2. 中国科学院大学, 北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049
多环芳烃(PAHs)是一类在自然界分布广泛的持久性有机污染物, 具有显著的致癌、致突变和毒性作用.内分泌干扰物(EDCs)是一类能够影响生物体内天然激素产生、分泌、运输、代谢、结合、作用和消除, 从而影响生物体的内稳态和发育的外源性物质, 其在低至ng·L-1浓度水平即具有内分泌干扰性(李建忠, 2013).在多环芳烃中, 菲的分子量较低, 通过3个苯环以稠环形式相连, 在诸多环境领域的研究中都被当作一种典型的PAHs (Zhang et al., 1997; Zhan et al., 2015; Zindler et al., 2016;Li et al., 2018).菲的“湾”区和“K”区可能有一定的致癌作用, 因此, 菲被用作人体代谢研究的模型PAHs (Bücker et al., 1979;Schrlau et al., 2017).菲也是土壤和沉积物中普遍检出且浓度较高的一种PAHs污染物(Mai et al., 2000; Tang et al., 2005; Chen et al., 2006; Guo et al., 2006).此外, 国内外许多学者通过菲来研究空间位阻、微孔填充、平面芳香结构和π-π电子供受体作用在吸附过程中的重要性(Ran et al., 2004; Cornelissen et al., 2004; Zhu et al., 2005; Nguyen et al., 2007).壬基酚也是一种典型的内分泌干扰物, 由于其具有明显的雌激素效应和生物毒性而受到高度关注(程广焕, 2015; Lou et al., 2014).因此, 本研究选择菲和壬基酚作为吸附质来研究其在环境介质中吸附行为.
沉积有机质对HOCs的吸附作用是控制其在环境中的分配、迁移和归宿的主导因素, 因此沉积物对HOCs吸附机理的研究已经受到了广泛关注(Kang et al., 2005; Ran et al., 2007; Wang et al., 2011; Yang et al., 2011; Sun et al., 2013; Jin et al., 2015; Zhang et al., 2016).沉积物主要是由粘土矿物、碳酸盐、类脂、多糖、木质素和腐殖质组成(Zhang et al., 2016), 在漫长的地质历史中, 由于沉积环境的差异造成不同地区之间的沉积物间存在巨大差异, 各组分的含量不尽相同.通过化学处理从原始沉积物中逐步除去矿物、类脂、多糖和蛋白质后得到非水解有机质(NHC)级分.
许多研究已经强调了沉积有机质中的NHC级分的重要性以及沉积有机质的结构性质是影响吸附行为的主要因素(Ran et al., 2007; Sun et al., 2012; Han et al., 2014; Jin et al., 2015; Zhang et al., 2016).例如:沉积有机质的微孔和表面积显著影响HOCs的吸附能力(Ran et al., 2013; Han et al., 2014; Zhang et al., 2016; Huang et al., 2017).有机质的整体和表面极性及极性官能团也是影响HOCs和有机质吸附域之间可及性的重要因素(Huang and Weber, 1997; Yang et al., 2011; Jin et al., 2015), 但也有研究报道整体或表面极性及微孔与吸附能力之间没有单一的相关性(Sun et al., 2013; Lattao et al., 2014).Foster等观察到碳质材料在吸附高浓度的苯和丙酮时, 其比表面越高吸附容量越大, 但在低浓度下观察到相反的现象(Foster et al., 1992).因此, 沉积有机质的各种性质(如极性、结构及微孔等)在HOCs吸附过程中的影响仍需进一步研究.目前的研究大多强调单一机制的重要性, 关于沉积有机质表面羧酸碳对HOCs吸附行为影响的研究也较少(Jin et al., 2015; Jin et al., 2017a, 2017b), 因此, 本研究综合考虑了沉积有机质和HOCs间的多种作用机理(疏水作用、微孔填充和π-π电子供受体作用), 有助于进一步理解沉积有机质与有机污染物之间的吸附机制, 对于水环境的治理与修复具有重要意义.
此外, 由于沉积物的高度异质性, 其吸附HOCs并不是一个浓度独立的过程, 特别是低浓度下表现出明显的非线性吸附(Sun et al., 2012).已经有多篇研究观察到不同浓度下有机质的吸附行为和规律差异(Huang and Weber, 1998; Xia and Pignatello, 2001; Liu et al., 2011; Pan et al., 2012).因此, 在本文的研究中, 选用ce=0.005Sw、ce=0.05Sw和ce=0.5Sw下的分配系数Kd(Kd=qe/ce)来探讨菲和壬基酚在沉积物原始样品及其非水解有机质组分上的吸附机理, 其中qe为固相吸附剂上吸附质的浓度(μg·g-1), ce为平衡溶液中吸附质的浓度(μg·g-1).
2 材料和方法(Materials and methods) 2.1 样品前处理在2012年9月国家自然科学基金委支持的春季航次中, 使用箱式采样器采集南海陆架和陆坡区的表层(0~5 cm)沉积物.采样点分布如图 1所示, 其中E1~E4属于大陆架沉积物, 水深为44~208 m;E5属于大陆坡沉积物, 水深1468 m.将沉积物样品冷冻干燥, 研磨过80目筛, 每个沉积物样品取160 g, 根据文献中的方法来提取NHC级分(Gélinas et al., 2001; Zhang et al., 2016), 其方法简述如下:冷冻干燥后的样品依次用1 mol·L-1 HCl+10% HF混合酸溶液去矿、二氯甲烷和甲醇(2: 1)混合溶剂超声抽提、不同浓度三氟醋酸和盐酸水解, 最后得到NHC级分.固体样品用超纯水清洗至中性, 冷冻干燥并研磨后用于仪器表征和吸附实验.
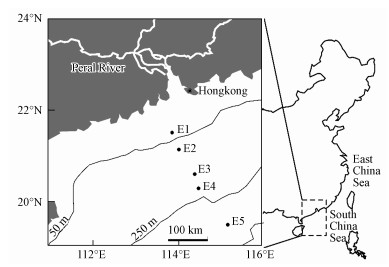 |
| 图 1 5个海洋沉积物采样点分布图 Fig. 1 The sampling locations for the five marine sediments |
所有样品用Vario EL CUBE (Elementar, 德国)和Vario ELIII (Elementar, 德国)元素分析仪检测C、H、N和O.
N2和CO2气体等温线采用Micromeritics ASAP 2460表面积和孔径分析仪, 在77 K温度条件下, N2在0.05~0.995个相对大气压的条件下测定;CO2在273 K条件下, 1×10-6~0.03个相对大气压的条件下测定.氮气的表面积(N2-SSA)采用BET公式计算.二氧化碳表面积(CO2-SSA)和微孔体积(CO2-V0)采用Dubinin-Radushkavich(DR)模型拟合, 使用密度泛函理论(Density Functional Theory, DFT)计算出累计表面积(CSA)和0~1.1 nm的微孔体积V0.
NHC级分的表面元素(C、N、O、Si和Al)的相对含量通过X射线光电子能谱仪(K-Alpha Thermo Fisher Scientific, 英国)测试.具体方法是先将样品颗粒均匀地平铺在铝箔纸上的双面胶上, 在压片机上压实, 压片机的压力设置为10 kg, 压片时间15 s.将压好的样品剪成1 cm × 1 cm的大小, 粘在样品台上, 揭下表面的铝箔纸, 用洗耳球吹扫测试面以去除未粘牢的样品颗粒.分析前将XPS分析测试腔抽到超高真空(≤5×10-8 mbar).采用Al-Kα作为X射线激发源, 激发电压为1468.6 eV.谱图收集范围为0~1350 eV, X射线光斑大小为400 μm, 全谱通能和窄谱通能分别为100 eV和30 eV, 能量分辨率高于0.5 eV (Liu et al., 2016).利用Avantage专业软件对C1s峰进行分峰拟合以确定与碳相连接的O官能团和不同的碳形式, 其中结合能为284.7 eV, 属于C—C和C—H类碳的特征峰, 对应于芳烃或芳烃的取代烷烃;结合能为285.5 eV, 属于sp3杂化中C—O类碳的特征峰;结合能为288.2 eV, 属于C=O/O—C=O类碳的特征峰.
2.3 吸附实验及模型采用色谱纯菲(纯度>98%, Aldrich)和壬基酚(纯度>94%, Aldrich)为吸附质.菲和壬基酚在水中溶解度分别为1.12 mg·L-1和5.43 mg·L-1.菲和壬基酚的辛醇水分配系数logKow分别为4.57和5.76 (Xia and Ball, 1999; Heidler and Halden, 2008).背景溶液包含0.01 mol·L-1 CaCl2、200 mg·L-1 NaN3和5 mg·L-1 NaHCO3, 其中CaCl2是矿物成分, NaN3为生物活性抑制剂, NaHCO3是为了控制pH的值等于7.根据预实验的结果, 将适量的吸附剂置于20 mL安瓿瓶中使体系表观平衡浓度达到初始浓度的30%~70%.在加有吸附剂并预先称重的玻璃安瓿瓶中加入不同浓度的菲和壬基酚水溶液, 其中菲的初始浓度分别为:10、20、40、80、100、150、250、500、750、1000 μg·L-1;壬基酚的初始浓度分别为:250、500、1000、1500、2000、2500、3000、3500、4000、4500、5000 μg·L-1.每个浓度梯度均设置了空白对照组.火焰封口避免蒸发损失, 并在摇床上以125 r·min-1振荡28 d达到吸附平衡, Ran等进行长达4个月的沉积有机质对菲的吸附实验和超过2个月的解吸实验, 实验结果表明超过7 d的固液反应时间足以达到吸附和解吸平衡(Ran et al., 2004).控制温度在(25±1) ℃, 避光保存.色谱柱采用C18反相柱(150 mm × 4.6 mm × 5 μm), 柱温箱温度设置为40 ℃, 检测器使用荧光检测器.进样体积为10 μL, 菲的流动相为V(超纯水): V(乙腈)=10: 90, 流速为1.0 mL·min-1, 其荧光检测器的激发和发射波长分别为250 nm和364 nm.壬基酚的流动相为V(超纯水): V(乙腈)=30: 70, 其荧光检测器的激发和发射波长分别为277 nm和300 nm.吸附结果使用Freundlich模型进行拟合, 模型公式如下:
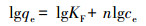
|
(1) |
式中, qe为固相吸附剂上吸附质的浓度(μg·L-1), ce为平衡溶液中吸附质的浓度(μg·L-1), KF为Freundlich吸附容量参数((μg·g-1)/(μg·L-1)n), n为Freundlich非线性因子.
3 结果与分析(Results and analysis) 3.1 沉积物有机质的整体和表面元素组成由表 1可知, 原始样品的有机碳含量为0.202%~0.678%, 当样品灰分含量过高, 氧和氢元素的测量会受结合水的影响(Lyon and Rhodes, 1993; Ran et al., 2007; Jin et al., 2017), 所以原始样品的氧和氢元素含量没有列出.与原始样品OS相比, 由于大量的无机矿物被去除, NHC级分的C含量显著增加;三氟醋酸水解去除了大量的蛋白质导致NHC级分的C/N比显著增加.NHC级分的O/C和H/C比值与前期的研究一致(Ran et al., 2007; Jin et al., 2015; Zhang et al., 2016).沉积物有机质的表面元素组成列在表 2中, 可以看出NHC级分表面的元素组成是相似的, 主要元素是碳, 其次是氧, 仅有微量的硅元素检出, 说明本实验方法可以有效的去除原始沉积物中的硅酸盐.除了NHC-E4, 其余NHC级分整体的O/C和XPS观测到的结果非常接近.表面元素分析的碳含量显著高于整体元素分析的结果, 以及NHC级分表面非常低的硅含量(表 2), 表明原始样品经过去矿处理后, 有机质可能包裹在残余矿物的表面(Mikutta et al., 2009; Yang et al., 2011).此外, 从表 1可以观察到大陆架沉积物E1~E4, 随着水深的增加原始样品和NHC级分的C/N比均呈现逐渐减小的趋势, 指示了海相迁移过程中, 陆源有机质的贡献逐渐变小(Hu et al., 2006; Zhang et al., 2014).
| 表 1 沉积物原始样品和NHC级分的元素含量及元素比 Table 1 Elemental compositions of the original sediment samples and NHC fractions |
| 表 2 沉积物NHC级分表面元素组成 Table 2 The surface element composition of NHC samples |
图 2为沉积物原始样品和NHC级分CO2和N2吸附等温线.使用DR模型拟合CO2吸附等温线得到的微孔体积(<2 nm)及比表面积(Dubinin, 1989), 根据密度泛函数理论(Density functional Theory, DFT)分析样品在0~1.1 nm的微孔孔径分布和累计表面积(CSA) (Mastalerz et al., 2013; Zhang et al., 2017).DFT方法属于反映多孔样品的孔中流体热力学性质的分子动力学方法, 不仅提供了吸附的微观模型, 而且比传统的热力学方法可以更准确地反映孔径分布(李恒超, 2017).氮气吸附测试孔径范围在1.7~300 nm, 主要测试了介孔和宏孔, 根据Brunauer-Emmet-Teller(BET)法提取比表面积.上述模型可以分别准确地表征沉积物在微孔、介孔及宏孔范围内的孔隙结构特征, 从而合理地研究HOCs的吸附行为与微孔、介孔及宏孔范围内的孔隙结构特征的相关性.从表 3可以观察到, 去除了矿物、游离脂、多糖和蛋白质后得到的NHC级分的微孔和比表面积明显增加.此外, 以DFT模型为例, 有机碳含量与CO2等温线计算的微孔体积均呈现出显著的正相关关系(图 3a).而与氮气测得介孔和宏孔的比表面积相关性很差(图 3b), 可能是因为沉积有机质内部多发育微孔结构, 而不是介孔和宏孔(李恒超, 2017).
 |
| 图 2 沉积物原始样品和NHC级分的CO2和N2吸附等温线 Fig. 2 CO2 and N2 adsorption isotherms on the original sediment samples and NHC fractions |
| 表 3 基于不同模型计算的沉积物原始样品及其NHC级分的氮气和二氧化碳吸附结果 Table 3 CO2 and N2 adsorption results of the bulk sediments and NHC fractions derived from different models |
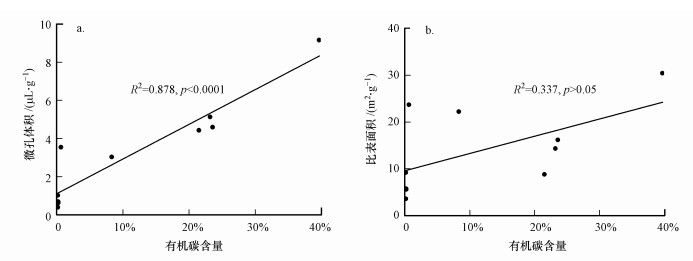 |
| 图 3 沉积物原始样品和NHC级分有机碳含量与V0 (0~1.1 nm)及N2-SSA之间的关系 Fig. 3 Correlations among organic carbon content and microvoid volume V0 (0~1.1 nm) and N2-SSA for bulk samples and NHC fractions |
由表 4可知, 所有样品对菲和壬基酚的吸附可以很好地用Freundlich模型进行拟合, 且对菲的吸附为典型的非线性吸附.因此, 选用ce= 0.005Sw、ce=0.05Sw和ce= 0.5Sw下的Kd来探讨菲和壬基酚在沉积物原始样品及其非水解有机质组分上的吸附机理.原始沉积物对菲的吸附参数与之前文献中报道的6个珠江口沉积物的结果一致(Ran et al., 2007).原始样品对壬基酚的吸附行为与之前文献中报道河流沉积物的结果基本一致(Navarro et al., 2009).NHC级分对菲的非线性因子为0.601~0.661, 与珠江口沉积物NHC级分报道的n值(0.593~0.666)一致(Zhang et al., 2016), 低于Ran等的研究结果(0.708~0.751) (Ran et al., 2007).与原始样品相比, NHC级分的吸附能力、微孔和有机碳均显著增加.
| 表 4 沉积物原始样品及其NHC级分对菲和壬基酚吸附的Freundlich参数和Kd值 Table 4 Freundlich isotherm parameters and concentration-dependent distribution coefficients(Kd) of phenanthrene (Phen) and nonylphenol (NP) on the original sediments and NHC fractions |
不同浓度下Phen和NP的Kd值与0~1.1 nm的微孔呈显著的正相关关系(图 4a~4b), 且随着吸附质浓度的增加, 相关系数逐渐减小, 表明微孔填充机理在低浓度下更加重要.之前的研究也显示了在低浓度下微孔填充占总吸附量的68%, 在最高的浓度下也占据了45% (Xing and Pignatello, 1997).Wang等报道了在低浓度下, 萘优先占据生物炭微孔中的高能点位, 而在高浓度下, 萘的吸附机制主要是分配作用(Wang et al., 2017).我们也观察到在低浓度时, 原始沉积物对菲的吸附能力均大于对壬基酚的Kd值, 随着浓度的增加, 这种趋势逐渐减小, 当ce= 0.5Sw或更高浓度时, 原始样品对壬基酚的吸附能力均大于对菲的吸附能力.这可能是因为菲的分子直径(7.85 Å)小于壬基酚(9.03 Å), 所以在低浓度时, 与壬基酚相比, 菲进入微孔的空间位阻更小, 展示出更高的吸附能力;前人已经报道了微孔填充机制中的空间位阻效应与吸附质分子和孔径密切相关(Ran et al., 2004; Nguyen et al., 2007; Zhang et al., 2010; Wang et al., 2016).随着浓度的进一步增加, 原始沉积物有机质内部的微孔数量接近饱和, 微孔填充机制的作用减少, 疏水分配作用可能占据了更主要的地位, 考虑到壬基酚的logKow更大, 因此其吸附能力更强.对于NHC级分, 其微孔体积显著大于原始样品, 在实验浓度下, 微孔中的吸附点位可能没有达到饱和状态, 微孔填充机制一直处于主导地位.所以NHC级积分对菲的吸附能力一直高于分子尺寸较大的壬基酚.
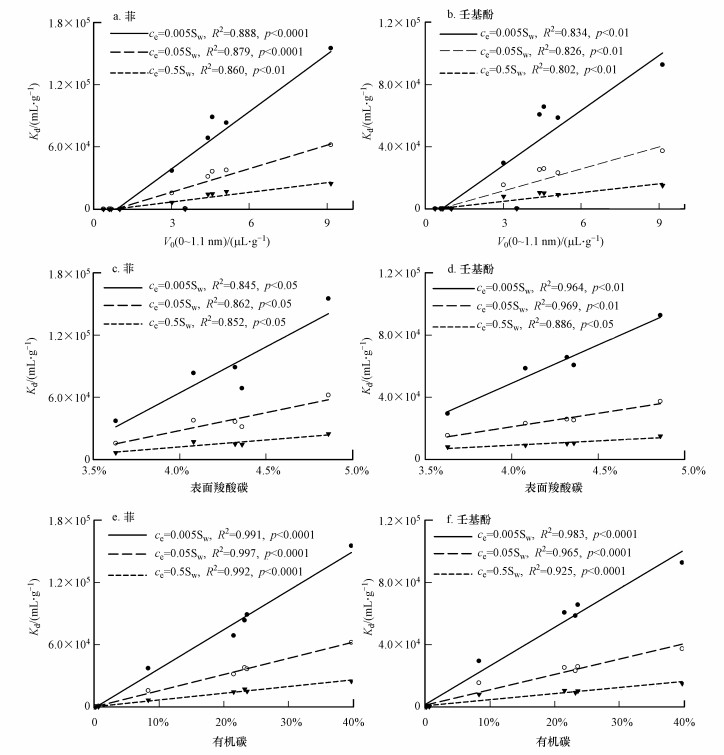 |
| 图 4 沉积物原始样品和NHC级分对菲和壬基酚的吸附能力Kd与V0 (0~1.1 nm)、表面羧酸碳和有机碳含量之间的关系 Fig. 4 Correlations among Kd values of phenanthrene (Phen) and nonylphenol (NP) and microvoid volume V0 (0~1.1 nm), surface carboxyl and organic carbon content for bulk samples and NHC fractions |
图 4c~4d为NHC级分对菲和壬基酚不同浓度下的Kd值与表面羧酸碳含量的关系.已经有多篇文章报道了较高的有机质的整体或表面极性指数(O+N/C)会降低HOCs的吸附容量(Kang and Xing, 2005; Chen et al., 2008; Wang et al., 2011; Yang et al., 2011; Jin et al., 2015).主要因为有机质中大量的亲水基团可以通过氢键为其表面的“水簇”提供结合位点.“水簇”可以降低有机质的表面疏水性和HOCs对于吸附区域的可及性, 并且与吸附质竞争吸附点位, 从而降低了HOCs的吸附容量(Wang et al., 2011).但最近也有研究发现生物炭或者NHC级分的表面羧酸碳含量与菲的吸附能力呈正相关关系(Jin et al., 2015; Jin et al., 2017a; 2017b).原因在于这些有机质中的芳香碳含量较高(>40%), 是一种良好的π受体, 菲是一种具有3个苯环的π供体, 位于芳香区域边缘的羧酸碳具有很强的吸电子能力(Zhu et al., 2004), 因此表面羧酸碳含量的增加, 会同时引起菲和有机质之间的π-π电子供受体作用力增强(Jin et al., 2015; Jin et al., 2017a; 2017b)和有机质表面“水簇”增加导致的吸附抑制作用.图 4c~4d展示的显著正相关关系和前期的研究均表明这些吸电子基团含量增加对HOCs可及性的影响较小, π-π电子供受体作用强度可能比氢键还要大(Keiluweit and Kleber, 2009).此外, 图 4c~4d显示随着吸附质浓度的增加, NHC级分对菲和壬基酚的Kd值与表面羧酸碳含量的相关系数先增大后减小, 可能是由于中低浓度时, NHC级分上的芳香区域内的吸附点位较多, π-π电子供受体作用占据主要地位, 随着浓度的进一步增加, NHC级分上的芳香区域内的吸附点位接近饱和, 菲和有机质之间的π-π电子供受体作用强度减小.不同浓度下Phen和NP的Kd值与有机碳含量始终呈显著的正相关关系(图 4e~4f), 再一次表明沉积有机质在吸附HOCs过程中的重要作用.上述内容从宏观角度讨论了吸附质在不同平衡浓度下, 微孔填充机理、π-π电子供受体作用机制在NHC吸附HOCs中产生的差异变化.
4 结论(Conclusions)1) 对海洋表层沉积物进行去矿、抽提和水解等化学处理得到非水解有机质级分, CO2和N2吸附实验表明, 与原始样品相比, NHC级分的微孔体积和比表面积显著增加.菲和壬基酚吸附模拟实验结果表明所有对菲的吸附为典型的非线性吸附, 沉积有机质对HOCs的吸附能力与有机碳含量之间存在显著正相关关系.
2) 微孔、XPS分析以及吸附实验结果表明, 微孔填充和π-π电子供受体作用机制在SOM吸附疏水性有机物污染物中占据了至关重要的地位.
Bucker M, Glatt H R, Platt K L, et al. 1979. Mutagenicity of phenanthrene and phenanthrene K-region derivatives[J]. Mutation Research/Genetic Toxicology, 66: 337–348.
DOI:10.1016/0165-1218(79)90044-2
|
Chen B, Zhou D, Zhu L. 2008. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures[J]. Environmental Science & Technology, 42: 5137–5143.
|
Chen S J, Luo X J, Mai B X, et al. 2006. Distribution and mass inventories of polycyclic aromatic hydrocarbons and organochlorine pesticides in sediments of the Pearl River Estuary and the Northern South China Sea[J]. Environmental Science & Technology, 40: 709–714.
|
程广焕2015. 黑碳对沉积物中壬基酚吸附/解吸和微生物降解的影响[D]. 杭州: 浙江大学
|
Cornelissen G, Elmquist M, Groth I, et al. 2004. Effect of sorbate planarity on environmental black carbon sorption[J]. Environmental Science & Technology, 38: 3574–3580.
|
Dubinin M M. 1989. Fundamentals of the theory of adsorption in micropores of carbon adsorbents:Characteristics of their adsorption properties and microporous structures[J]. Carbon, 27: 457–467.
DOI:10.1016/0008-6223(89)90078-X
|
Foster K L, Fuerman R G, Economy J, et al. 1992. Adsorption characteristics of trace volatile organic-compounds in gas streams onto activated carbon-fibers[J]. Chemistry of Materials, 4: 1068–1073.
DOI:10.1021/cm00023a026
|
Gelinas Y, Prentice K M, Baldock J A, et al. 2001. An improved thermal oxidation method for the quantification of soot/graphitic black carbon in sediments and soils[J]. Environmental Science & Technology, 35: 3519–3525.
|
Guo Z, Lin T, Zhang G, et al. 2006. High-resolution depositional records of polycyclic aromatic hydrocarbons in the central continental shelf mud of the East China Sea[J]. Environmental Science & Technology, 40: 5304–5311.
|
Han L, Sun K, Jin J, et al. 2014. Role of structure and microporosity in phenanthrene sorption by natural and engineered organic matter[J]. Environmental Science & Technology, 48: 11227–11234.
|
Heidler J, Halden R U. 2008. Meta-analysis of mass balances examining chemical fate during wastewater treatment[J]. Environmental Science & Technology, 42: 6324–6332.
|
Hu J, Zhang H, Peng P A. 2006. Fatty acid composition of surface sediments in the subtropical Pearl River estuary and adjacent shelf, Southern China[J]. Estuarine, Coastal and Shelf Science, 66: 346–356.
DOI:10.1016/j.ecss.2005.09.009
|
Huang W L, Weber W J. 1997. A distributed reactivity model for sorption by soils and sediments. Relationships between desorption, hysteresis, and the chemical characteristics of organic domains[J]. Environmental Science & Technology, 31: 2562–2569.
|
Huang W L, Weber W J. 1998. A distributed reactivity model for sorption by soils and sediments. 11. Slow concentration dependent sorption rates[J]. Environmental Science & Technology, 32: 3549–3555.
|
Huang Y, Zhang D, Duan D, et al. 2017. Importance of the structure and nanoporosity of organic matter on the desorption kinetics of benzo a pyrene in sediments[J]. Environmental Pollution, 225: 628–636.
DOI:10.1016/j.envpol.2017.03.032
|
Jin J, Sun K, Wang Z, et al. 2017a. Effects of chemical oxidation on phenanthrene sorption by grass-and manure-derived biochars[J]. Science of The Total Environment, 598: 789–796.
DOI:10.1016/j.scitotenv.2017.04.160
|
Jin J, Sun K, Wang Z, et al. 2015. Characterization and phthalate esters sorption of organic matter fractions isolated from soils and sediments[J]. Environmental Pollution, 206: 24–31.
DOI:10.1016/j.envpol.2015.06.031
|
Jin J, Sun K, Wang Z, et al. 2017b. Characterization and phenanthrene sorption of natural and pyrogenic organic matter fractions[J]. Environmental Science & Technology, 51: 2635–2642.
|
Kang S H, Xing B S. 2005. Phenanthrene sorption to sequentially extracted soil humic acids and humins[J]. Environmental Science & Technology, 39: 134–140.
|
Keiluweit M, Kleber M. 2009. Molecular-Level interactions in soils and sediments:The role of aromatic π-systems[J]. Environmental Science & Technology, 43: 3421–3429.
|
Lattao C, Cao X, Mao J, et al. 2014. Influence of molecular structure and adsorbent properties on sorption of organic compounds to a temperature series of wood chars[J]. Environmental Science & Technology, 48: 4790–4798.
|
李恒超2017. 构造挤压对页岩孔隙特征及含气性的影响[D]. 广州: 中国科学院广州地球化学研究所
|
Li J, Luo C, Zhang D, et al. 2018. Autochthonous bioaugmentation-modified bacterial diversity of phenanthrene degraders in PAH-Contaminated Wastewater as revealed by DNA-Stable isotope probing[J]. Environmental Science & Technology, 52: 2934–2944.
|
李建忠2013. 典型内分泌干扰物在土壤中迁移转化规律研究[D]. 北京: 清华大学
|
Liping L, Guanghuan C, Jingyou D, et al. 2014. Mechanism of and relation between the sorption and desorption of nonylphenol on black carbon-inclusive sediment[J]. Environmental Pollution, 190: 101–108.
DOI:10.1016/j.envpol.2014.03.027
|
Liu H, Wei G, Xu Z, et al. 2016. Quantitative analysis of Fe and Co in Co-substituted magnetite using XPS:The application of non-linear least squares fitting (NLLSF)[J]. Applied Surface Science, 389: 438–446.
DOI:10.1016/j.apsusc.2016.07.146
|
Liu W, Li W, Xing B, et al. 2011. Sorption isotherms of brominated diphenyl ethers on natural soils with different organic carbon fractions[J]. Environmental Pollution, 159: 2355–2358.
DOI:10.1016/j.envpol.2011.06.032
|
Lyon W G, Rhodes D E. 1993. Molecular-size exclusion by soil organic materials estimated from their swelling in organic-solvents[J]. Environmental Toxicology and Chemistry, 12: 1405–1412.
DOI:10.1002/etc.v12:8
|
Mai B X, Fu J M, Sheng G Y, et al. 2002. Chlorinated and polycyclic aromatic hydrocarbons in riverine and estuarine sediments from Pearl River Delta, China[J]. Environmental Pollution, 117: 457–474.
DOI:10.1016/S0269-7491(01)00193-2
|
Mastalerz M, Schimmelmann A, Drobniak A, et al. 2013. Porosity of devonian and mississippian new albany shale across a maturation gradient:Insights from organic petrology, gas adsorption, and mercuty intrusion[J]. Aapg Bulletin, 97: 1621–1643.
DOI:10.1306/04011312194
|
Mikutta R, Schaumann G E, Gildemeister D, et al. 2009. Biogeochemistry of mineral-organic associations across a long-term mineralogical soil gradient (0.3-4100 kyr), Hawaiian Islands[J]. Geochimica Et Cosmochimica Acta, 73: 2034–2060.
DOI:10.1016/j.gca.2008.12.028
|
Navarro A, Endo S, Gocht T, et al. 2009. Sorption of alkylphenols on Ebro River sediments:Comparing isotherms with field observations in river water and sediments[J]. Environmental Pollution, 157: 698–703.
DOI:10.1016/j.envpol.2008.08.007
|
Nguyen T H, Cho H H, Poster D L, et al. 2007. Evidence for a pore-filling mechanism in the adsorption of aromatic hydrocarbons to a natural wood char[J]. Environmental Science & Technology, 41: 1212–1217.
|
Pan B, Wang P, Wu M, et al. 2012. Sorption kinetics of ofloxacin in soils and mineral particles[J]. Environmental Pollution, 171: 185–190.
DOI:10.1016/j.envpol.2012.07.037
|
Ran Y, Sun K, Yang Y, et al. 2007. Strong sorption of phenanthrene by condensed organic matter in soils and sediments[J]. Environmental Science & Technology, 41: 3952–3958.
|
Ran Y, Xing B S, Rao P S C, et al. 2004. Importance of adsorption (hole-filling) mechanism for hydrophobic organic contaminants on an aquifer kerogen isolate[J]. Environmental Science & Technology, 38: 4340–4348.
|
Ran Y, Yang Y, Xing B S, et al. 2013. Evidence of micropore filling for sorption of nonpolar organic contaminants by condensed organic matter[J]. Journal of Environmental Quality, 42: 806–814.
DOI:10.2134/jeq2012.0286
|
Schrlau J E, Kramer A L, Chlebowski A, et al. 2017. Formation of developmentally toxic phenanthrene metabolite mixtures by Mycobacterium sp. ELW1[J]. Environmental Science & Technology, 51: 8569–8578.
|
Sun K, Gao B, Ro K S, et al. 2012. Assessment of herbicide sorption by biochars and organic matter associated with soil and sediment[J]. Environmental Pollution, 163: 167–173.
DOI:10.1016/j.envpol.2011.12.015
|
SUN K, Jin J, Kang M J, et al. 2013. Isolation and characterization of different organic matter fractions from a same soil source and their phenanthrene sorption[J]. Environmental Science & Technology, 47: 5138–5145.
|
Tang L, Tang X Y, Zhu Y G, et al. 2005. Contamination of polycyclic aromatic hydrocarbons (PAHs) in urban soils in Beijing, China[J]. Environment International, 31: 822–828.
DOI:10.1016/j.envint.2005.05.031
|
Wang F, Sun H, Ren X, et al. 2017. Effects of humic acid and heavy metals on the sorption of polar and apolar organic pollutants onto biochars[J]. Environmental Pollution, 231: 229–236.
DOI:10.1016/j.envpol.2017.08.023
|
Wang J, Chen B, Xing B. 2016. Wrinkles and folds of activated graphene nanosheets as fast and efficient adsorptive sites for hydrophobic organic contaminants[J]. Environmental Science & Technology, 50: 3798–3808.
|
Wang X, Guo X, Yang Y, et al. 2011. Sorption mechanisms of phenanthrene, lindane, and atrazine with various humic acid fractions from a single soil sample[J]. Environmental Science & Technology, 45: 2124–2130.
|
Xia G S, Ball W P. 1999. Adsorption-partitioning uptake of nine low-polarity organic chemicals on a natural sorbent[J]. Environmental Science & Technology, 33: 262–269.
|
Xia G S, Pignatello J J. 2001. Detailed sorption isotherms of polar and apolar compounds in a high-organic soil[J]. Environmental Science & Technology, 35: 84–94.
|
Xing B S, Pignatello J J. 1997. Dual-mode sorption of low-polarity compounds in glassy poly(vinyl chloride) and soil organic matter[J]. Environmental Science & Technology, 31: 792–799.
|
Yang Y, Shu L, Wang X, et al. 2011. Impact of de-ashing humic acid and humin on organic matter structural properties and sorption mechanisms of phenanthrene[J]. Environmental Science & Technology, 45: 3996–4002.
|
Zhan X, Yi X, Yue L, et al. 2015. Cytoplasmic pH-stat during phenanthrene uptake by wheat roots:A mechanistic consideration[J]. Environmental Science & Technology, 49: 6037–6044.
|
Zhang D N, Duan D D, Huang Y D, et al. 2016. Role of structure, accessibility and microporosity on sorption of phenanthrene and nonylphenol by sediments and their fractions[J]. Environmental Pollution, 219: 456–465.
DOI:10.1016/j.envpol.2016.05.052
|
Zhang J, Sequaris J M, Narres H D, et al. 2010. Pyrene and phenanthrene sorption to model and natural geosorbents in single-and binary-solute systems[J]. Environmental Science & Technology, 44: 8102–8107.
|
Zhang L, Xiong Y Q, Li Y, et al. 2017. DFT modeling of CO2 and Ar low-pressure adsorption for accurate nanopore structure characterization in organic-rich shales[J]. Fuel, 204: 1–11.
DOI:10.1016/j.fuel.2017.05.046
|
Zhang Y, Kaiser K, Li L, et al. 2014. Sources, distributions, and early diagenesis of sedimentary organic matter in the Pearl River region of the South China Sea[J]. Marine Chemistry, 158: 39–48.
DOI:10.1016/j.marchem.2013.11.003
|
Zhang Y, Maier W J, Miller R M. 1997. Effect of rhamnolipids on the dissolution, bioavailability, and biodegradation of phenanthrene[J]. Environmental Science & Technology, 31: 2211–2217.
|
Zhu D Q, Hyun S H, Pignatello J J, et al. 2004. Evidence for pi-pi electron donor-acceptor interactions between pi-donor aromatic compounds and pi-acceptor sites in soil organic matter through pH effects on sorption[J]. Environmental Science & Technology, 38: 4361–4368.
|
Zhu D Q, Pignatello J J. 2005. Characterization of aromatic compound sorptive interactions with black carbon (charcoal) assisted by graphite as a model[J]. Environmental Science & Technology, 39: 2033–2041.
|
Zindle F, Glomstad B, Altin D, et al. 2016. Phenanthrene bioavailability and toxicity to daphnia magna in the presence of carbon nanotubes with different physicochemical properties[J]. Environmental Science & Technology, 50: 12446–12454.
|
 2018, Vol. 38
2018, Vol. 38


