2. 中国科学院大学, 北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049
干酪根是沉积岩中的不溶性大分子有机质(Organic Matter, OM), 是迄今为止地球上最丰富的有机质形式(Duan et al., 2016; Vandenbroucke et al., 2007), 在全球碳循环中起着重要的作用.这种有机质经历了地球化学成岩作用, 是石油和天然气的最重要的来源(Durand, 1980).由于其化学结构的异质性、复杂性和不溶性, 它们也被作为吸附疏水性有机污染物(Hydrophobic Organic Contaminants, HOCs)的吸附材料(Ran et al., 2004; Song et al., 2002).
近年来, 一些研究者对碳质材料进行化学活化和氧化, 以期达到改变其化学特性和结构特性的目的, 扩大其在催化剂、能源储存及环境保护方面的应用(Liu et al., 2015).通常人们选用ZnCl2和KOH作为活化剂(Blasi et al., 2015; Lillo-Ródenas et al., 2003), 对碳质材料的化学结构和表面性质进行调控;选用H2O2、KMnO4及NaClO等为氧化剂(Lutfalla et al., 2013; Mikutta et al., 2011; Siregar et al., 2005; Song et al., 2014), 对碳质材料表面进行修饰, 使其表面添加上一些含氧官能团, 以提高其交换容量、表面性质、络合容量等(Lehmann et al., 2011; Liu et al., 2015), 以便同时处理水土环境中的非极性、极性、离子化有机污染物及重金属等复合污染.然而, 目前尚未见将活化处理和氧化处理结合起来研究干酪根等一些碳质材料对极性、非极性有机污染物的吸附机理的报道;碳质材料的纳米孔及其表面性质的定量方法也不成熟;碳质材料和有机质的结构、成分、表面极性及其与HOCs的吸附机理的关系仍需要进一步研究, 最终建立分子水平的吸附机理(Chefetz et al., 2017; Ran et al., 2013; Wang et al., 2014;Zhu et al., 2005).因此, 本研究选取茂名油页岩为前体物质, 通过去矿处理获得干酪根, 前期有文献(张代男, 2015)报道油页岩仅利用去矿处理就可以提取干酪根.然后通过ZnCl2和KOH活化, 以及H2O2和NaClO氧化等手段对干酪根样品进行处理, 研究处理前后干酪根样品的微孔性质和有机官能团的变化, 以及处理前后对菲和壬基酚的吸附行为的变化, 并探讨其吸附机理.
2 材料和方法(Materials and methods) 2.1 活化和氧化干酪根的制备ZnCl2活化:选取茂名油页岩为前体物质, 油页岩经去矿处理(张代男, 2015)后得到原始干酪根OS, 样品经研磨过60目筛后备用;称取一定量的干酪根样品OS于烧杯中, 加入一定量质量分数为20%的ZnCl2溶液(Kula et al., 2008), 使之与ZnCl2的质量比为1(Ahmadpour et al., 1997; Mohanty et al., 2005; Oliveira et al., 2009; Yang et al., 2009; 2010), 在室温下静置12 h(He et al., 2013), 之后放于烘箱中105 ℃过夜烘干并研磨均匀;然后先用0.1 mol·L-1 HCl清洗, 再用去离子水清洗至中性, 冷冻干燥并研磨备用.
KOH活化:称取一定量的干酪根样品OS于烧杯中, 加入一定浓度的KOH溶液, 使KOH与OS的质量比为1(Ahmadpour et al., 1997; Diaz-Terán et al., 2003; Lillo-Ródenas et al., 2008; 王会涛, 2015), 在60 ℃下搅拌2 h(Lozano-Castelló et al., 2007), 之后放于烘箱中105 ℃烘干, 研磨均匀;先用5 mol·L-1 HCl清洗, 然后用去离子水清洗至中性, 冷冻干燥并研磨备用.
H2O2氧化(Zhang et al., 2011):分别取0.5 g OS及ZnCl2、KOH活化后的样品于50 mL的特氟龙离心管中, 加入20 mL去离子水和10 mL质量分数为30%的H2O2, 放于摇床中振荡反应24 h, 取出后用去离子水清洗5次, 冷冻干燥研磨备用, 该样品依次命名为OH、ZH、KH.
NaClO氧化(Siregar et al., 2005):分别取0.5 g OS及ZnCl2、KOH活化后的样品于50 mL的特氟龙离心管中, 加入10 mL质量分数为6%的NaClO溶液并用HCl调节pH为8, 于摇床中振荡反应6 h (125 r·min-1, 25 ℃), 离心去除上清液, 重复氧化3次, 第3次处理后, 离心去除上清液并用1 mol·L-1 NaCl清洗, 接着用去离子水清洗至电导率小于40 μS·cm-1, 冷冻干燥研磨备用, 该样品依次命名为ON、ZN、KN.
2.2 活化和氧化干酪根的表征 2.2.1 元素分析所有样品用Elementar Vario EL EL Ⅲ ( (Hanau, 德国)和Heraeus CHN-O-RAPID (Hanau, 德国)元素分析仪检测C、H、N和O.
2.2.2 傅里叶变换红外分析将0.1 mg活化和氧化的样品研磨后与80 mg光谱纯KBr压片, 用Bruker VERTEX-70傅里叶变换红外分析仪测定, 谱图分辨率为4 cm-1, 波数测定范围为4000~400 cm-1.
2.2.3 CO2气体吸附CO2气体等温线是在273 K下, 采用Micromeritics ASAP 2460表面积和孔径分析仪, 在1×10-6~0.03个相对大气压的条件下测定.表面积(SSA)和微孔体积(Vo)采用DR(Dubinin-Radushkavich)(Ran et al., 2013)模型拟合, 孔径分布用密度泛函理论(Density Functional Theory, DFT)来计算.
2.3 吸附实验及模型采用色谱纯菲(纯度>98%, Aldrich)和壬基酚(纯度>94%, Aldrich)为吸附质.菲的辛醇水分配系数logKOW为4.57, 水中溶解度为1.12 mg·L-1.壬基酚的辛醇水分配系数logKOW为4.48, 水中溶解度为5.43 mg·L-1.背景溶液为0.01 mol·L-1 CaCl2、200 mg·L-1 NaN3、5 mg·L-1 NaHCO3.将0.1~0.5 mg质量不等的吸附剂置于20 mL玻璃安瓿瓶中, 控制温度在(25±1) ℃.在加有吸附剂并预先称重的玻璃安瓿瓶中加入不同浓度的菲水溶液和壬基酚水溶液, 用蓝色火焰进行封口, 并在摇床上以125 r·min-1振荡28 d达到吸附平衡.色谱柱采用反相Inertsil ODS-SP(150 cm×4.6 mm×5 μm)柱, 检测器使用荧光检测器.进样体积为10 μL, 菲的流动相为90%乙腈/10%水, 流速为1.0 mL·min-1, 其荧光检测器的激发和发射波长分别为250 nm和364 nm.壬基酚的流动相为70%乙腈/30%水, 其荧光检测器的激发和发射波长分别为277 nm和300 nm.
修正的Freundlich吸附容量参数(K′F)可以对污染物的吸附能力进行直接比较, 其计算公式如式(1) 所示.

|
(1) |
式中, qe为固相吸附剂上菲的含量(μg·g-1);K′F为修正的Freundlich吸附容量(μg·g-1);Cr为无量纲水相中浓度, n为Freundlich非线性因子.K′F和KF的关系见式(2)~(3).
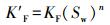
|
(2) |

|
(3) |
式中, Sw和Sscl分别是指在一定温度条件下, 溶质在水中的溶解度和固体有机化合物的过冷液体状态的溶解度(mg·L-1).
Polanyi-Dubinin(PD)模型和结合线性的Linear Partitioning and Polanyi-Dubinin(LPPD)模型可以用来描述纳米孔固体材料在液相中的吸附平衡, 拟合样品的吸附体积(Kujawinski et al., 2002; Ran et al., 2004), 同时, LPPD模型是考虑了分配贡献后的吸附模型, 其公式分别如式(4)~(6) 所示.
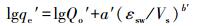
|
(4) |

|
(5) |

|
(6) |
式中, qe′为单位吸附剂上吸附溶质的体积(cm3·kg-1), Qo′为最大吸附体积(cm3·kg-1), εsw为有效吸附势(cal·mol-1), Vs为溶质的摩尔体积(cm3·mol-1), a′和b′为拟合参数, ρ为溶质的密度(g·cm-3), R为理想气体常数, Sw和Ce分别为溶质在温度T(K)时的溶解度(mg·L-1)和平衡浓度(μg·L-1), Kd是由KOC的经验公式计算(Kujawinski et al., 2002), 即当溶质的KOW在2~6之间时, lgKOC=0.99lgKow-0.35.
3 结果与分析(Results and analysis) 3.1 活化和氧化干酪根的元素组成表 1为活化和氧化干酪根样品的元素含量, 样品的总有机碳含量为61.82%~67.87%, H/C比值为1.04~1.17, 其O/C比值和(N+O)/C比值分别为0.21~0.26和0.24~0.29.样品的H/C比值较高, 表明其脂肪性较高;O/C比值较低, 说明其含氧官能团较少.
| 表 1 活化和氧化样品的元素含量 Table 1 Physicochemical properties of the activated and oxidized samples |
与原始样品OS相比, 除ZH样品外, 其余样品的C含量均有所下降;所有样品的O含量均有所增加;此外, H/C有不同程度的下降, 表明在处理后其脂肪性下降;而O/C比值和(N+O)/C比值均有所增加, 表明其极性增加(Wang et al., 2007), 同时氧化处理中引入了含氧官能团, 该推断可由红外分析的结果证实.同时, KH和KN样品的氧含量均比ZH和ZN样品大, 这与活化剂的性质有关, KOH本身也具有一定的氧化能力.此外, 用NaClO氧化的样品其碳含量均比H2O2氧化样品的小.
3.2 傅里叶红外分析图 1为活化和氧化干酪根的红外光谱图, 可以定性地判断有机官能团的变化.与原始样品OS相比, 除ZH样品外, 其它样品在1710 cm-1处的吸收峰均有所增强, 该峰是C=O的伸缩振动吸收峰, 表明氧化过程引入了含氧官能团.对于ZH样品, 虽然在1710 cm-1处的吸收峰有所减弱, 但其在3436 cm-1处的羟基官能团吸收峰明显增强, 同时在1110 cm-1处明显出现了吸收峰(Song et al., 2014), 可能是酯类或内酯的C—O吸收峰, 使氧含量增加.此外, 所有样品在2920 cm-1的甲基C—H和2846 cm-1的亚甲基C—H伸缩振动吸收峰(Zhang et al., 2014)及1620 cm-1处的芳环和多环芳烃的C=C伸缩振动吸收峰没有明显变化.
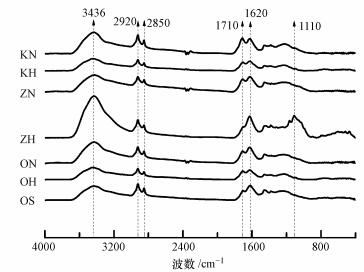 |
| 图 1 活化和氧化样品的红外光谱图 Fig. 1 FTIR spectra of the activated and oxidized samples |
图 2为活化和氧化样品的CO2吸附等温线及其孔径分布图, 结果表明, DR模型可以很好地拟合CO2气体的吸附等温线(R2>0.9999), 且可以很直观地看到对CO2吸附量的大小.表 2为活化和氧化样品的比表面积(SSA)及纳米孔体积(Vo).由表 2可知, 与OS相比, OH、ZH及ZN样品的纳米孔体积和比表面积有所增加, 其中, ZH样品的Vo和SSA最大, 分别为41.67 μL·g-1和104.0 m2·g-1;KN样品的Vo和SSA最小, 分别为30.68 μL·g-1和76.57 m2·g-1.此外, 用NaClO氧化的样品Vo和SSA值均比H2O2氧化样品的小.由图 3可知, 除OS样品外, 其他样品的Vo和SSA值与样品的有机碳(OC)含量呈显著正相关关系(p < 0.05).
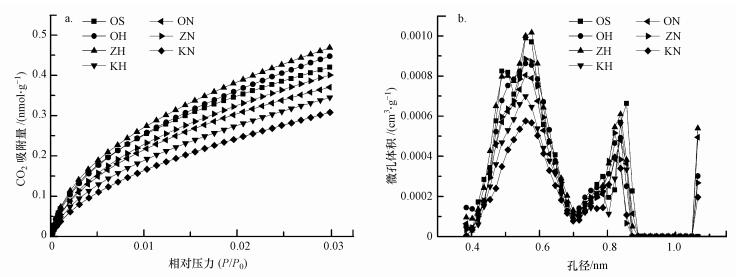 |
| 图 2 活化和氧化样品的CO2吸附等温线(a)及其孔径分布(b) Fig. 2 Carbon dioxide adsorption fitted by the Dubinin-Radushkevitch equation on the activated and oxidized samples |
| 表 2 活化和氧化样品的纳米孔体积和比表面积 Table 2 The nanopore volumes and specific surface areas of the activated and oxidized samples |
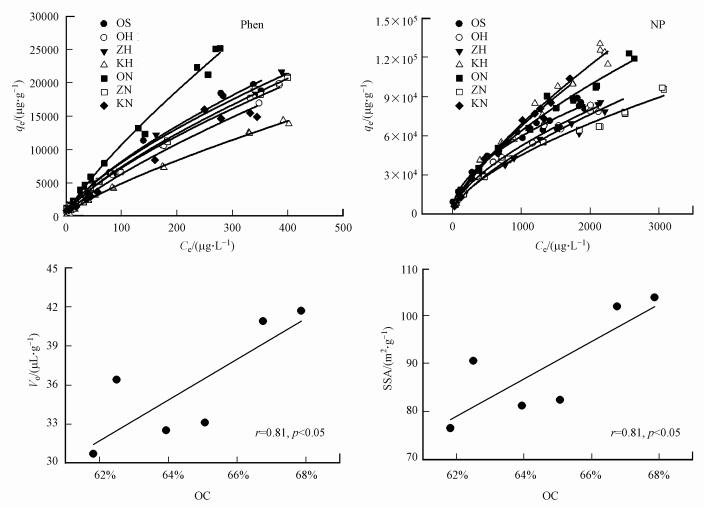 |
| 图 3 活化和氧化样品对菲和壬基酚的吸附等温线及Vo、SSA与OC之间的关系 Fig. 3 The adsorption isotherms for phenanthrene (Phen) and nonylphenol (NP) on the activated and oxidized samples and the relationship between OC with Vo and SSA |
由孔径分布图可知(图 2b), 在0~1.1 nm孔径范围, 纳米孔主要集中在0.4~0.7 nm和0.7~1.1 nm范围;经活化氧化处理后, 所有样品的孔径在0.4~0.7 nm范围的分布减少, 在0.7~1.1 nm范围的分布增加, 孔径分布趋于均匀.同时经活化氧化处理后, 所有样品在大于1.1 nm孔径分布的比例增大, 表明孔径有增大的趋势.
3.4 活化和氧化干酪根对菲和壬基酚的吸附作用表 3为菲和壬基酚在活化和氧化干酪根上吸附的Freundlich及修正的Freundlich参数和KOC值, 图 3为样品对菲和壬基酚的吸附等温线.由表 3可知, 所有样品对菲和壬基酚的吸附都可以很好地用Freundlich模型进行拟合, 且吸附为典型的非线性吸附.其中, 对菲的非线性因子n为0.678~0.797, 大于其对壬基酚吸附的n值(0.530~0.748), 这与文献(Zhang et al., 2015)中藻类非水解性有机碳(NHC)的研究结果一致.所有样品对菲的吸附容量值K′F为435~1297 μg·g-1, 对壬基酚的吸附容量值K′F为1358~3750 μg·g-1, 对壬基酚的吸附容量明显大于对菲的吸附容量, 这与文献(Zhang et al., 2015)中报导的藻类对壬基酚和菲的吸附现象相一致.
| 表 3 活化和氧化样品对菲和壬基酚吸附的Freundlich及修正的Freundlich参数和Koc值 Table 3 Freundlich isotherm parameters and modified Freundlich isotherm parameters for phenanthrene (Phen) and nonylphenol (NP) on the activated and oxidized samples |
图 4为样品对菲和壬基酚的吸附参数lgK′F值与O/C和(N+O)/C值的关系.除原始样品OS外, 其他样品对菲的吸附参数lgK′F值与O/C的相关性不显著(p=0.08), 而与(N+O)/C值呈显著负的相关关系(p < 0.05);而其他样品对壬基酚的吸附参数lgK′F值与O/C呈显著负的相关关系(p < 0.05), 与(N+O)/C值的相关性不显著(p=0.08).表明极性在样品对菲和壬基酚的吸附过程中起着的重要作用, 这与前期的研究结果相一致(Zhang et al., 2015).
 |
| 图 4 活化和氧化样品对菲和壬基酚吸附的lg K′F值与O/C、(N+O)/C之间的关系 Fig. 4 The relationship between lg K′F values of phenanthrene (Phen) and nonylphenol (NP) with O/C and (N+O)/C values |
图 5为PD、LPPD模型拟合的纳米孔填充体积(Vo)及Freundlich模型拟合的最大吸附体积(Vmax).表 4为PD、LPPD模型对菲和壬基酚吸附的拟合参数, 图 6为PD、LPPD模型对菲和壬基酚的吸附等温线.由图 5可知, 对于菲的吸附, Freundlich模型拟合的最大吸附体积与CO2气体吸附测定的纳米孔体积(Vo-CO2)基本吻合;PD模型拟合的纳米孔填充体积均大于Vo-CO2;而LPPD模型拟合的纳米孔填充体积除ON和ZN样品外, 其它样品的Vo-LPPD与Vo-CO2相差不大, 表明对于活化和氧化干酪根样品对菲的吸附, 纳米孔填充是重要的吸附机制.样品中Vo-PD均超过Vo-CO2, 表明PD模型可能高估了吸附贡献;当扣除分配贡献后, ON和ZN样品上菲的吸附容量在所有样品中是最大的, 而且菲的纳米孔填充体积高于V-CO2, 可能与干酪根的膨胀等性质有关, 需要作进一步研究.对于壬基酚的吸附, Freundlich模型拟合的最大吸附体积与Vo-PD和Vo-LPPD相差不大, 但都远大于Vo-CO2, 表明除了微孔填充体积机制外, 还存在别的吸附机理, 有研究报导, 藻类对壬基酚的吸附存在氢键作用, 使得藻类对壬基酚的吸附容量大于对菲的吸附容量(Zhang et al., 2015).经过活化和氧化后, NP的微孔填充体积在OH、ZH、ZN处理上比在未处理样品上都有不同程度的下降, 但在KH、ON、KN处理上都有明显的提高;一方面可能与孔径分布的变化有关, 另一方面可能与NP与表面—OH、—COOH、—NH2等基团形成氢键有关.
 |
| 图 5 不同模型下干酪根样品对菲和壬基酚的吸附体积及CO2吸附微孔体积(Vmax是在平衡浓度为溶解度时(Ce=Sw), 用Freundlich模型计算的吸附体积;Vo-PD是用PD模型拟合的吸附体积;Vo-LPPD是用LPPD模型拟合的吸附体积;Vo-CO2是用CO2吸附测定的微孔体积) Fig. 5 Adsorption volumes of phenanthrene and nonylphenol of kerogen samples in different models and CO2 adsorption micropore volumes |
| 表 4 PD模型和LPPD模型对菲和壬基酚吸附的拟合参数 Table 4 The Polanyi-Dubinin(PD) model and LPPD model fitting curves for the adsorption of Phen and NP |
 |
| 图 6 PD、LPPD模型对菲和壬基酚的吸附等温线(D=RTln(Sscl/Ce)/Vs) Fig. 6 The adsorption isotherms of Phen and NP by PD and LPPD models |
1) 对干酪根样品进行活化和氧化实验, 元素分析结果表明, 样品的极性增加;红外图谱表明, 氧化后样品引入了新的含氧官能团;CO2气体吸附则表明, 样品的微孔体积和比表面积发生了变化, 其中, OH、ZH及ZN样品的纳米孔体积和比表面积增加.
2) 对疏水性有机污染物菲和壬基酚进行了吸附模拟实验, 结果表明, 所有样品对菲和壬基酚的吸附均为典型的非线性吸附, 且所有样品对壬基酚的吸附容量参数K′F均大于对菲的K′F值, 非线性吸附因子n值均小于菲的n值.
3) 纳米孔填充模型拟合表明, 活化和氧化的干酪根样品对菲和壬基酚的吸附中, 纳米孔填充机制很重要, 对壬基酚的吸附可能还有氢键作用.一些样品上菲和壬基酚的纳米孔填充体积高于V-CO2, 机理尚不清楚.
Ahmadpour A, Do D D. 1997. The preparation of activated carbon from macadamia nutshell by chemical activation[J]. Carbon, 35(12): 1723–1732.
DOI:10.1016/S0008-6223(97)00127-9
|
Blasi C D, Branca C, Galgano A, et al. 2015. Modifications in the thermicity of the pyrolysis reactions of ZnCl2-loaded wood[J]. Industrial & Engineering Chemistry Research, 54(51): 12741–12749.
|
Chefetz B, Xing B. 2017. Relative role of aliphatic and aromatic moieties as sorption domains for organic compounds: A review[J]. Environmental Science & Technology, 43(6): 1680–1688.
|
Diaz-Terán J, Nevskaia D M, Fierro J L G, et al. 2003. Study of chemical activation process of a lignocellulosic material with KOH by XPS and XRD[J]. Microporous & Mesoporous Materials, 60(1/3): 173–181.
|
Duan D D, Zhang D N, Ran Y, et al. 2016. Chemical and structural characterization of thermally simulated kerogen and its relationship with microporosity[J]. Marine & Petroleum Geology.
DOI:10.1016/j.marpetgeo.2016.12.016
|
Durand B. 1980. Kerogen:Insoluble Organic Matter from Sedimentary Rocks[M]. Paris: Editions Technip.
|
He X, Ling P, Yu M, et al. 2013. Rice husk-derived porous carbons with high capacitance by ZnCl2 activation for supercapacitors[J]. Electrochimica Acta, 105(26): 635–641.
|
Kujawinski E B, Freitas M A, Xu Z, et al. 2002. The application of electrospray ionization mass spectrometry (ESI MS) to the structural characterization of natural organic matter[J]. Organic Geochemistry, 33(3): 171–180.
DOI:10.1016/S0146-6380(01)00149-8
|
Kula I, Uğurlu M, Karaoğlu H, et al. 2008. Adsorption of Cd(Ⅱ) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation[J]. Bioresource Technology, 99(3): 492–501.
DOI:10.1016/j.biortech.2007.01.015
|
Lehmann J, Rillig M C, Thies J, et al. 2011. Biochar effects on soil biota-A review[J]. Soil Biology and Biochemistry, 43(9): 1812–1836.
DOI:10.1016/j.soilbio.2011.04.022
|
Lillo-Ródenas M A, Cazorla-Amorós D, Linares-Solano A. 2003. Understanding chemical reactions between carbons and NaOH and KOH:An insight into the chemical activation mechanism[J]. Carbon, 41(2): 267–275.
DOI:10.1016/S0008-6223(02)00279-8
|
Lillo-Ródenas M A, Ros A, Fuente E, et al. 2008. Further insights into the activation process of sewage sludge-based precursors by alkaline hydroxides[J]. Chemical Engineering Journal, 142(2): 168–174.
DOI:10.1016/j.cej.2007.11.021
|
Liu W J, Jiang H, Yu H Q. 2015. Development of biochar-based functional materials:Toward a sustainable platform carbon material[J]. Chemical Reviews, 115(22): 125–128.
|
Lozano-Castelló D, Calo J M, Cazorla-Amorós D, et al. 2007. Carbon activation with KOH as explored by temperature programmed techniques, and the effects of hydrogen[J]. Carbon, 45(13): 2529–2536.
DOI:10.1016/j.carbon.2007.08.021
|
Lutfalla S, Chenu C, Barré P. 2013. Are chemical oxidation methods relevant to isolate a soil pool of centennial carbon[J]. Biogeochemistry, 118(1): 135–139.
|
Mikutta R, Kaiser K. 2011. Organic matter bound to mineral surfaces:Resistance to chemical and biological oxidation[J]. Soil Biology & Biochemistry, 43(8): 1738–1741.
|
Mohanty K, Das D, Biswas M N. 2005. Adsorption of phenol from aqueous solutions using activated carbons prepared from Tectona grandis sawdust by ZnCl2 activation[J]. Chemical Engineering Journal, 115(1): 121–131.
|
Oliveira L C, Pereira E, Guimaraes I R, et al. 2009. Preparation of activated carbons from coffee husks utilizing FeCl3 and ZnCl2 as activating agents[J]. Journal of Hazardous Materials, 165(1/3): 87–94.
|
Ran Y, Xing B, Rao P S C, et al. 2004. Importance of adsorption (hole-filling) mechanism for hydrophobic organic contaminants on an aquifer kerogen isolate[J]. Environmental Science & Technology, 38(16): 4340–4348.
|
Ran Y, Yang Y, Xing B, et al. 2013. Evidence of micropore filling for sorption of nonpolar organic contaminants by condensed organic matter[J]. Journal of Environmental Quality, 42(3): 806–814.
DOI:10.2134/jeq2012.0286
|
Siregar A, Kleber M, Mikutta R, et al. 2005. Sodium hypochlorite oxidation reduces soil organic matter concentrations without affecting inorganic soil constituents[J]. European Journal of Soil Science, 56(56): 481–490.
|
Song J, Peng P, Huang W. 2002. Black carbon and kerogen in soils and sediments1.Quantification and characterization[J]. Environmental Science & Technology, 36(18): 3960–3967.
|
Song Z, Lian F, Yu Z, et al. 2014. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution[J]. Chemical Engineering Journal, 242: 36–42.
DOI:10.1016/j.cej.2013.12.061
|
Vandenbroucke M, Largeau C. 2007. Kerogen origin, evolution and structure[J]. Organic Geochemistry, 38(5): 719–833.
DOI:10.1016/j.orggeochem.2007.01.001
|
Wang F, Haftka J J, Sinnige T L, et al. 2014. Adsorption of polar, nonpolar, and substituted aromatics to colloidal graphene oxide nanoparticles[J]. Environmental Pollution, 186C: 226–233.
|
王会涛. 2015. KOH活化法制备石油焦基活性炭[D]. 大连: 大连理工大学. 19-20
|
Wang X, Xing B. 2007. Sorption of organic contaminants by biopolymer-derived chars[J]. Environmental Science & Technology, 41(24): 8342–8348.
|
Yang J, Qiu K Q. 2009. Preparation of activated carbon by chemical activation under vacuum[J]. Environmental Science & Technology, 43(9): 3385–3390.
|
Yang J, Qiu K Q. 2010. Preparation of activated carbons from walnut shells via vacuum chemical activation and their application for methylene blue removal[J]. Chemical Engineering Journal, 165(1): 209–217.
DOI:10.1016/j.cej.2010.09.019
|
Zhang D, Ran Y, Cao X, et al. 2015. Biosorption of nonylphenol by pure algae, field-collected planktons and their fractions[J]. Environmental Pollution, 198C: 61–69.
|
Zhang W, Wang L, Sun H. 2011. Modifications of black carbons and their influence on pyrene sorption[J]. Chemosphere, 85(8): 1306–1311.
DOI:10.1016/j.chemosphere.2011.07.042
|
Zhang Y, Ma X, Ran Y. 2014. Sorption of phenanthrene and benzene on differently structural kerogen:Important role of micropore-filling[J]. Environmental Pollution, 185C: 213–218.
|
Zhu D, Kwon S, Pignatello J J. 2005. Adsorption of single-ring organic compounds to wood charcoals prepared under different thermochemical conditions[J]. Environmental Science & Technology, 39(11): 3990–3998.
|
张代男. 2015. 不同类型天然有机质的结构组成及其与菲和壬基酚的吸附行为的关系[D]. 北京: 中国科学院大学. 6, 94-95
http://cdmd.cnki.com.cn/Article/CDMD-80165-1015361477.htm |
 2017, Vol. 37
2017, Vol. 37


