2. 江西省兽药饲料监察所, 南昌 330096;
3. 南昌大学, 分析测试中心, 南昌 330047;
4. 江西省烟草科学研究所, 南昌 330000;
5. 广东省科学院, 广州 510070
2. Jiangxi Institute of Veterinary and Feed Control, Nanchang 330096;
3. Analysis and testing center, Nanchang University, Nanchang 330047;
4. Tobacco Science Institute of Jiangxi Province, Nanchang 330000;
5. Guangdong Academy of Sciences, Guangzhou 510070
随着社会经济的快速发展, 对能源的消耗急剧增加, 伴随而来的是日益突出的全球环境污染.有毒有害的污染物曾被肆意排放到自然环境中, 给全球生态和人类健康带来了严重的威胁.因此, 人类的未来的发展就是向利用绿色能源要动力, 太阳光能是一种最绿色环保的清洁能源, 且具有成本低廉、储备量无限及可再生等优点.其中, 光催化降解技术能直接利用太阳光能将污染物降解为无毒无害的物质, 具有广泛应用于解决水体、大气中污染物处理的潜力而赢得了广泛的研究.
目前研究已经研制合成了大批具有光催化活性的光催化剂, 如以TiO2为代表的过渡金属氧化物、SrTiO3为代表的钙钛矿、CuAl2O4为代表的尖晶石及LaVO4代表的白钨矿等化合物具有较高的光催化活性(Asahi et al., 2001; Zou et al., 2001; Maeda et al., 2006; Wang et al., 2009; Wang et al., 2009; Yi, et al., 2010; Chen et al., 2011; Cao et al., 2011).其中, 以Bi2O3(Zhou et al., 2010)、BiVO4(Lei et al., 2014)、Bi2WO6 (Li et al., 2007)及BiFeO3(Gao et al., 2010)等为典型代表的Bi为基底的半导体光催化剂具有可见光相应性能、较窄的带隙能而被广泛应用于光催化降解有机污染物.BiVO4以具有2.3~2.5 eV较低的间带能、可见光相应活性高、高效地光电子传导性、良好的刚弹性、颜色形变属性等独特而受到最广泛的研究(David et al., 1983; HIROTA et al., 1992; Kudo et al., 1998; Zhang et al., 2006; Zhao et al., 2010).
然而, BiVO4较窄的禁带宽度导致光生电子和光生空穴极容易复合而光催化效率较低.因此, 探索一种有效的技术途径来抑制光生电子和光生空穴的复合提高其光催化性能尤其重要.Pt/Au/Ag等贵金属已经被证明是解决问题的一种非常有效理想的途径(Sayama et al., 2006).在可见光条件下, 金属W掺杂的BiVO4大幅提高了双氧水的光电化学(PEC)氧化速率(Ye et al., 2015);多金属掺杂(M为Ag, Co, Ni)明显提高了BiVO4对有机污染物的光催化降解活性(Zhou et al., 2011).Au修饰后形成的BiVO4异质纳米复合结构微管和微片对有机染料的光降解活性和水的氧化能力大大提高(Cao et al., 2012).Pt纳米粒子负载BiVO4光催化剂之后, 对甲基橙的光降解效率大幅度提高(Ge, 2008).Sara等报道了Y-BiVO4复合光催化剂对罗丹明(MB)的催化效率大大提高了(Usai et al., 2013).
但贵金属的含量极低导致大规模的实际应用成本昂贵.因此, 迫切地要求我们探索一种更加低廉有效的途径来替代贵金属修饰.将两种或多种半导体氧化物材料耦合并构筑复合界面形成一个可变能带宽度的p-n异质结构, 该结构能够有效抑制光生电子和光生空穴的复合而大大提高光催化效率(Yan et al., 2009; Zhang et al., 2011; Kim et al., 2011).如BiVO4/CuCr2O4(Bajaj et al., 2013), BiVO4/Bi2O3(Guan et al., 2011)和Bi2S3/BiVO4(Ma et al., 2012)等复合催化剂都已经被证明能够很好地使e-与h+复合, 因而促使复合材料的光催化性能得到很大提高.
因此, 本文合成了一种纯金红石型Ti0.7W0.3O2, 通过表征显示其具有与贵金属类似的良好导电性和耐腐蚀性, 可与BiVO4形成p-n复合异质结构.基于以上考虑, 以斜立方相BiVO4为载体构筑了Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂.探究了其在可见光照射下催化降解苯酚的性能;同时, 提出了苯酚在Ti0.7W0.3O2/BiVO4纳米复合界面上可能的降解机理.
2 材料和方法(Materials and methods) 2.1 仪器与试剂Waters ZQ 4000/2695型高效液相色谱-质谱联用仪;Bruker D8 Advance型X射线衍射仪日本JSM-6700F型冷场发射电子扫描显微镜(SEM);Perkin Eimer紫外可见分光光度计;DHG-9000型恒温干燥箱;西安比郎光化学反应仪;马弗炉;钼丝碳管炉;SK64-12型开启式管式炉;78-2双向磁力加热搅拌器;超声波清洗器;赫西离心机;HY-3多功能振荡器:Milli-Q超纯水纯化系统;METTLER TOLEDO ME104分析天平.
四氯化钛、六氯化钨、矾酸铋、偏钒酸铵、苯酚、聚乙二醇4000及无水乙醇为AR级,; 甲醇、乙腈为HPLC级;0.22 μm PTFE膜针头过滤器;实验用水为Milli-Q超纯水.
2.2 样品的制备 2.2.1 金红石型Ti0.7W0.3O2的制备在冰水浴强磁力搅拌条件下, 缓慢地将1 mL TiCl4滴加到5 mL蒸馏水中, 得到澄清的溶液;称量1.55 g WCl6溶解到10 mL无水乙醇, 然后将两种溶液在磁力搅拌下充分混合, 在40 ℃恒温水浴锅中机械搅拌大约24 h得到白色凝胶体, 100 ℃的烘箱中烘干并研磨成粉末;将上述的粉末置于具有适量无水乙醇溶液的高压反应釜中180 ℃/8.5 h反应后得到的淡蓝色沉淀, 置于100 ℃的烘干得到淡蓝色粉末.最后, 将淡蓝色粉末在1300 ℃ PVA真空低压煅烧4 h, 即得到纯金红石相Ti0.7W0.3O2.
2.2.2 BiVO4纳米颗粒的制备在磁力搅拌下, 将2 mmol Bi(NO3)3 · 5H2O、2 mmol NH4VO3和4.0 g的聚乙二醇4000充分溶解于320 mL蒸馏水中, 保持20 ℃/3 h, 得到橘黄色胶体溶液;然后转入高压反应釜:120 ℃/24 h.待反应完成后, 抽滤、洗涤得滤饼, 烘干.最后, 在N2为保护气, 将粉末在管式炉中400 ℃煅烧2 h.
2.2.3 Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂在磁力搅拌下, 将2 mmol Bi(NO3)3 · 5H2O、2 mmol NH4VO3和4.0 g的聚乙二醇4000充分溶解于320 mL蒸馏水中, 保持20 ℃/3 h, 得到橘黄色胶体溶液;然后将一定量的Ti0.7W0.3O2纳米粒子和黄色胶体一并转入高压反应釜中:120 ℃/24 h.待反应完成后, 抽滤、洗涤得滤饼, 烘干.在N2为保护气, 将粉末在管式炉中400 ℃煅烧2 h, 即构筑得到1%、3%、5%、7%、10%、20% Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂, 待用.
同时, 不停负载比例的Pt/BiVO4光催化剂制备方法如下:0.5 g BiVO4光粉末加入到20 mL蒸馏水搅拌超声分散10 min.加入2 mL异丙醇和一定量的H2PtCl6溶液, 搅拌30 min达到吸附脱附平衡.混合溶液转入石英管中, 在强磁力搅拌高压汞灯照射下光还原反应4 h.在强光照射下, 光生空穴(h+)氧化成无机物, 同时产生的光生电子(e-)会将+4价态的铂离子(Pt4+)还原成金属单质态(Pt0).反应混合溶液的颜色由淡黄色变成浅灰色, 也说明了Pt价态的变化.最终制备了Pt掺杂含量为0.1%、0.3%、0.4%、0.5%、0.75%、1.0%和1.5%Pt/BiVO4光催化剂.
2.3 样品的表征采用Bruker D8 Advance型X射线衍射仪对合成样品进行结构、晶型表征.Cu Kα靶(λ=0.154056 nm), 管流40 mA, 管压45 kV, 步长0.04°, 2θ的扫描速度为2.4 ° · min-1, 范围为10°~80°.采用Malvern Zetasizer Nano-ZS90 and Omecls-601A型电位粒径分析仪测试样品的平均粒径.采用日本JSM-6700F型冷场发射电子扫描显微镜(SEM)(配有EDS (energy dispersive spectrometer))观察样品的表面形貌.采用Escalab 250 Xi(单色Al目标)型X射线光电子能谱(XPS)仪分析样品表面的元素和存在状态.采用Cary 100 UV-vis-NIR型分光光度计测量样品在200~800 nm范围的紫外-可见漫反射光谱(DRS).采用Hitachi F-4500型荧光分光光度计分析样品荧光光谱(FL), 羟基对苯二甲酸的荧光强度在425 nm, 激发波长为(315±11) nm, 扫描波长范围为400~600 nm.采用美国珀金埃尔默Lambda 35型紫外-可见分光光谱仪用于测定不同时间段降解液中苯酚的浓度和光谱图.采用Rosemount-Dohrmann DC-190型总有机碳分析仪测定不同时间段苯酚降解液中的总有机碳含量.
2.3 光催化性能测试利用光反应仪(150 W超高压氙灯)对进行光催化降解苯酚实验研究样品的光催化性能.苯酚的起始浓度是95 ppm, pH值为4.5, 0.45 g催化剂加入装有1000 mL苯酚溶液的石英管中, 在黑暗环境中, 磁力搅拌30 min, 充分混合使催化剂和苯酚达到吸附脱附平衡.随后取5 mL悬浮液, 离心取上清液, 作为苯酚的起始浓度C0(0 min); 开启光反应仪, 分别在光照30、60、90、120、150、180、210、240、270、300、330及360 min时刻取反应溶液, 离心得上清液, 用于紫外检测.
Pt/BiVO4复合光催化剂降解苯酚作为对照组实验, 条件与上述条件完全一致.
2.4 降解中间产物分析Pt/BiVO4和Ti0.7W0.3O2/BiVO4p-n复合异质结构界面光催化剂降解苯酚的中间产物通过超高效液相色谱-质谱联用仪(UHPLC-MS)检测.色谱条件:流动相为水/乙腈=65/35, 使用前超声脱气;流速为0.6 mL · min-1;检测波长为190~400 nm;进样量:10 μL;C18色谱柱(250 mm×4.6 mm, Diamonsil), 柱温:35 ℃.质谱条件:离子源温度为110 ℃;脱溶剂气温度为350 ℃;毛细管电压:3.20 kV, 锥孔电压:30 V;保护气:N2.ESI+/ESI-同时监测.
3 结果与讨论(Results and discussion) 3.1 样品的XRD分析图 1为纯金红石相Ti0.7W0.3O2、叶形BiVO4纳米颗粒及不同比例Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂的粉末X射线衍射图(PXRD).由图可知Ti0.7W0.3O2样品为纯金红石型(JCPDS No.21-1272).合成的叶子型BiVO4纳米颗粒为纯单斜晶相(JCPDS NO.14-0688).根据Ti0.7W0.3O2的(110)晶面(2θ=27.1°)和BiVO4的(130)晶面(2θ=30.5°)估算出平均粒径大小分别约为1009.23 nm和22.53 nm (Bodoardo et al., 1997).各复合异质结构的所有衍射峰均能与金红石型Ti0.7W0.3O2和单斜晶型BiVO4的特征晶面吻合.在经过400 ℃煅烧后, Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂都保持具有最高催化活性的晶型.另外, 可能是负载的Ti0.7W0.3O2的量比较低及BiVO4均匀地分散在Ti0.7W0.3O2纳米颗粒表面, 直到3wt%添加Ti0.7W0.3O2的吸收峰才比较明显.
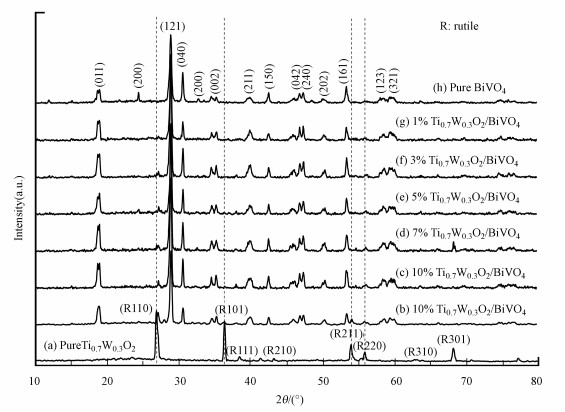 |
| 图 1 样品的XRD衍射图 Fig. 1 PXRD patterns of samples |
纯单斜晶型BiVO4、金红石相Ti0.7W0.3O2纳米颗粒的粒径分析结果见图 2.Ti0.7W0.3O2纳米颗粒的平均粒径约为1189.21 nm, 这与德拜-谢乐(Debye-Scherrer)公式进行估算的结果比较接近.但BiVO4纳米颗粒的粒径大小约为33.87 nm, 这与德拜-谢乐(Debye-Scherrer)公式估算的结果偏大, 可能的原因有两个方面:第一, 在高温条件下, BiVO4纳米颗粒发生了团聚现象.第二, 两种方法测定的原理不同, 粒径分析是先将样品分散到水中, BiVO4纳米颗粒在水中的分散不是很好, 会发生部分团聚现象, 最终导致粒径偏大.
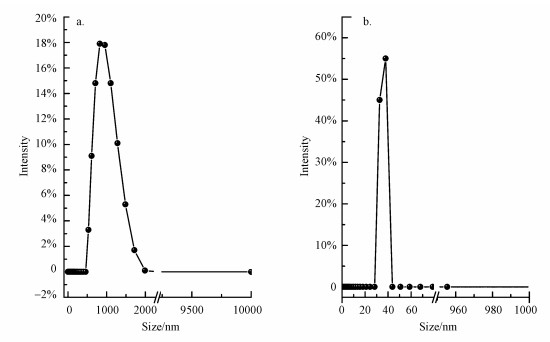 |
| 图 2 样品的粒径分布图(a.纯金红石相Ti0.7W0.3O2纳米颗粒, b.纯叶子形BiVO4纳米颗粒) Fig. 2 Diameter distribution histograms of the as-fabricated samples (a.Ti0.7W0.3O2 NPs, b.BiVO4 NPs) |
纯单斜晶型BiVO4、金红石相Ti0.7W0.3O2纳米颗粒和5%Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂的扫描电镜(SEM)如图 3所示.图 3(a)和(a-1)分别为BiVO4纳米颗粒的整体和局部放大形貌图, BiVO4纳米颗粒呈现大小均匀的叶子形状.图 3(b)和(b-1)为金红石相Ti0.7W0.3O2纳米颗粒的总体和局部放大形貌图, Ti0.7W0.3O2纳米粒子呈现质地致密无规则的块状.图 3(c)和(c-1)为5%Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面的总体和局部放大形貌图, BiVO4纳米粒子均匀地分布在Ti0.7W0.3O2纳米颗粒的表面, 且Ti0.7W0.3O2与BiVO4结合紧密构筑了复合异质结构界面, 保证其具有优越的光催化性能.
 |
| 图 3 叶子形BiVO4纳米颗粒(a, a-1), 金红石Ti0.7W0.3O2纳米颗粒(b, b-1)和5 % Ti0.7W0.3O2/BiVO4p-n复合异质结构界面光催化剂(c, c-1)的SEM图 Fig. 3 SEM images of the pure rutile Ti0.7W0.3O2 nanoparticles (a, a-1), pure BiVO4 nano-leaves (b, b-1) and 5 % Ti0.7W0.3O2/BiVO4, (c, c-1), respective |
为探究纯金红石型Ti0.7W0.3O2的结构特征和成分组成, XPS表征结果见图 4.图 4a为XPS的全扫描光谱, 纯金红石Ti0.7W0.3O2是Ti、W和O 3种元素组成.同时, 根据Ti和W的XPS测定峰面积和原子敏感性因子计算出其相对浓度之比约为2.14, 这与理论摩尔比值2.33接近.纯金红石Ti0.7W0.3O2中O 1s, W 4f和Ti 2p的XPS高分辨扫描光谱如图 4b~4c所示.由图可知, O 1s峰位于532.0 eV为Ti0.7W0.3O2中氧的结合能, Ti 2p1/2和Ti 2p3/2峰位于464.6和458.8 eV为Ti0.7W0.3O2中Ti4+的结合能, W 4f5/2和W 4f7/2峰位于34.2和33.1 eV为Ti0.7W0.3O2中W4+的结合能, 这证明Ti0.7W0.3O2中W6+完全被还原成为了W4+.
 |
| 图 4 纯金红石型Ti0.7W0.3O2的XPS全扫描光谱(a), O 1s(b), W 4f(c)和Ti 2p(d)窄扫描光谱图 Fig. 4 The XPS survey scan spectra of rutile Ti0.7W0.3O2(a), the XPS narrow scans of O 1s(b), W 4f (c) and Ti 2p(d) of the rutile Ti0.7W0.3O2 |
同时, 5% Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂的XPS表征结果见图 5.XPS全扫描光谱如图 5a可知, 合成的Ti0.7W0.3O2/BiVO4由Ti、W、Bi和O 4种元素组成.同时, Ti和W元素的吸收峰强度相对其他元素较弱, 这与较低的负载比例有关.图 5b~5c为金红石型Ti0.7W0.3O2中W 4f、O 1s和Ti 2p的XPS高分辨扫描光谱.由图可知, O 1s峰位于532.0 eV为Ti0.7W0.3O2中氧的结合能, Ti 2p1/2和Ti 2p3/2峰位于464.6和458.8 eV为Ti0.7W0.3O2中Ti4+的结合能, W 4f5/2和W 4f7/2峰位于34.2和33.1 eV为Ti0.7W0.3O2中W4+的结合能.单斜晶型BiVO4中Bi 4f和V 2p的XPS高分辨扫描光谱如图 5e~5f所示.Bi 4f5/2和W 4f7/2峰分别位于164.8和159.4 eV为BiVO4中Bi4+的结合能.V 2p1/2和V 2p3/2峰分别位于528.4和517.2 eV为BiVO4中V5+的结合能.这表明在构筑Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂时两种单体材料的晶型都未发生改变, 从而保证高效的光催化活性.
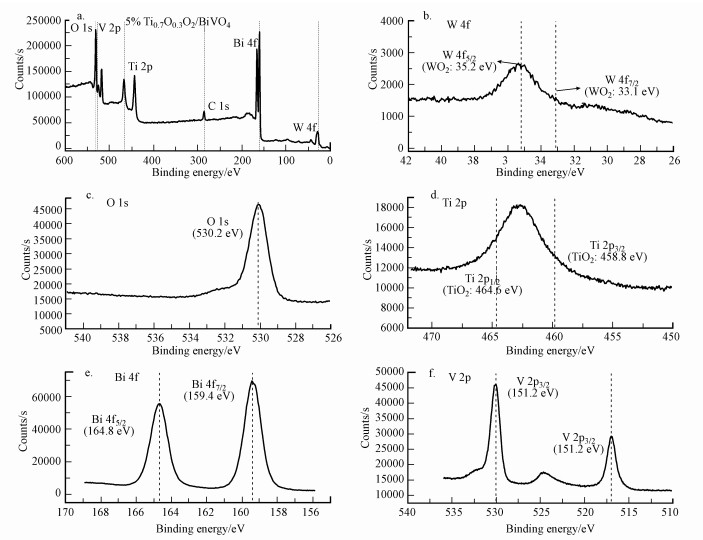 |
| 图 5 5 % Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂(a.XPS全扫描光谱, b.W 4f, c. O 1s, d.Ti 2p, e. Bi 4f, f. V 2p) Fig. 5 The XPS survey scan spectra of (a) rutile Ti0.7W0.3O2, the XPS narrow scans of W 4f(b), O 1s(c), Ti 2p(d), Bi 4f(e) and V 2p(f) of 5% Ti0.7W0.3O2/BiVO4 p-n CHIP |
纯BiVO4、纯Ti0.7W0.3O2、1%、3%、5%、10%和20% Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂的紫外-可见漫反射结果见图 6a.同时, 由公式(F(R∞)hv)1/2计算得到的各催化剂的能带宽度结果见图 6b.纯单斜晶型BiVO4和金红石型Ti0.7W0.3O2对可见光响应光谱波长分别为550.5 nm和610.8 nm, 其能带宽度分别为2.41 eV和2.09 eV.纯金红石型Ti0.7W0.3O2具有较低的能带宽度可能会使其具有更好的导电性, 能迅速转移光生电子而有效抑制光生电子-空穴复合提高可见光催化活性(Wang et al., 2015).
 |
| 图 6 纯BiVO4, 纯金红石型Ti0.7W0.3O2和1%、3%、5%、10%、20% Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂紫外-可见漫反射图(a)及能带宽度吸收光谱图(b) Fig. 6 DRS(a) and plots of the energy of absorbed light(b) for the pure BiVO4, pure rutile Ti0.7W0.3O2 and 1%, 3%, 5%, 10%, 20% Ti0.7W0.3O2/BiVO4 p-n CHIP, respectively |
同时, 随着Ti0.7W0.3O2负载比例增加(1%~20%), Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂对光的响应范围从550.5 nm扩展到602.7 nm, 能带宽度从2.41 eV降到2.17 eV.这表明复合异质结构界面光催化剂对可见光的利用效率比纯BiVO4高, 能有效抑制光生电子-空穴复合提高光催化效率.
3.5 样品的XPS价带谱分析纯单斜晶BiVO4和金红石型Ti0.7W0.3O2的XPS价带扫描光谱如图 7所示.纯BiVO4和Ti0.7W0.3O2的价带位置分别为1.79和2.65 eV, 根据DRS能带宽度的结果, 可以推算其导带位置分别为-0.62和0.56 eV, 结果与文献报道中是一致的(Sayama et al., 2006).因此, Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂的能级图见图 14.
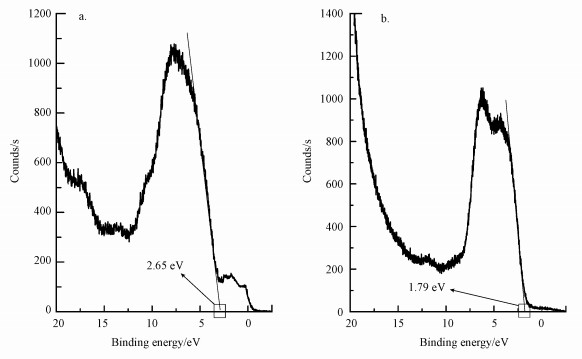 |
| 图 7 纯金红石型Ti0.7W0.3O2(a)和纯BiVO4(b)的XPS价带扫描光谱 Fig. 7 The XPS valence band scan spectrum of pure rutile Ti0.7W0.3O2 (a) and BiVO4 (b) |
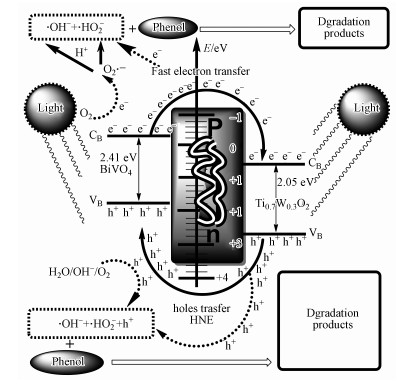 |
| 图 14 Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂光生电子和空穴分离及降解苯酚的过程示意图 Fig. 14 Schematic diagram for the possible charge separation process and the photodegradative process of phenol at Ti0.7W0.3O2/BiVO4 p-n CHIP |
为探究苯酚降解溶液的最佳初始pH, 研究了不同pH对光催化活性的影响(3.5~10.0), 结果见图 8a.5% Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂在pH为4.5时, 降解苯酚的活性最强;且在酸性溶液中的降解活性高于自然、中性和碱性溶液, 这与Khataee等研究的结论一致.分析可能的原因:一方面, 在酸性条件下羟基自由基的氧化能力更强;因为在碱性溶液中HCO3-和CO32-的形成会直接影响污染物和羟基自由基之间的反应而减弱其氧化能力和催化活性.另一方面, 由于形成了以HCO3-和CO32-为主的低吸附性负电荷系统将抑制超氧化物自由基阴离子(O2-)的生成, 从而降低氧化能力(Khataee et al., 2017).同时, 在pH为3.5酸性过强的条件下苯酚光降解效率也较低, 可能的原因是Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面被部分破坏导致催化活性低.因此, 最佳的苯酚初始pH值为4.5.
 |
| 图 8 不同光催化条件对Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂催化活性的影响(a.苯酚溶液的初始ph值, b.苯酚的起始浓度, c.催化剂的用量, d.苯酚的连续6次催化降解降解率) Fig. 8 The effect of the initial pH value(a), initial catalyst dosage(b), initial phenol concentration (c) on phenol photocatalytic degradation and Recyclability of Ti0.7W0.3O2/BiVO4 p-n CHIP in phenol photodegradation process under visible light illumination through 6 cycles at initial concentration(d) |
探究了不同的苯酚初始浓度(50~125 mg · L-1)的光催化效果, 结果见图 8b.苯酚完全降解的时间随着苯酚起始浓度的增大而增加.当苯酚浓度为95 mg · L-1时, 在330 min就可以完全彻底降解完成, 当苯酚浓度低于95 mg · L-1时, 完全降解时间太短, 没有达到催化剂降解饱和状态.相反, 苯酚初始浓度增大到110 mg · L-1及125 mg · L-1时, 光照360 min后苯酚的降解率分别为89.6%和84.8%, 苯酚不能完全降解.这表明既定光催化条件下, 苯酚的初始浓度存在有一个最佳值, 且苯酚的最佳初始浓度为95 mg · L-1.分析原因是:一方面, 苯酚浓度太高, 过多的苯酚分子及中间产物会与催化剂分子形成对光的竞争, 阻碍了催化剂对光的吸收, 从而影响光响应效率导致催化效率下降.另一方面, 过多的苯酚分子及中间产物会占据分布在催化剂表面的活性位点, 导致接受光辐射的活性位点数量下降, 导致催化剂光电子效率较低;而较低的光电子效率将直接影响羟基自由基和超氧化物自由基阴离子的形成, 最终导致光催化活性下降(Taufik et al., 2017; Jorfi et al., 2018).
催化剂用量对Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂催化活性的影响结果如图 8c所示.催化剂用量从0.15 g · L-1增加到0.60 g · L-1时, 催化剂表面的反应位点和比表面积会增多, 从而产生更多的羟基自由基和超氧化物自由基阴离子, 苯酚光催化效率从35.7%增加100%.但继续增加催化剂用量, 苯酚的降解效率不增反而会下降.可能的原因是:第一, 过量的催化剂会导致溶液透明度降低, 光的散射增强, 催化剂粒子的光响应效率降低.第二, 过多的催化剂粒子会阻碍苯酚分子与自由基之间的有效碰撞.此外, 催化剂的有效比表面积和孔隙容积也会随着催化剂用量过量而下降, 最终导致较低的光催化活性.因次, 最佳的催化剂用量为0.60 g · L-1.
所有非均相光催化剂的稳定性和循环利用性对于大规模污水处理的实际应用非常重要.Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂每完成一次苯酚降解实验之后, 催化剂被重新收集, 用50%的乙醇水清洗, 并在105 ℃条件下烘干4 h.经过连续六次重复使用, 苯酚降解效率结果如图 8d所示.结果显示, 在经过6次循环之后, 苯酚的降解效率仍高于90%, 表明Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂具有很好的稳定性和循环利用性.
3.6.2 光催化降解性能研究不同负载比例的Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂和Pt/BiVO4纳米光催化剂降解苯酚的紫外-可见光谱见图 9.由图可知, 不同Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂降解苯酚时, 苯酚的特征吸收波长(270 nm)强度随着光照时间的延长下降速度不一致, 表明苯酚被降解的速率不同.纯Ti0.7W0.3O2在经过360 min照射后, 特征峰强度下降很少, 降低部分可能与Ti0.7W0.3O2的吸附作用及辐射光本身的降解作用有关, 表明纯Ti0.7W0.3O2对苯酚无光催化降解作用.5% Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂的苯酚特征吸收峰强度下降最快, 表明其光催化活性最高.同时, Pt/BiVO4纳米光催化剂与Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂对苯酚的降解规律相似, Pt纳米粒子的最佳负载值为0.75%, 具有最高催化活性.5% Ti0.7W0.3O2/BiVO4比0.75% Pt/BiVO4对苯酚的降解活性更高, 表明在修饰提高BiVO4对苯酚光催化降解活性方面, Ti0.7W0.3O2纳米粒子比贵金属Pt更具优势, 甚至具有替代贵金属而大规模应用于光催化降解污染物的可能.
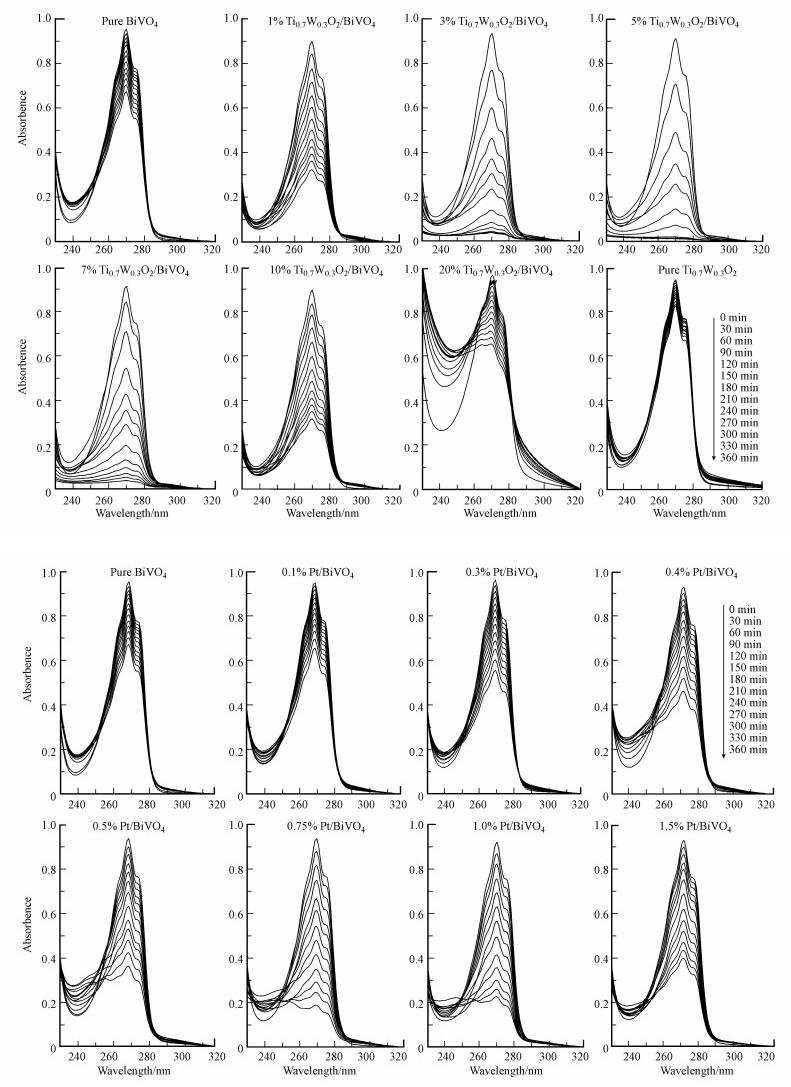 |
| 图 9 不同样品光催化剂降解苯酚的紫外-可见光谱图 Fig. 9 UV-Vis spectra of phenol degradation catalyzed by different samples |
另外, 从苯酚的紫外-可见光谱中不难看出, 可能是苯酚降解过程中产生的一些带环中间产物导致其特征吸收峰出现不同位置的蓝移现象(Xiong et al., 2010).Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂出现了波长为257.5和263.5 nm蓝移吸收峰, 而Pt/BiVO4蓝移波长为245.5和254.5 nm, 可能是中间降解产物不同;但这些蓝移的吸收峰最后都消失, 表明最终都被完全降解矿化为CO2和H2O.
研究了两种催化剂对苯酚降解的动力学, 苯酚相对浓度对数ln(c0/c)与光照时间(t)的关系及降解速率常数(K)如图 10所示.5%Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂和0.75%Pt/BiVO4具有最大降解速率常数, 且5% Ti0.7W0.3O2/BiVO4光降解苯酚的速率常数分别约为纯BiVO4和0.75% Pt/BiVO4的12和3.5倍.这表明在BiVO4负载适量的Ti0.7W0.3O2能够提高光催化效率, 且比负载适量的Pt纳米粒子更具优势.
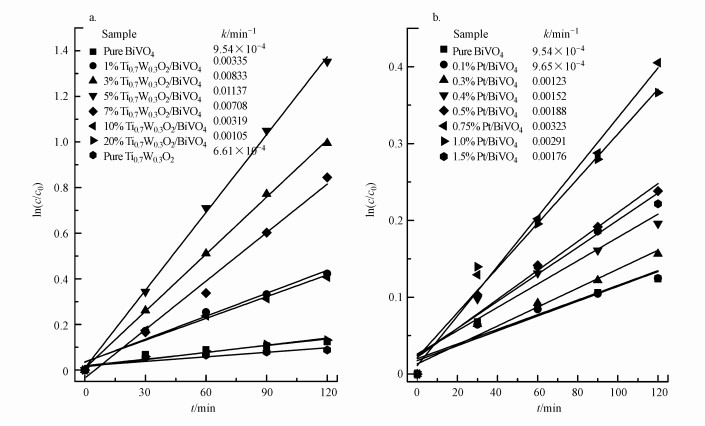 |
| 图 10 不同Ti0.7W0.3O2负载量的Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂和不同Pt负载量的Pt/BiVO4纳米复合界面材料的苯酚相对浓度对数ln(c0/c)与光照时间(t)的关系 Fig. 10 The dependence of ln(c0/c) on the irradiation time (t) in Pt/BiVO4 and Ti0.7W0.3O2/BiVO4 p-n CHIP, respectively |
为了更好地评估合成Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂的活性, 对比了相关文献中报道关于BiVO4复合光催化材料光催化降解苯酚的性能, 结果如表 1所示.通过对比很容易发现, Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂具有明显的光催化性能优势:在相当的光照时间、最低的光照能量及最少量的催化剂光催化降解最高浓度的苯酚污染物.因此, 在优化后的光催化降解参数条件下, 制备的Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂是降解低浓度酚类污染物最有效的催化剂之一.
| 表 1 Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂的苯酚光催化降解效率与文献报道中的催化效率比较 Table 1 Comparison of photocatalytic degration of phenol results of Ti0.7W0.3O2/BiVO4 p-n CHIP with the open literature |
苯酚在通过不同种类的光催化剂降解时可能会产生不同的中间产物, 对应不同的降解机理.Xiong等提出了用TiO2-xBx降解苯酚时, 苯酚先被羟基自由基反应和空穴反应两种降解机理氧化成邻、对苯二酚和2, 4-己二烯二酸等中间产物(Xiong et al., 2010).Liu等发现3D的TiO2降解苯酚产生了对二苯酚、对苯醌和4, 4-二羟基联苯等中间降解产物, 最终这些中间产物都会被矿化为CO2和H2O(Liu et al., 2008).
由图 9结果可知两种光催化剂降解苯酚的中间产物不完全相同, 并取两种催化剂降解苯酚180 min反应溶液, 注入HPLC-MS分析苯酚降解过程中产生的中间产物.通过分析质谱图谱的核质比(m/z)及比对标准数据库, 推测了Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂和Pt/BiVO4纳米复合光催化剂在苯酚降解过程中可能产生了8和7种中间产物, 中间产物可能的结构见图 11.
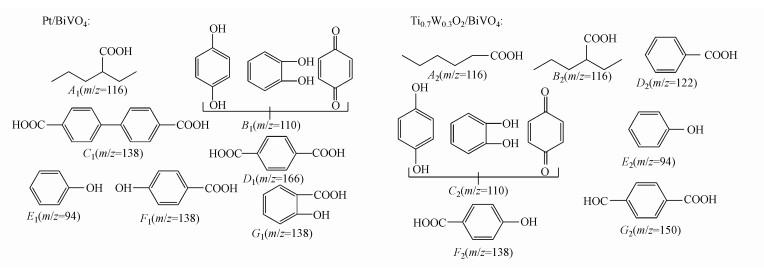 |
| 图 11 Pt/BiVO4和Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂的苯酚降解中间产物可能结构示意图 Fig. 11 Proposed possible structures for intermediates of phenol degradation over Pt/BiVO4 and Ti0.7W0.3O2/BiVO4 p-n CHIP, respectively |
Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂对苯酚的降解过程中产生的羟自由基(· OH-)、过氧化物自由基阴离子(· O2 -)和光生空穴(h+)分别用异丙醇、对苯醌及乙二胺四乙酸二钠作为捕获剂进行捕获实验.实验条件为:pH为4.5, 催化剂用量为0.60 g · L-1, 苯酚浓度为95 mg · L-1, 光照时间为240 min.三组实验组分别加入10 mL异丙醇、10 mL对苯醌及10 mL乙二胺四乙酸二钠(0.01 mol · L-1)作为捕获剂, 同时设置没有添加任何捕获剂作为对照组开展光催化降解苯酚, 结果如图 12A所示.在光照240 min之后, 苯酚的降解效率分别为(异丙醇)28.42%、59.60%(对苯醌)、68.71%(乙二胺四乙酸二钠)及98.92%(没有添加任何捕获剂).这说明在苯酚的光催化降解过程中, 三种自由基均参加了苯酚的氧化降解, 且氧化能力由强到弱顺序为:羟自由基(· OH-)>过氧化物自由基阴离子(· O2 -)>光生空穴(h+).同时, 更进一步证明Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂对苯酚的光催化降解速率快慢由羟自由基(· OH-)机理决定.
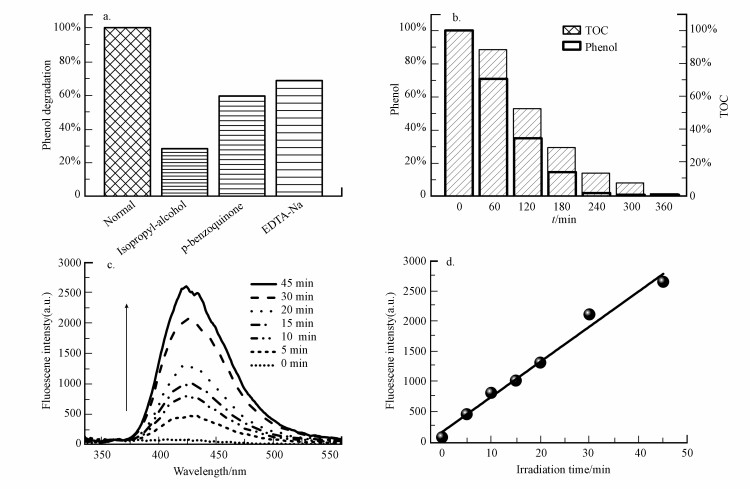 |
| 图 12 Ti0.7W0.3O2/BiVO4的光催化降解苯酚(a.不同自由基捕获剂条件下的苯酚光催化效率, b.不同光照时间降解悬浮液中苯酚和总有机碳含量, c.在含0.01 mmol · L-1氢氧化钠和3 mmol · L-1对苯二甲酸的上清液荧光光谱强度与光照时间的关系(激发波长为315 nm), d.对苯二甲酸的荧光强度(激发发射波长为425 nm)与光照时间的关系) Fig. 12 The photocatalytic degradation of phenol at Ti0.7W0.3O2/BiVO4 p-n CHIP (a.the photocatalytic degradation of phenol with different radical scavengers, b.the phenol and TOC content in suspension solution under different illumination time, c. Fluorescence spectral changes observed during illumination in 0.01 mol · L-1 NaOH and 3 mM terephthalic acid solution (under 315 nm excitation), d.plots showing the induced fluorescence intensities (425 nm) against light illumination time for terephthalic acid) |
同时, Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂降解苯酚的过程中, 不同光照时间之后降解悬浮溶液中苯酚的含量和总有机碳(TOC)测定结果如图 12b所示.可知, 悬浮溶液中苯酚的含量是一直低于TOC的含量, 且苯酚在光照催化240 min后基本降解完全, TOC则在光照360 min之后才降解完全, 这说明苯酚在降解的过程中是先产生了中间降解产物, 最后这些中间产物再矿化为无机的CO2和H2O.
3.7.3 羟自由基(· OH-)的测定羟自由基(· OH-)可以与对苯二甲酸(TA)反应生成羟基对苯二甲酸(TAOH), 生成的TAOH在315 nm处激发时, 会在425 nm左右的产生荧光强度(Andersson et al., 2002; HIRAKAWA et al., 2002; Wodka et al., 2010; Ramalingam et al., 2016; Li et al., 2016).图 12c是Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面催化剂光催化降解苯酚悬浮溶液(含有0.01 mmol · L-1氢氧化钠和3 mmol · L-1 TA)的上层清液在不同光照射时间之后产生的荧光光谱.由图 12d可以清楚看到TAOH的荧光强度随着光照时间的推移不断增强, 说明在Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂降解苯酚的反应中生成了大量的羟自由基(· OH-), 这也进一步说明Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂降解苯酚是羟自由基(· OH-)降解机理.
3.7.4 光催化降解机理的研究根据实验结果及相关文献报道(Liu et al., 2008;Xiong et al., 2010;Castillo et al., 2010;郑龙珍等, 2014;Li et al. 2016; 陈苗等, 2019), 我们提出了苯酚在Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面和Pt/BiVO4纳米复合光催化剂表面中可能的降解机理如图 13所示.
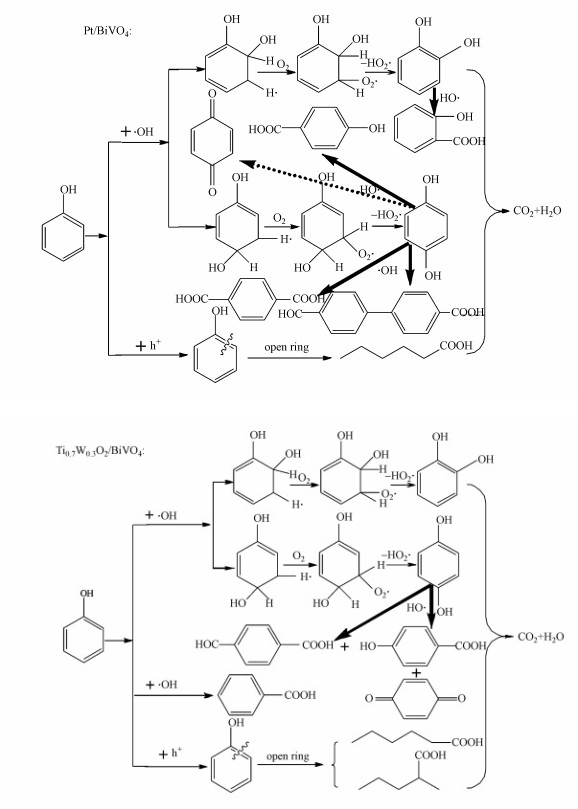 |
| 图 13 Pt/BiVO4和Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂降解苯酚的可能机理示意图 Fig. 13 Proposed mechanisms of phenol degradation over Pt/BiVO4 and Ti0.7W0.3O2/BiVO4 p-n CHIP, respectively |
由图可知, 两种复合界面光催化剂降解苯酚时, 羟基自由基反应和空穴反应机理同时进行, 且这两个机理反应是一个竞争的过程, 两种光催化剂降解苯酚的中间产物不完全一样.同时, 空穴反应机理可能会随着Pt和Ti0.7W0.3O2负载量的增加而增强.在两种机理作用条件下, 所有的中间产物最后都被矿化成了无毒无害的CO2和H2O.
3.8 Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化活性提高的分析根据整个实验结果, Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂对苯酚降解包括两个竞争且同时进行的羟基自由基反应和空穴反应机理.苯酚在p-n复合异质结构界面上的可能降解过程如下图 14所示. Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂具有较高的光催化活性主要原因:首先, 叶子型BiVO4为纳米级颗粒具有较大比表面积能吸附更多的污染物分子, 且高结晶度产生微晶尺寸效应能使光生电荷载体迅速扩散到催化剂表面, 能快速与吸附在其表面的污染物反应(Tsuji et al., 2004; Bian et al., 2008; Sivaranjani et al., 2011).其次, 具有一定导电性的p型半导体Ti0.7W0.3O2与n型半导体BiVO4之间可能形成了p-n复合异质结构界面新肖特基势垒, BiVO4表面产生的光生电子能迅速转移到Ti0.7W0.3O2表面, Ti0.7W0.3O2表面产生的光生空穴能迅速转移到BiVO4表面, 从而达到有效抑制光生电子与光生空穴复合, 抑制光腐蚀现象(Linsebigler et al., 1995; Hosseini et al., 2007).同时, 在转移的过程中更多有效光生电子和空穴会加速向溶解氧分子转移, 结合更多水分子生成更多的自由基粒子, 从而提高了污染物降解速率(郑龙珍等, 2014).再次, Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂的稳定性优越, 在经历了6次循环光照催化降解实验后, 仍能保持较高的光催化活性(Zeng et al., 2008; Wang et al., 2010).因此, 制备的Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂是降解低浓度酚类污染物有机废水的一种理想材料.
4 结论(Conclusions)1) 纯单斜晶BiVO4和金红石型Ti0.7W0.3O2的价带位置分别为1.79和2.65 eV, 能带宽度分别为2.41和2.09 eV, 其导带位置分别为-0.62和0.56 eV;Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂的光响应范围扩展到586.5 nm, 能带宽度降到2.33 eV.
2) 在可见光照射条件下, 构筑Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂是提高BiVO4光催化性能的一种有效途径;且苯酚起始浓度为95 mg · L-1, pH为4.5, 催化剂的用量为0.60 g · L-1为最佳催化条件.
3) 5% Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂表现出最高的苯酚催化活性、很好的稳定性和可循环利用性, 且符合一级动力学降解规律, 其初始降解速率常数(k)分别约为纯BiVO4和0.75%Pt/BiVO4的12和3.5倍, 纯金红石型Ti0.7W0.3O2纳米粒子比贵金属Pt纳米粒子在修饰BiVO4光催化性能方面更具优势.
4) Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂降解苯酚过程中产生了8种中间降解产物, 机理为同时进行且具有竞争关系的羟基自由基反应和空穴自由基反应, 且羟基自由基反应为主要反应机理.制备的Ti0.7W0.3O2/BiVO4 p-n复合异质结构界面光催化剂是降解含低浓度酚类污染物废水的一种理想光催化剂.
Andersson M, Osterlund L, Ljungstrom S, et al. 2002. Preparation of nanosize anatase and rutile TiO2 by hydrothermal treatment of microemulsions and their activity for photocatalytic wet oxidation of phenol[J]. Journal of Physical Chemistry B, 106(41): 10674-10679. DOI:10.1021/jp025715y |
Asahi R, Morikawa T, Ohwaki T, et al. 2001. Visible-light photocatalysis in nitrogen-doped titanium oxides[J]. Science, 293(5528): 269-271. DOI:10.1126/science.1061051 |
Bajaj R, Sharma M, Bahadur D. 2013. Visible light-driven novel nanocomposite (BiVO4/CuCr2O4) for efficient degradation of organic dye[J]. Dalton Transactions, 42(19): 6736-6744. DOI:10.1039/c2dt32753h |
Bian Z F, Zhu J, Wang S, et al. 2008. Self-assembly of active Bi2O3/TiO2 visible photocatalyst with ordered mesoporous structure and highly crystallized anatase[J]. Journal of Physical Chemistry C, 112(16): 6258-6262. DOI:10.1021/jp800324t |
Bodoardo S, Maja A M, Penazzi A N, et al. 1997. Oxidation of hydrogen on WC at low temperature[J]. Electrochimica Acta, 42(17): 2603-2609. DOI:10.1016/S0013-4686(96)00434-3 |
Cao L, Sahu S, Anilkumar P, et al. 2011. Carbon nanoparticles as visible-light photocatalysts for efficient CO2 conversion and beyond[J]. Journal of the American Chemical Society, 133(13): 4754-4757. DOI:10.1021/ja200804h |
Cao S W, Yin Z, Barber J, et al. 2012. Preparation of Au-BiVO4, heterogeneous nanostructures as highly efficient visible-light photocatalysts[J]. ACS Applied Materials & Interfaces, 4(1): 418-423. |
Castillo N C, Ding L, Heel A, et al. 2010. On the photocatalytic degradation of phenol and dichloroacetate by BiVO4:The need of a sacrificial electron acceptor[J]. Journal of Photochemistry and Photobiology A:Chemistry, 216(2/3): 221-227. |
陈苗, 郭昌胜, 吴琳琳, 等. 2019. Ag/P-g-C3N4复合材料可见光催化降解双酚AF的机理研究[J]. 环境科学学报, 39(5): 1497-1508. |
Chen X, Lei L, Yu P Y, et al. 2011. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals[J]. Science, 331(6018): 746-750. DOI:10.1126/science.1200448 |
David W I F, Wood I G. 1983. Ferroelastic phase transition in BiVO4:VI. Some comments on the relationship between spontaneous deformation and domain walls in ferroelastics[J]. Journal of Physics C:Solid State Physics, 16(26): 5149-5166. DOI:10.1088/0022-3719/16/26/009 |
Gao F, Chen X Y, Yin K B, et al. 2010. Visible-light photocatalytic properties of weak magnetic BiFeO3 nanoparticles[J]. Cheminform, 38(49): 2889-2892. |
Gao X M, Fu F, Li W H. 2013. Photocatalytic degradation of phenol over Cu loading BiVO4 metal composite oxides under visible light irradiation[J]. Physica B, 412: 126-131. DOI:10.1016/j.physb.2012.11.031 |
Guan M L, Ma D K, Hu S W, et al. 2011. From Hollow Olive-Shaped BiVO4 to n-p Core-Shell BiVO4@Bi2O3 Microspheres:Controlled Synthesis and Enhanced Visible-Light-Responsive Photocatalytic Properties[J]. Inorganic Chemistry, 50(3): 800-805. DOI:10.1021/ic101961z |
HIRAKAWA T N, et al. 2002. Properties of O2·- and OH·formed in TiO2 aqueous suspensions by photocatalytic reaction and the influence of H2O2 and some ions[J]. Langmuir, 18(18): 3247-3254. |
Hirota K, Komatsu G, Yamashita M, et al. 1992. Formation, characterization and sintering of alkoxy-derived bismuth vanadate[J]. Materials Research Bulletin, 27(7): 823-830. DOI:10.1016/0025-5408(92)90177-2 |
Hosseini S N, Borghei S M, Vossough M, et al. 2007. Immobilization of TiO2 on perlite granules for photocatalytic degradation of phenol[J]. Applied Catalysis B Environmental, 74(1): 53-62. |
Jiang H, Meng X, Dai H, et al. 2012. High-performance porous spherical or octapod-like single-crystalline BiVO4 photocatalysts for the removal of phenol and methylene blue under visible-light illumination[J]. Journal of Hazardous Materials, 217-218: 92-99. DOI:10.1016/j.jhazmat.2012.02.073 |
Jorfi S, Pourfadakari S, Kakavandi B. 2018. A new approach in sono-photocatalytic degradation of recalcitrant textile wastewater using MgO@Zeolite nanostructure under UVA irradiation[J]. Chemical Engineering Journal, 343(24): 95-107. |
Khataee A, Kayan B, Gholami P, et al. 2017. Sonocatalytic degradation of an anthraquinone dye using TiO2-biochar nanocomposite[J]. Ultrasonics Sonochemistry, 39(Pt A): 120. |
Kim H I, Kim J, Kim W, et al. 2011. Enhanced photocatalytic and photoelectrochemical activity in the ternary hybrid of CdS/TiO2/WO3 through the cascadal electron transfer[J]. The Journal of Physical Chemistry C, 115(19): 9797-9805. DOI:10.1021/jp1122823 |
Kudo A, Ueda K, Kato H, et al. 1998. Photocatalytic O2 evolution under visible light irradiation on BiVO4 in aqueous AgNO3 solution[J]. Catalysis Letters, 53(3/4): 229-230. DOI:10.1023/A:1019034728816 |
Lei B X, Zeng L L, Zhang P, et al. 2014. Hydrothermal synthesis and photocatalytic properties of visible-light induced BiVO 4 with different morphologies[J]. Advanced Powder Technology, 25(3): 946-951. DOI:10.1016/j.apt.2014.01.014 |
Lei Ge. 2008. Novel visible-light-driven Pt/BiVO4 photocatalyst for efficient degradation of methyl orange[J]. Journal of Molecular Catalysis A:Chemical, 282(1/2): 62-66. |
Li R, Mansukhani N D, Guiney L M, et al. 2016. Identification and optimization of carbon radicals on hydrated graphene oxide for ubiquitous antibacterial coatings[J]. ACS Nano, 10(12): 10966-10980. DOI:10.1021/acsnano.6b05692 |
Li Y Y, Liu J P, Huang X T, et al. 2007. Hydrothermal synthesis of Bi2WO6 uniform hierarchical microspheres[J]. Crystal Growth & Design, 7(7): 1350-1355. |
Linsebigler A L, Lu G Q, Yates J T. 1995. Photocatalysis on TiO2 Surfaces:Principles, mechanisms, and selected results[J]. Chemical Reviews, 95(3): 735-758. |
Liu L, Liu H J, Zhao Y P, Wang Y Q, et al. 2008. Directed synthesis of hierarchical nanostructured TiO2 catalysts and their morphology-dependent photocatalysis for phenol degradation[J]. Environmental Science & Technology, 42(7): 2342-2348. |
Long M, Cai W M, Cai J, et al. 2006. Efficient photocatalytic degradation of phenol over Co3O4/BiVO4 composite under visible light irradiation[J]. Journal of Physics and Chemistry B, 110(41): 20211-20216. DOI:10.1021/jp063441z |
Ma D K, Guan M L, Liu S S, et al. 2012. Controlled synthesis of olive-shaped Bi2S3/BiVO4 microspheres through a limited chemical conversion route and enhanced visible-light-responding photocatalytic activity[J]. Dalton Transactions, 41(18): 5581-5586. DOI:10.1039/c2dt30099k |
Maeda K, Teramura K, Lu D, et al. 2006. Photocatalyst releasing hydrogen from water[J]. Nature, 440(7082): 295. DOI:10.1038/440295a |
Obregón S and Colón G, 2014.A ternary Er3+-BiVO4/TiO2 complex heterostructure with excellent photocatalytic performance[J]. RSC Advances, 4(14): 6920-6926
|
Ramalingam B, Parandhaman T, Das S K. 2016. Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of gram-negative bacteria viz.Escherichia coli and Pseudomonas aeruginosa[J]. Acs Applied Materials & Interfaces, 8(7): 4963-4976. |
Sayama K, Nomura A, Arai T, et al. 2006. Photoelectrochemical decomposition of water into H2 and O2 on porous BiVO4 thin-film electrodes under visible light and significant effect of Ag ion treatment[J]. Journal of Physical Chemistry B, 110(23): 11352-11360. DOI:10.1021/jp057539+ |
Sayama K, Nomura A, Arai T, et al. 2006. Photoelectrochemical decomposition of water into H2 and O2 on porous BiVO4 thin-film electrodes under visible light and significant effect of Ag ion treatment[J]. Journal of Physical Chemistry B, 110(23): 11352-11360. DOI:10.1021/jp057539+ |
Sivaranjani K, Gopinath C, Sivaranjani K, et al. 2011. Porosity driven photocatalytic activity of wormhole mesoporous TiO2-xNx in direct sunlight[J]. Journal of Materials Chemistry, 21(8): 2639-2647. DOI:10.1039/c0jm03825c |
Taufik A, Albert A, Saleh R. 2017. Sol-gel synthesis of ternary CuO/TiO2/ZnO nanocomposites for enhanced photocatalytic performance under UV and visible light irradiation[J]. Journal of Photochemistry & Photobiology A Chemistry, 344(1): 149-162. |
Tsuji I, Kobayashi H, Kudo A, et al. 2004. Photocatalytic H2 evolution reaction from aqueous solutions over band structure-controlled (AgIn)xZn2(1-x)S2 solid solution photocatalysts with visible-light response and their surface nanostructures[J]. Journal of the American Chemical Society, 126(41): 13406-13413. DOI:10.1021/ja048296m |
Usai S, Obregón, Sergio, Becerro A I, et al. 2013. Monoclinic-tetragonal heterostructured BiVO4, by yttrium doping with improved photocatalytic activity[J]. The Journal of Physical Chemistry C, 117(46): 24479-24484. DOI:10.1021/jp409170y |
Wang C X, Yin L W, Zhang L Y, et al. 2010. Platinum-nanoparticle-modified TiO2 nanowires with enhanced photocatalytic property[J]. Acs Applied Materials & Interfaces, 2(11): 3373-3377. |
Wang F, Shao M, Cheng L, et al. 2009. The synthesis of monoclinic bismuth vanadate nanoribbons and studies of photoconductive, photoresponse, and photocatalytic properties[J]. Materials Research Bulletin, 44(8): 1687-1691. DOI:10.1016/j.materresbull.2009.04.005 |
Wang M, Zheng H, Liu J, et al. 2015. Enhanced visible-light-driven photocatalytic activity of B-doped BiVO4 synthesized using a corn stem template[J]. Materials Science in Semiconductor Processing, 30: 307-313. DOI:10.1016/j.mssp.2014.09.031 |
Wang X, Maeda K, Thomas A, et al. 2009. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nature Materials, 8(1): 76-80. DOI:10.1038/nmat2317 |
Wodka D, Bielańska E, Socha R P, et al. 2010. Photocatalytic activity of titanium dioxide modified by silver nanoparticles[J]. Applied Surface Science, 319(7): 173-180. |
Xia L, Bai J, Li J, et al. 2017. High-performance BiVO4 photoanodes cocatalyzed with an ultrathin α-Fe2O3 layer for photoelectrochemical application[J]. Applied Catalysis B, 204: 127-133. DOI:10.1016/j.apcatb.2016.11.015 |
Xiong L Y, Zheng L Z, Xu J P, et al. 2010. Photocatalytic degradation of phenol with mesoporous TiO2-xBx[J]. Environmental Chemistry Letter, 39(2): 1201-1208. |
Yan J, Zhang L, Yang H, et al. 2009. CuCr2O4/TiO2 heterojunction for photocatalytic H2 evolution under simulated sunlight irradiation[J]. Solar Energy, 83(9): 1534-1539. DOI:10.1016/j.solener.2009.05.004 |
Ye H, Park H S, Bard A J. 2015. Screening of electrocatalysts for photoelectrochemical water oxidation on W-Doped BiVO4 photocatalysts by scanning electrochemical microscopy[J]. Journal of Physical Chemistry C, 115(25): 12464-12470. |
Yi Z, Ye J, Kikugawa N, et al. 2010. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation[J]. Nature Materials, 9(7): 559-564. DOI:10.1038/nmat2780 |
Zeng H B, Cai W P, Liu P S, et al. 2008. ZnO-based hollow nanoparticles by selective etching:elimination and reconstruction of metal-semiconductor interface, improvement of blue emission and photocatalysis[J]. Acs Nano, 2(8): 1661-1670. DOI:10.1021/nn800353q |
Zhang H, Fan X, Quan X, et al. 2011. Graphene sheets grafted Ag@AgCl hybrid with enhanced plasmonic photocatalytic activity under visible light[J]. Environmental Science & Technology, 45(13): 5731-5736. |
Zhang K, Liu Y, Deng J, et al. 2018. Co-Pd/BiVO4:High-performance photocatalysts for the degradation of phenol under visible light irradiation[J]. Applied Catalysis B:Environmental, 224: 350-359. DOI:10.1016/j.apcatb.2017.10.044 |
Zhang L, Chen D, Jiao X. 2006. Monoclinic structured BiVO4 nanosheets:hydrothermal preparation, formation mechanism, and coloristic and photocatalytic properties[J]. Journal of Physical Chemistry B, 110(6): 2668-2673. DOI:10.1021/jp056367d |
Zhang X F, Quan X, Chen S, et al. 2010. Effect of Si doping on photoelectrocatalytic decomposition of phenol of BiVO4 film under visible light[J]. Journal of Hazardous Materials, 177(1/3): 914-917. |
Zhang X F, Zhang Y, Quan X, et al. 2009. Preparation of Ag doped BiVO4 film and its enhanced photoelectrocatalytic (PEC) ability of phenol degradation under visible light, Journal of Hazardous Materials[J]. Journal of Hazardous Materials, 167(1/3): 911-914. |
Zhao Y, Xie Y, Zhu X, et al. 2010. Surfactant-free synthesis of hyperbranched monoclinic bismuth vanadate and its applications in photocatalysis, gas sensing, and lithium-ion batteries[J]. Chemistry, 14(5): 1601-1606. |
Zhou B, Zhao X, Liu H, et al. 2011. Synthesis of visible-light sensitive M-BiVO4 (M=Ag, Co and Ni) for the photocatalytic degradation of organic pollutants[J]. Separation & Purification Technology, 77(3): 275-282. |
Zhou L, Prof W W, Xu H, et al. 2010. Bi2O3 Hierarchical nanostructures:controllable synthesis, growth mechanism, and their application in photocatalysis[J]. Chemistry-A European Journal, 15(7): 1776-1782. |
Zou Z, Ye J, Sayama K, et al. 2001. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst[J]. Nature, 414(6864): 625-627. DOI:10.1038/414625a |
郑龙珍, 董泽民, 熊乐艳, 等. 2014. WC/TiO2纳米复合界面光催化剂制备及其光催化降解酚类污染物研究[J]. 环境科学学报, 34(11): 2806-2814. |
 2020, Vol. 40
2020, Vol. 40


