogeneration of H2O2 utilizing HNO3 modified graphite/polytetrafluoroethylene cathode with exterior hydrophobic film[J]. Journal of Colloid and Interface Science, 533: 471-480. |
He J, Li N, Zhang D, et al. 2020. Real-time monitoring of ciprofloxacin degradation in an electro-Fenton-like system using electrochemical-mass spectrometry[J]. Environmental Science:Water Research & Technology, 6(1): 181-188. |
Hoang N T, Holze R. 2020. Degradation of pesticide Cartap in Padan 95SP by combined advanced oxidation and electro-Fenton process[J]. Journal of Solid State Electrochemistry, 1(3): 23-34. |
Hsiao Y L, Nobe K. 1993. Hydroxylation of chlorobenzene and phenol in a packed bed flow reactor with electrogenerated Fenton's reagent[J]. Journal of Applied Electrochemistry, 23(9): 943-946. |
Huang A, Zhi D, Tang H, et al. 2020. Effect of Fe2+, Mn2+ catalysts on the performance of electro-Fenton degradation of antibiotic ciprofloxacin, and expanding the utilizing of acid mine drainage[J]. Science of the Total Environment, 720: 137560-137579. |
Huang B, Qi C, Yang Z, et al. 2017. Pd/Fe3O4 nanocatalysts for highly effective and simultaneous removal of humic acids and Cr(Ⅵ) by electro-Fenton with H2O2 in situ electro-generated on the catalyst surface[J]. Journal of Catalysis, 352: 337-350. |
韩佳佳, 王淼, 田苗, 等. 2019. 生物质基碳材料的制备及其在电芬顿降解体系中的应用[J]. 功能材料, 50(2): 2094-2100. |
Huong Le T X, Bechelany M, Cretin M. 2017. Carbon felt based-electrodes for energy and environmental applications:A review[J]. Carbon, 122: 564-591. |
Iglesias O, Meijide J, Bocos E, et al. 2015. New approaches on heterogeneous electro-Fenton treatment of winery wastewater[J]. Electrochimica Acta, 169: 134-141. |
Jiang K, Back S, Akey A J, et al. 2019. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination[J]. Nature Communications, 10(1): 3997-4015. |
Jiang W, Ding Y, Haider M R, et al. 2020. A novel TiO2/graphite felt photoanode assisted electro-Fenton catalytic membrane process for sequential degradation of antibiotic florfenicol and elimination of its antibacterial activity[J]. Chemical Engineering Journal, 391: 123503-123520. |
Jiang Y, Ni P, Chen C, et al. 2018. Selective electrochemical H2O2 production through two-electron oxygen electrochemistry[J]. Advanced Energy Materials, 8(31): 1801909-1801920. |
Jiao Y, Ma L, Tian Y, et al. 2020. A flow-through electro-Fenton process using modified activated carbon fiber cathode for orange Ⅱ removal[J]. Chemosphere, 252: 126483-126495. |
Jung E, Shin H, Lee B, et al. 2020. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production[J]. Nature Materials, 19: 436-442. |
Kamaraj R, Vasudevan S. 2019. Sulfur-doped carbon chain network as high-performance electrocatalyst for electro-Fenton system[J]. ChemistrySelect, 4(8): 2428-2435. |
Khataee A R, Safarpour M, Zarei M, et al. 2011. Electrochemical generation of H2O2 using immobilized carbon nanotubes on graphite electrode fed with air:Investigation of operational parameters[J]. Journal of Electroanalytical Chemistry, 659(1): 63-68. |
Khataee A, Sajjadi S, Hasanzadeh A, et al. 2017a. One-step preparation of nanostructured martite catalyst and graphite electrode by glow discharge plasma for heterogeneous electro-Fenton like process[J]. Journal of Environmental Management, 199: 31-45. |
Khataee A, Sajjadi S, Rahim Pouran S, et al. 2017b. Efficient electrochemical generation of hydrogen peroxide by means of plasma-treated graphite electrode and activation in electro-Fenton[J]. Journal of Industrial and Engineering Chemistry, 56: 312-320. |
Ko Y, Kim H, Seid M G, et al. 2018. Ionic-liquid-derived nitrogen-doped carbon electrocatalyst for peroxide generation and divalent iron regeneration:Its application for removal of aqueous organic compounds[J]. ACS Sustainable Chemistry & Engineering, 6(11): 14857-14865. |
Kornienko V L, Kolyagin G A. 2003. Indirect oxidation of organic substances by intermediates of the oxygen reduction[J]. Russian Journal of Electrochemistry, 39(12): 1308-1316. DOI:10.1023/B:RUEL.0000009097.40909.a4 |
Kourdali S, Badis A, Boucherit A, et al. 2018. Electrochemical disinfection of bacterial contamination:Effectiveness and modeling study of E. coli inactivation by electro-Fenton, electro-peroxi-coagulation and electrocoagulation[J]. Journal of Environmental Management, 226: 106-119. |
Kuang C, Xu Y, Lai W, et al. 2019. Novel electrodes for cathode electro-Fenton oxidation coupled with anodic oxidation system for advanced treatment of livestock wastewater[J]. Electrochimica Acta, 321: 134605-134623. |
Kusvuran E, Gulnaz O, Irmak S, et al. 2004. Comparison of several advanced oxidation processes for the decolorization of Reactive Red 120 azo dye in aqueous solution)[J]. Journal of Hazardous Materials, 109(1): 85-93. |
Labiadh L, Ammar S, Kamali A R. 2019. Oxidation/mineralization of AO7 by electro-Fenton process using chalcopyrite as the heterogeneous source of iron and copper catalysts with enhanced degradation activity and reusability[J]. Journal of Electroanalytical Chemistry, 853: 113532-113548. |
Lai W, Xie G, Dai R, et al. 2020. Kinetics and mechanisms of oxytetracycline degradation in an electro-Fenton system with a modified graphite felt cathode[J]. Journal of Environmental Management, 257: 109968-109989. DOI:10.1016/j.jenvman.2019.109968 |
Lang Z, Zhou M, Zhang Q, et al. 2020a. Comprehensive treatment of marine aquaculture wastewater by a cost-effective flow-through electro-oxidation process[J]. Science of the Total Environment, 722(3): 137812-137834. |
Lang Z, Zhou M, Zhang Q, et al. 2020b. Comprehensive treatment of marine aquaculture wastewater by a cost-effective flow-through electro-oxidation process[J]. Science of the Total Environment, 722(3): 137812-137834. |
Li K, Liu J, Li J, et al. 2018. Effects of N mono- and N/P dual-doping on H2O2, OH generation, and MB electrochemical degradation efficiency of activated carbon fiber electrodes[J]. Chemosphere, 193: 800-810. |
罗万江, 兰新哲, 宋永辉. 2009. 煤的电化学脱硫技术研究及进展[J]. 选煤技术, (3): 64-67. |
Li Z, Shen C, Liu Y, et al. 2020a. Carbon nanotube filter functionalized with iron oxychloride for flow-through electro-Fenton[J]. Applied Catalysis B:Environmental, 260: 118204-118423. |
Li S, Hua T, Yuan C, et al. 2020b. Degradation pathways, microbial community and electricity properties analysis of antibiotic sulfamethoxazole by bio-electro-Fenton system[J]. Bioresource Technology, 298: 122501-122518. |
Liang J, Zhang Y, Song C, et al. 2018. Double-potential electro-Fenton:A novel strategy coupling oxygen reduction reaction and Fe2+/Fe3+ recycling[J]. Electrochemistry Communications, 94: 55-58. |
Lin H, Wu J, Zhang H. 2014. Degradation of clofibric acid in aqueous solution by an EC/Fe3+/PMS process[J]. Chemical Engineering Journal, 244: 514-521. |
Liu F, Liu Y, Yao Q, et al. 2020a. Supported atomically-precise gold nanoclusters for enhanced flow-through electro-Fenton[J]. Environmental Science & Technology, 54(9): 5913-5921. |
Liu F, Oturan N, Zhang H, et al. 2020b. Soil washing in combination with electrochemical advanced oxidation for the remediation of synthetic soil heavily contaminated with diesel[J]. Chemosphere, 249: 126176-126186. |
Liu H Y, Hou Z F, Hu C H, et al. 2012. Electronic and magnetic properties of fluorinated graphene with different coverage of fluorine[J]. The Journal of Physical Chemistry C, 116(34): 18193-18201. |
Liu K, Yu M, Wang H, et al. 2019. Multiphase porous electrochemical catalysts derived from iron-based metal-organic framework compounds[J]. Environmental Science & Technology, 53(11): 6474-6482. |
Liu S, Wang P, Zhao X. 2010. The degradation of organics by electro-fenton system using graphite and graphene electrode as cathode[J]. 1st Conference on Environmental Pollution and Public Health Location, 1(3): 79-80. |
Liu T, Wang K, Song S, et al. 2016. New electro-Fenton gas diffusion cathode based on nitrogen-doped graphene@carbon nanotube composite materials[J]. Electrochimica Acta, 194: 228-238. |
Liu W, Ai Z, Zhang L. 2012. Design of a neutral three-dimensional electro-Fenton system with foam nickel as particle electrodes for wastewater treatment[J]. Journal of Hazardous Materials, 243: 257-264. |
Liu X, Yang D, Zhou Y, et al. 2017. Electrocatalytic properties of N-doped graphite felt in electro-Fenton process and degradation mechanism of levofloxacin[J]. Chemosphere, 182: 306-315. |
Liu Y, Chen S, Quan X, et al. 2015. Efficient mineralization of prfluorooctanoate by electro-Fenton with H2O2 eelectro-generated on hierarchically porous carbon[J]. Environmental Science & Technology, 49(22): 13528-13533. |
Liu Z, Gao Z, Lu X. 2020. An Integrated approach to remove PAHs from highly contaminated soil:Electro-Fenton process and bioslurry treatment[J]. Water, Air, & Soil Pollution, 231(6): 314-329. |
Lou Y, Geneste F, Soutrel I, et al. 2020. Alachlor dechlorination prior to an electro-Fenton process:Influence on the biodegradability of the treated solution[J]. Separation and Purification Technology, 232: 115936-115956. |
Lü A, Shen Y, Gong X, et al. 2016. Effects of electrolyte recycling on desulfurization from bauxite water slurry electrolysis[J]. Transactions of Nonferrous Metals Society of China, 26(6): 1714-1720. |
Luo H, Li C, Wu C, et al. 2015. Electrochemical degradation of phenol by in situ electro-generated and electro-activated hydrogen peroxide using an improved gas diffusion cathode[J]. Electrochimica Acta, 186: 486-493. |
Luo T, Feng H, Tang L, et al. 2020. Efficient degradation of tetracycline by heterogeneous electro-Fenton process using Cu-doped Fe@Fe2O3:Mechanism and degradation pathway[J]. Chemical Engineering Journal, 382: 122970-122990. DOI:10.1016/j.cej.2019.122970 |
Ma L, Zhou M, Ren G, et al. 2016. A highly energy-efficient flow-through electro-Fenton process for organic pollutants degradation[J]. Electrochimica Acta, 200: 222-230. |
Ma R, Lin G, Zhou Y, et al. 2019. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts[J]. npj Computational Materials, 5(1): 78-89. |
Márquez A A, Sirés I, Brillas E, et al. 2020. Mineralization of methyl orange azo dye by processes based on H2O2 electrogeneration at a 3D-like air-diffusion cathode[J]. Chemosphere, 259: 127466. DOI:10.1016/j.chemosphere.2020.127466 |
Matyszczak G, Fidler A, Polesiak E, et al. 2020a. Application of sonochemically synthesized SnS and SnS2 in the electro-Fenton process:Kinetics and enhanced decolorization[J]. Ultrasonics Sonochemistry, 68: 105186-105199. |
Matyszczak G, Sędkowska A, Kuś S. 2020b. Comparative degradation of metanil yellow in the electro-Fenton process with different catalysts:A simplified kinetic model study[J]. Dyes and Pigments, 174: 108076-108098. |
Mcginnis D F, Little J C. 2002. Predicting diffused-bubble oxygen transfer rate using the discrete-bubble model[J]. Water Research, 36(18): 4627-4635. |
Meinero S, Zerbinati O. 2006. Oxidative and energetic efficiency of different electrochemical oxidation processes for chloroanilines abatement in aqueous medium[J]. Chemosphere, 64(3): 386-392. |
Monteil H, Péchaud Y, Oturan N, et al. 2019. A review on efficiency and cost effectiveness of electro- and bio-electro-Fenton processes:Application to the treatment of pharmaceutical pollutants in water[J]. Chemical Engineering Journal, 376: 119577-119599. |
Moraleda I, Oturan N, Saez C, et al. 2020. A comparison between flow-through cathode and mixed tank cells for the electro-Fenton process with conductive diamond anode[J]. Chemosphere, 238: 124854-124876. |
Nazari P, Setayesh S R. 2019. Effective degradation of Reactive Red 195 via heterogeneous electro-Fenton treatment:theoretical study and optimization[J]. International Journal of Environmental Science and Technology, 16(10): 6329-6346. |
Oloman C, Watkinson A P. 1975. The electroreduction of oxygen to hydrogen peroxide on fluidized cathodes[J]. The Canadian Journal of Chemical Engineering, 53(3): 268-273. |
Olvera-Vargas H, Gore-Datar N, Garcia-Rodriguez O, et al. 2021. Electro-Fenton treatment of real pharmaceutical wastewater paired with a BDD anode:Reaction mechanisms and respective contribution of homogeneous and heterogeneous OH[J]. Chemical Engineering Journal, 404: 126524-126545. |
Oriol R, Sirés I, Brillas E, et al. 2019. A hybrid photoelectrocatalytic/photoelectro-Fenton treatment of Indigo Carmine in acidic aqueous solution using TiO2 nanotube arrays as photoanode[J]. Journal of Electroanalytical Chemistry, 847: 113088-113101. |
Oturan M A, Oturan N, Lahitte C, et al. 2001. Production of hydroxyl radicals by electrochemically assisted Fenton's reagent:Application to the mineralization of an organic micropollutant, pentachlorophenol[J]. Journal of Electroanalytical Chemistry, 507(1): 96-102. DOI:10.1016/S0022-0728(01)00369-2 |
Oturan M A, Peiroten J, Chartrin P, et al. 2000. Complete destruction of p-nitrophenol in aqueous medium by electro-Fenton method[J]. Environmental Science & Technology, 34(16): 3474-3479. |
özcan A, şahin Y, Savaş Koparal A, et al. 2008. Carbon sponge as a new cathode material for the electro-Fenton process:Comparison with carbon felt cathode and application to degradation of synthetic dye basic blue 3 in aqueous medium[J]. Journal of Electroanalytical Chemistry, 616(1): 71-78. DOI:10.1016/j.jelechem.2008.01.002 |
Pan G, Sun X, Sun Z. 2020. Fabrication of multi-walled carbon nanotubes and carbon black co-modified graphite felt cathode for amoxicillin removal by electrochemical advanced oxidation processes under mild pH condition[J]. Environmental Science and Pollution Research, 27(8): 8231-8247. DOI:10.1007/s11356-019-07358-2 |
Pan Z, Wang K, Wang Y, et al. 2018. In-situ electrosynthesis of hydrogen peroxide and wastewater treatment application:A novel strategy for graphite felt activation[J]. Applied Catalysis B:Environmental, 237: 392-400. DOI:10.1016/j.apcatb.2018.05.079 |
Park J, Nabae Y, Hayakawa T, et al. 2014. Highly selective two-electron oxygen reduction catalyzed by mesoporous nitrogen-doped carbon[J]. ACS Catalysis, 4(10): 3749-3754. |
Peng Q, Zhang Z, Huang Z, et al. 2014. N-Doped ordered mesoporous carbon grafted onto activated carbon fibre composites with enhanced activity for the electro-Fenton degradation of Brilliant Red X3B dye[J]. RSC Advances, 4(104): 60168-60175. |
Peng Q, Zhang Z, Huang Z, et al. 2014. N-Doped ordered mesoporous carbon grafted onto activated carbon fibre composites with enhanced activity for the electro-Fenton degradation of Brilliant Red X3B dye[J]. RSC Advances, 4(104): 60168-60175. |
Perazzolo V, Durante C, Gennaro A. 2016. Nitrogen and sulfur doped mesoporous carbon cathodes for water treatment[J]. Journal of Electroanalytical Chemistry, 782: 264-269. |
Pérez J F, Llanos J, Sáez C, et al. 2017. The jet aerator as oxygen supplier for the electrochemical generation of H2O2[J]. Electrochimica Acta, 246: 466-474. |
Pérez J F, Sabatino S, Galia A, et al. 2018a. Effect of air pressure on the electro-Fenton process at carbon felt electrodes[J]. Electrochimica Acta, 273: 447-453. |
Pérez J F, Llanos J, Sáez C, et al. 2018b. The pressurized jet aerator:A new aeration system for high-performance H2O2 electrolyzers[J]. Electrochemistry Communications, 89: 19-22. |
Pérez J F, Llanos J, Sáez C, et al. 2019. On the design of a jet-aerated microfluidic flow-through reactor for wastewater treatment by electro-Fenton[J]. Separation and Purification Technology, 208: 123-129. |
Qiu S, Yu L, Tang D, et al. 2018. Rapidly enhanced electro-Fenton efficiency by in situ electrochemistry-activated graphite cathode[J]. Industrial & Engineering Chemistry Research, 57(14): 4907-4915. |
Radwan M, Gar Alalm M, El-Etriby H K. 2019. Application of electro-Fenton process for treatment of water contaminated with benzene, toluene, and p-xylene (BTX) using affordable electrodes[J]. Journal of Water Process Engineering, 31: 100837-100846. |
Raschitor A, Llanos J, Rodrigo M A, et al. 2019. Combined electrochemical processes for the efficient degradation of non-polar organochlorine pesticides[J]. Journal of Environmental Management, 248: 109289-109300. |
Rekik R, Hamza M, Jaziri M, et al. 2020. Electrochemical oxidation of vanillic acid by electro-Fenton process:Toward a novel route of protocatechuic acid electrosynthesis[J]. Arabian Journal of Chemistry, 13(1): 357-365. |
Ren G, Zhou M, Su P, et al. 2019a. Simultaneous sulfadiazines degradation and disinfection from municipal secondary effluent by a flow-through electro-Fenton process with graphene-modified cathode[J]. Journal of Hazardous Materials, 368: 830-839. |
Ren G, Zhou M, Su P, et al. 2019b. Simultaneous sulfadiazines degradation and disinfection from municipal secondary effluent by a flow-through electro-Fenton process with graphene-modified cathode[J]. Journal of Hazardous Materials, 368: 830-839. |
Ren W, Tang D, Lu X, et al. 2016. Novel multilayer ACF@rGO@OMC cathode composite with enhanced activity for electro-Fenton degradation of phthalic acid esters[J]. Industrial & Engineering Chemistry Research, 55(42): 11085-11096. |
Ren Z, Chen F, Wen K, et al. 2020. Enhanced photocatalytic activity for tetracyclines degradation with Ag modified g-C3N4 composite under visible light[J]. Journal of Photochemistry and Photobiology A:Chemistry, 389: 112217-112245. |
Samy M, Ibrahim M G, Gar Alalm M, et al. 2020. Effective photocatalytic degradation of sulfamethazine by CNTs/LaVO4 in suspension and dip coating modes[J]. Separation and Purification Technology, 235: 116138-116145. DOI:10.1016/j.seppur.2019.116138 |
Santos G D O S, Eguiluz K I B, Salazar-Banda G R, et al. 2020. Testing the role of electrode materials on the electro-Fenton and photoelectro-Fenton degradation of clopyralid[J]. Journal of Electroanalytical Chemistry, 871: 114291-114312. |
Scialdone O, Galia A, Gattuso C, et al. 2015. Effect of air pressure on the electro-generation of H2O2 and the abatement of organic pollutants in water by electro-Fenton process[J]. Electrochimica Acta, 182: 775-780. |
Scialdone O, Galia A, Sabatino S. 2013. Electro-generation of H2O2 and abatement of organic pollutant in water by an electro-Fenton process in a microfluidic reactor[J]. Electrochemistry Communications, 26: 45-47. |
Sheng Y, Zhao Y, Wang X, et al. 2014. Electrogeneration of H2O2 on a composite acetylene black-PTFE cathode consisting of a sheet active core and a dampproof coating[J]. Electrochimica Acta, 133: 414-421. |
Song S, Wu M, Liu Y, et al. 2015. Efficient and stable carbon-coated nickel foam cathodes for the electro-Fenton process[J]. Electrochimica Acta, 176: 811-818. |
Souza F L, Rocha R S, Ferreira N G, et al. 2019. Effects of coupling hybrid processes on the treatment of wastewater containing a commercial mixture of diuron and hexazinone herbicides[J]. Electrochimica Acta, 328: 135013-135034. |
Sudoh M, Kodera T, Sakai K, et al. 1986. Oxidative degradation of aqueous phenol effluent with electrogenerated Fenton's reagent[J]. Journal of Chemical Engineering of Japan, 19(6): 513-518. |
Suquet C, Warren J J, Seth N, et al. 2010. Comparative study of HOCl-inflicted damage to bacterial DNA ex vivo and within cells[J]. Archives of Biochemistry and Biophysics, 493(2): 135-142. |
孙康. 2007. 氢氧化钠体系中煤的电化学脱硫研究[J]. 能源技术与管理, 1(3): 79-80. |
Tan A, Wan K, Wang Y, et al. 2018. N, S-containing MOF-derived dual-doped mesoporous carbon as a highly effective oxygen reduction reaction electrocatalyst[J]. Catalysis Science & Technology, 8(1): 335-343. |
Tang J, Wang J. 2019. MOF-derived three-dimensional flower-like FeCu@C composite as an efficient Fenton-like catalyst for sulfamethazine degradation[J]. Chemical Engineering Journal, 375: 122007-122023. |
Tang Q, Wang D, Yao D M, et al. 2015. Highly efficient electro-generation of hydrogen peroxide using NCNT/NF/CNT air diffusion electrode for electro-Fenton degradation of p-nitrophenol[J]. Water Science and Technology, 73(7): 1652-1658. |
Teymori M, Khorsandi H, Aghapour A A, et al. 2019. Electro-Fenton method for the removal of Malachite Green:Effect of operational parameters[J]. Applied Water Science, 10(1): 39-56. |
Thiam A, Salazar R, Brillas E, et al. 2020. In-situ dosage of Fe2+ catalyst using natural pyrite for thiamphenicol mineralization by photoelectro-Fenton process[J]. Journal of Environmental Management, 270: 110835-110856. |
Thiam A, Salazar R. 2019. Fenton-based electrochemical degradation of metolachlor in aqueous solution by means of BDD and Pt electrodes:influencing factors and reaction pathways[J]. Environmental Science and Pollution Research, 26(3): 2580-2591. |
Thiam A, Sirés I, Salazar R, et al. 2018. On the performance of electrocatalytic anodes for photoelectro-Fenton treatment of synthetic solutions and real water spiked with the herbicide chloramben[J]. Journal of Environmental Management, 224: 340-349. |
Thor S, Ho L, Ong S, et al. 2020. Explicating the importance of aeration and pH for Amaranth degradation and electricity generation in a viable hybrid system of photocatalytic fuel cell and electro-Fenton process[J]. Separation and Purification Technology, 239: 116535-116545. |
Tian Y, Zhou M, Pan Y, et al. 2020. Pre-magnetized Fe0 as heterogeneous electro-Fenton catalyst for the degradation of p-nitrophenol at neutral pH[J]. Chemosphere, 240: 124962-124977. |
Tomat R, Vecchi E. 1971. Electrocatalytic production of OH radicals and their oxidative addition to benzene[J]. Journal of Applied Electrochemistry, 1(3): 185-188. |
Tran M H, Nguyen H C, Le T S, et al. 2019. Degradation of glyphosate herbicide by an electro-Fenton process using carbon felt cathode[J]. Environmental Technology: 1-10. |
Tzedakis T, Savall A, Clifton M J. 1989. The electrochemical regeneration of Fenton's reagent in the hydroxylation of aromatic substrates:batch and continuous processes[J]. Journal of Applied Electrochemistry, 19(6): 911-921. |
王文波, 石生敏, 汪树军, 等. 2005. 汽油的电化学-化学氧化耦合法脱硫[J]. 石油学报(石油加工), 21(5): 41-47. |
王宇晶. 2011.微波原位增强电芬顿氧化降解偶氮染料废水的研究[C].第六届全国环境化学大会暨环境科学仪器与分析仪器展览会.中国上海: 1
|
吴海珍, 韦聪, 于哲, 等. 2018. 废水好氧生物处理工艺中氧的传质与强化的理论与实践[J]. 化工进展, 37(10): 4033-4043. |
Wang A, Qu J, Ru J, et al. 2005. Mineralization of an azo dye Acid Red 14 by electro-Fenton's reagent using an activated carbon fiber cathode[J]. Dyes and Pigments, 65(3): 227-233. |
Wang Q, Cai Z, Huang L, et al. 2019. Intensified degradation and mineralization of antibiotic metronidazole in photo-assisted microbial fuel cells with Mo-W catalytic cathodes under anaerobic or aerobic conditions in the presence of Fe(Ⅲ)[J]. Chemical Engineering Journal, 376: 119566-119578. |
Wang Y, Liu Y, Li X, et al. 2013. A highly-ordered porous carbon material based cathode for energy-efficient electro-Fenton process[J]. Separation and Purification Technology, 106: 32-37. |
Wang Y, Liu Y, Wang K, et al. 2015. Preparation and characterization of a novel KOH activated graphite felt cathode for the electro-Fenton process[J]. Applied Catalysis B:Environmental, 165: 360-368. |
Wang Y, Zhao H, Gao J, et al. 2012. Rapid mineralization of azo-dye wastewater by microwave synergistic electro-Fenton oxidation process[J]. The Journal of Physical Chemistry C, 116(13): 7457-7463. |
Xia C, Back S, Ringe S, et al. 2020. Confined local oxygen gas promotes electrochemical water oxidation to hydrogen peroxide[J]. Nature Catalysis, 3: 125-134. |
Xia G, Lu Y, Gao X, et al. 2015. Electro-Fenton degradation of methylene blue using polyacrylonitrile-based carbon fiberbBrush cathode[J]. CLEAN-Soil, Air, Water, 43(2): 229-236. |
Xia G, Lu Y, Xu H. 2015a. An energy-saving production of hydrogen peroxide via oxygen reduction for electro-Fenton using electrochemically modified polyacrylonitrile-based carbon fiber brush cathode[J]. Separation and Purification Technology, 156: 553-560. |
Xia G, Lu Y, Xu H. 2015b. Electrogeneration of hydrogen peroxide for electro-Fenton via oxygen reduction using polyacrylonitrile-based carbon fiber brush cathode[J]. Electrochimica Acta, 158: 390-396. |
Xia C, Xia Y, Zhu P, et al. 2019a. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte[J]. Science, 366(6462): 226-256. DOI:10.1126/science.aay1844 |
Xia Y, Shang H, Zhang Q, et al. 2019b. Electrogeneration of hydrogen peroxide using phosphorus-doped carbon nanotubes gas diffusion electrodes and its application in electro-Fenton[J]. Journal of Electroanalytical Chemistry, 840: 400-408. |
Xue Y, Zhang Y, Zhang Y, et al. 2017a. Electrochemical detoxification and recovery of spent SCR catalyst by in-situ generated reactive oxygen species in alkaline media[J]. Chemical Engineering Journal, 325: 544-553. |
Xue Y, Wang Y, Zheng S, et al. 2017b. Efficient oxidative dissolution of V2O3 by the in situ electro-generated reactive oxygen species on N-doped carbon felt electrodes[J]. Electrochimica Acta, 226: 140-147. |
肖柏林. 2019.改性及电Fenton技术修复石油烃污染土壤研究[D].重庆: 重庆大学. 1-7
|
徐文迪, 郭书海, 李刚, 等. 2019. 电芬顿-生物泥浆法联合修复芘污染土壤[J]. 中国环境科学, 39(10): 4247-4253. |
徐艳辉. 2015. 电极过程动力学:基础, 技术与应用[M]. 北京市: 化学工业出版社, 24.
|
姚鹏城, 陈嘉瑜, 张永明, 等. 2019. 废水处理系统中抗生素抗性基因分布特征[J]. 环境科学, 40(11): 5024-5031. |
Yang W, Zhou M, Liang L. 2018. Highly efficient in-situ metal-free electrochemical advanced oxidation process using graphite felt modified with N-doped graphene[J]. Chemical Engineering Journal, 338: 700-708. |
Yang F, Ma X, Cai W, et al. 2019a. Nature of oxygen-containing groups on carbon for high-efficiency electrocatalytic CO2 reduction reaction[J]. Journal of the American Chemical Society, 141(51): 20451-20459. |
Yang W, Zhou M, Oturan N, et al. 2019b. Enhanced activation of hydrogen peroxide using nitrogen doped graphene for effective removal of herbicide 2, 4-D from water by iron-free electrochemical advanced oxidation[J]. Electrochimica Acta, 297: 582-592. |
Yang W, Zhou M, Oturan N, et al. 2020a. Highly efficient and stable FeⅡFeⅢ LDH carbon felt cathode for removal of pharmaceutical ofloxacin at neutral pH[J]. Journal of Hazardous Materials, 393: 122513-122536. |
Yang Y, Liu Y, Fang X, et al. 2020b. Heterogeneous Electro-Fenton catalysis with HKUST-1-derived Cu@C decorated in 3D graphene network[J]. Chemosphere, 243: 125423-125443. |
Ye Z, Guelfi D R V, álvarez G, et al. 2019. Enhanced electrocatalytic production of H2O2 at Co-based air-diffusion cathodes for the photoelectro-Fenton treatment of bronopol[J]. Applied Catalysis B:Environmental, 247: 191-199. |
Yu D, Nagelli E, Du F, et al. 2010. Metal-free carbon nanomaterials become more active than metal catalysts and last longer[J]. The Journal of Physical Chemistry Letters, 1(14): 2165-2173. |
Yu F, Wang Y, Ma H, et al. 2020. Hydrothermal synthesis of FeS2 as a highly efficient heterogeneous electro-Fenton catalyst to degrade diclofenac via molecular oxygen effects for Fe(Ⅱ)/Fe(Ⅲ) cycle[J]. Separation and Purification Technology, 248: 117022-117043. |
Yu F, Zhou M, Zhou L, et al. 2014. A novel electro-Fenton process with H2O2 generation in a rotating disk reactor for organic pollutant degradation[J]. Environmental Science & Technology Letters, 1(7): 320-324. |
Yu J, Liu T, Liu H, et al. 2016. Electro-polymerization fabrication of PANI@GF electrode and its energy-effective electrocatalytic performance in electro-Fenton process[J]. Chinese Journal of Catalysis, 37(12): 2079-2085. |
Yu X, Zhou M, Ren G, et al. 2015. A novel dual gas diffusion electrodes system for efficient hydrogen peroxide generation used in electro-Fenton[J]. Chemical Engineering Journal, 263: 92-100. |
Zahrani A A, Ayati B. 2020. Improving Fe-based heterogeneous Electro-Fenton nano catalyst using transition metals in a novel orbiting electrodes reactor[J]. Chemosphere, 256: 127049. DOI:10.1016/j.chemosphere.2020.127049 |
Zarei M, Beheshti Nahand F, Khataee A, et al. 2019. Removal of nalidixic acid from aqueous solutions using a cathode containing three-dimensional graphene[J]. Journal of Water Process Engineering, 32: 100978-100999. DOI:10.1016/j.jwpe.2019.100978 |
Zhang D, Liu T, Yin K, et al. 2020b. Selective H2O2 production on N-doped porous carbon from direct carbonization of metal organic frameworks for electro-Fenton mineralization of antibiotics[J]. Chemical Engineering Journal, 383: 123184-123199. DOI:10.1016/j.cej.2019.123184 |
Zhang G, Zhao S, Yang F, et al. 2009. Electrocatalytic reduction of oxygen at anthraquinonedisulfonate/polypyrrole composite film modified electrodes and its application to the electrochemical oxidation of azo dye[J]. Electroanalysis, 21(22): 2420-2426. |
Zhang Z, Meng H, Wang Y, et al. 2018a. Fabrication of graphene@graphite-based gas diffusion electrode for improving H2O2 generation in Electro-Fenton process[J]. Electrochimica Acta, 260: 112-120. |
Zhang J, Zhou W, Yang L, et al. 2018b. Co-N-doped MoO2 modified carbon felt cathode for removal of EDTA-Ni in electro-Fenton process[J]. Environmental Science and Pollution Research, 25(23): 22754-22765. |
Zhang S, Pang X, Yue Z, et al. 2020a. Sulfonamides removed from simulated livestock and poultry breeding wastewater using an in-situ electro-Fenton process powered by photovoltaic energy[J]. Chemical Engineering Journal, 397: 125466-125488. DOI:10.1016/j.cej.2020.125466 |
Zhao K, Quan X, Chen S, et al. 2018a. Enhanced electro-Fenton performance by fluorine-doped porous carbon for removal of organic pollutants in wastewater[J]. Chemical Engineering Journal, 354: 606-615. |
Zhao K, Su Y, Quan X, et al. 2018b. Enhanced H2O2 production by selective electrochemical reduction of O2 on fluorine-doped hierarchically porous carbon[J]. Journal of Catalysis, 357: 118-126. DOI:10.1016/j.jcat.2017.11.008 |
Zheng Y, Qiu S, Deng F, et al. 2019. Three-dimensional electro-Fenton system with iron foam as particle electrode for folic acid wastewater pretreatment[J]. Separation and Purification Technology, 224: 463-474. DOI:10.1016/j.seppur.2019.05.054 |
Zhou L, Hu Z, Zhang C, et al. 2013. Electrogeneration of hydrogen peroxide for electro-Fenton system by oxygen reduction using chemically modified graphite felt cathode[J]. Separation and Purification Technology, 111: 131-136. DOI:10.1016/j.seppur.2013.03.038 |
Zhou L, Zhou M, Hu Z, et al. 2014. Chemically modified graphite felt as an efficient cathode in electro-Fenton for p-nitrophenol degradation[J]. Electrochimica Acta, 140: 376-383. DOI:10.1016/j.electacta.2014.04.090 |
Zhou L, Zhou M, Zhang C, et al. 2013. Electro-Fenton degradation of p-nitrophenol using the anodized graphite felts[J]. Chemical Engineering Journal, 233: 185-192. DOI:10.1016/j.cej.2013.08.044 |
Zhou W, Meng X, Rajic L, et al. 2018a. "Floating" cathode for efficient H2O2 electrogeneration applied to degradation of ibuprofen as a model pollutant[J]. Electrochemistry Communications, 96: 37-41. DOI:10.1016/j.elecom.2018.09.007 |
Zhou W, Gao J, Ding Y, et al. 2018b. Drastic enhancement of H2O2 electro-generation by pulsed current for ibuprofen degradation:Strategy based on decoupling study on H2O2 decomposition pathways[J]. Chemical Engineering Journal, 338: 709-718. DOI:10.1016/j.cej.2017.12.152 |
Zhou W, Meng X, Gao J, et al. 2019a. Hydrogen peroxide generation from O2 electroreduction for environmental remediation:A state-of-the-art review[J]. Chemosphere, 225: 588-607. DOI:10.1016/j.chemosphere.2019.03.042 |
Zhou W, Meng X, Gao J, et al. 2019b. Hydrogen peroxide generation from O2 electroreduction for environmental remediation:A state-of-the-art review[J]. Chemosphere, 225: 588-607. DOI:10.1016/j.chemosphere.2019.03.042 |
Zhou W, Rajic L, Meng X, et al. 2019c. Efficient H2O2 electrogeneration at graphite felt modified via electrode polarity reversal:Utilization for organic pollutants degradation[J]. Chemical Engineering Journal, 364: 428-439. DOI:10.1016/j.cej.2019.01.175 |
Zhou Z, Chen A, Fan X, et al. 2019d. Hierarchical porous N-P-coupled carbons as metal-free bifunctional electro-catalysts for oxygen conversion[J]. Applied Surface Science, 464: 380-387. DOI:10.1016/j.apsusc.2018.09.095 |
Zhu Y, Qiu S, Ma F, et al. 2018. Melamine-derived carbon electrode for efficient H2O2 electro-generation[J]. Electrochimica Acta, 261: 375-383. DOI:10.1016/j.electacta.2017.12.122 |
赵苹苹, 胡锴, 蔡苹, 等. 2019. 氧还原反应前沿与进展[J]. 大学化学, 34(5): 33-41. |
赵旭, 冒冉. 2020. 电解法用于消毒的原理、技术特点与主要应用方式:电产次氯酸钠及电化学消毒[J]. 环境工程学报, (7): 1728-1734. |
周蕾, 周明华. 2013. 电芬顿技术的研究进展[J]. 水处理技术, 39(10): 6-11. |
朱红, 杨玉芬, 赵炜, 等. 2003. 电解还原法强化高硫煤浮选脱硫机理研究[J]. 中国矿业大学学报, 32(6): 650-654. |
 2020, Vol. 40
2020, Vol. 40


