2. 农业微生物技术教育部工程研究中心, 哈尔滨 150500
2. Engineering Research Center of Agricultural Microbiology Technology, Ministry of Education, Harbin 150500
漆酶(EC1.10.3 .2)是一种古老的氧化还原蛋白, 在自然界高等动、植物中都有存在, 因最早发现于日本紫胶漆树(Rhus verniciflera)中而得名(Yoshida, 1883), 属于蓝多铜氧化酶家族(Blue Multi-Copper Oxidase Family).漆酶底物宽泛, 可催化多种结构复杂底物的氧化, 同时伴随一步四电子反应生成水.漆酶在生物传感(Tominaga et al., 2016;Battista et al., 2017) 、污染修复(Murugesan et al., 2010) 、制浆漂白(Gupta et al., 2015;Nogueira et al., 2015) 、果汁澄清(Lettera et al., 2016)等领域有广泛应用.尤其是在染料脱色中, 漆酶作用的生色基团结构多样, 催化反应效率高, 对环境无二次污染, 相关工作一直是国内外环保领域的研究热点(Campos et al., 2016; Dai et al., 2016) .
真菌漆酶具有来源广、产量高、作用条件温和、底物宽泛等优点, 目前常用于漆酶理论与应用研究, 如担子菌(Basidiomycete)中的白灵侧耳(Pleurotus nebrodensis)(Yuan et al., 2016) 、蜂窝菌属Hexagonia hirta (Kandasamy et al., 2016) 、子囊菌(Ascomycete)中的深绿木霉(Trichoderma atroviride)(Adnan et al., 2015)和腐皮镰刀菌(Fusarium solani) X701(Wu et al., 2015)等.近年来, 半知真菌因生活史简单、发酵周期短、易于遗传改造等优点, 成为漆酶研究的新热点, 如拟盘多毛孢属菌株(Pestalotiopsis sp.) J63(Feng et al., 2013) 、棘孢木霉(Trichoderma asperellum)(陈今朝等, 2010)等, 但产酶活性普遍低于上述提到的担子菌和子囊菌.提高半知真菌产漆酶水平并开展应用探索, 对于拓展微生物漆酶来源、促进漆酶发酵及应用的工业化进程具有积极意义.
课题组前期已从伊春凉水国家级自然保护区土壤样品中分离到1株产漆酶半知真菌疣孢漆斑菌(Myrothecium verrucaria) NF-08, 通过单因素试验确定了产酶最优的培养基组分、发酵条件和诱导物浓度.该菌株产酶周期短、活性高, 培养6 d胞外漆酶活性即可达到16.82 U·mL-1(高冬妮等, 2015) . 本文在此基础上, 为提高M.verrucaria NF-08产酶水平, 探究其漆酶应用前景, 采用响应面方法优化该菌株产酶条件, 并考察该漆酶的粗酶浓度、反应温度和pH值对3种结构类型6种染料脱色效果的影响.以期为该菌株漆酶的工艺优化和染料脱色应用奠定基础.
2 材料与方法 (Materials and methods) 2.1 材料 2.1.1 供试菌株疣孢漆斑菌(M.verrucaria) NF-08分离自凉水国家级自然保护区土壤样品(高冬妮等, 2015) .
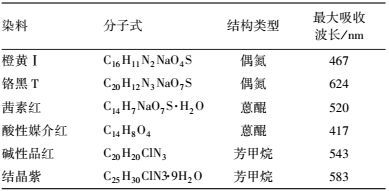
2.1.2 染料信息本研究中使用的染料信息如表 1所示.
| 表 1 本研究中使用的染料信息 Table 1 Dye information in this research |
PDA培养基:马铃薯20 g·L-1, 葡萄糖20 g·L-1, 琼脂20 g·L-1, pH自然, 用于供试菌活化及种子液制备;产酶培养基:马铃薯20 g·L-1, 葡萄糖40 g·L-1, 蛋白胨35 g·L-1, 没食子酸0.05 mmol·L-1, Cu2+ 0.1 mmol·L-1, pH=7.0.
2.2 方法 2.2.1 漆酶活性测定发酵液于10000 r·min-1下离心10 min, 上清液即为粗酶液.漆酶活性测定在Niladevi等(2009) 方法的基础上改良, 反应体系为适当稀释的粗酶液50 μL, 1 mmol·L-1的ABTS 1 mL, pH=4.0的磷酸氢二钠-柠檬酸缓冲液2.95 mL. 1个酶活单位(U)定义为每分钟催化1 μmol ABTS氧化的酶量.
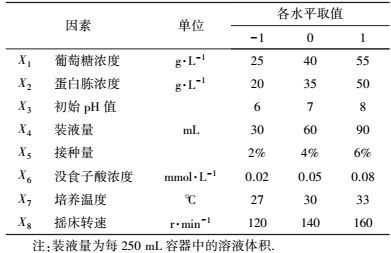
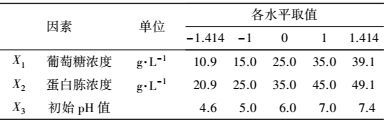
2.2.2 响应面试验利用Design Expert (Version 7.0.0, Stat-Ease, Inc.)软件进行试验设计及数据分析.依据高冬妮等(2015) 的方法, 进行供试菌活化、种子液制备并确定筛选试验的因素及水平(表 2), 以培养基组分和发酵条件相关参数为变量, 以漆酶活性为响应值, 采用两水平析因设计筛选对产漆酶影响显著的因素.考察葡萄糖浓度、蛋白胨浓度、初始pH值、装液量、接种量、没食子酸浓度、培养温度和摇床转速对供试菌产漆酶的影响, 确定影响显著因素.在此基础上, 利用中心组合设计确定显著因素的最优水平并进行验证.
| 表 2 两水平析因设计因素及水平 Table 2 The factors and levels in two level factorial design |
以2.2.2节中得到的最优发酵条件制备供试菌漆酶粗酶液.脱色反应体系体积3 mL, 缓冲体系为pH=4.0的磷酸-柠檬酸缓冲液, 粗酶终浓度为5 U·mL-1, 染料浓度为20 mg·mL-1, 25 ℃反应24 h.分别考察粗酶终浓度为2、5、8 U·mL-1, 温度为15、25、35 ℃, pH值为4、7和10时, 粗酶对表 1中3种结构类型6种染料的脱色效果.脱色率η的计算公式见式(1) .
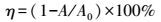
|
(1) |
式中, A为脱色反应后吸光值, A0为反应前吸光值, 测定波长见表 1.
2.2.4 数据处理每个试验处理均设置3个重复, 数据以均值±标准差形式表示, 统计检验的显著水平设定为0.05, 极显著水平设定为0.01. 利用JMP (Version 9.0.2, SAS, Inc.)软件进行方差分析及多重比较, 并用Sigmaplot (Version 10.0, Systat Software, Inc.)软件绘图.
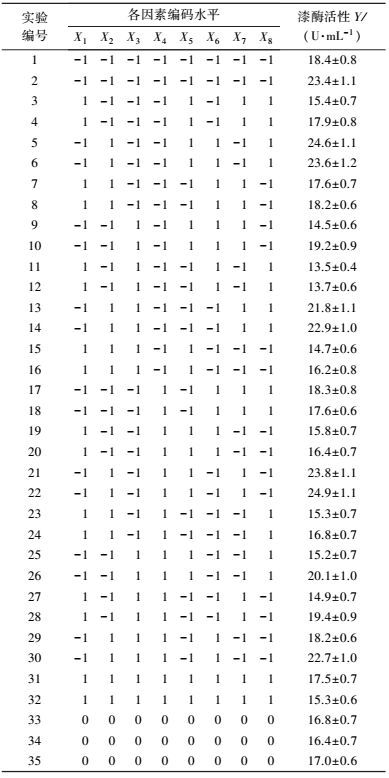
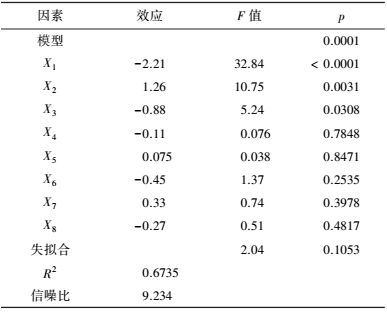
3 结果(Results) 3.1 供试菌M.verrucaria NF-08培养条件优化 3.1.1 显著因素筛选结果在对M.verrucaria NF-08产漆酶单因素条件研究的基础上(高冬妮等, 2015), 通过两水平析因设计筛选对产酶影响显著的因素, 变量的实际取值和编码水平如表 2所示, 试验设计和结果如表 3所示.回归分析结果如表 4所示, 两水平析因试验模型项极显著(p<0.0001<0.01), 失拟合项不显著(p=0.1053>0.05), R2=0.6735, 信噪比为9.234(大于4), 表明模型能够很好地拟合试验数据.各变量对响应值影响的显著程度依次为:X1>X2>X3>X6>X7>X8>X4>X5, 即葡萄糖浓度、蛋白胨浓度和初始pH值对M.verrucaria NF-08产漆酶分别有极显著(p<0.0001<0.01) 、极显著(p=0.0031<0.01)和显著(p=0.0308<0.05) 影响, 其他变量在本研究条件下对发酵液漆酶活性无显著影响, 后续研究依据表 2中心点水平取值. 变量为编码水平时, 模型拟合方程为:
| 表 3 两水平析因设计试验结果 Table 3 The experimental results of two level factorial design |
| 表 4 两水平析因设计分析结果 Table 4 The analysis results of two level factorial design |
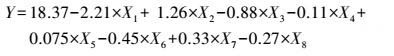
|
(2) |
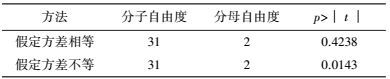
将表 3中的试验结果进行t检验, 比较试验点(处理1~32) 与中心点(处理33~35) 响应值间是否存在显著差异.等方差检验(表 5) 表明, 二者之间方差非齐性即p=0.0093<0.05, 因此, 根据表 6中方差不等结果即p=0.0143<0.05判定, 两水平析因试验中选取的中心点已接近最大响应区, 无需进行爬坡试验.
| 表 5 等方差检验 Table 5 Equality of variances |
| 表 6 t-检验结果 Table 6 The result of t-test |
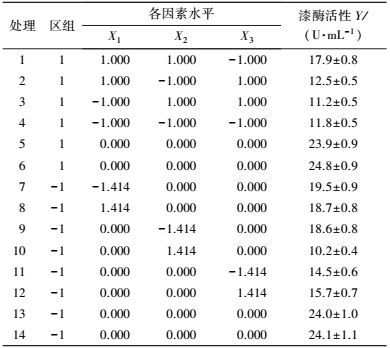
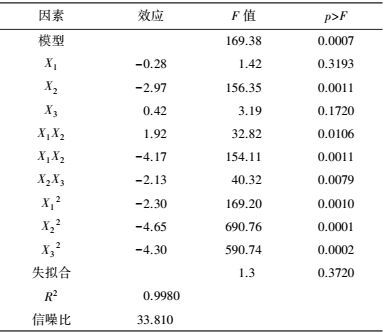
中心组合试验设计因素及水平如表 7所示, 试验结果见表 8. 由回归分析(表 9) 可知, 模型项显著(p=0.0007<0.05), 失拟合项不显著(p=0.3720>0.05), R2=0.9980, 信噪比为33.810(大于4), 表明模型能够很好地拟合试验数据. 除葡萄糖浓度和初始pH值一次项外, 蛋白胨浓度一次项和3个试验因素的二次项及交互项对产酶均有显著影响.
| 表 7 中心组合设计因素及水平 Table 7 The factors and levels in central composite design |
| 表 8 中心组合设计试验结果 Table 8 The experimental results of central composite design |
| 表 9 中心组合设计分析结果 Table 9 The analysis results of center composite design |
各因素及交互作用对M.verrucaria NF-08产漆酶影响如图 1所示.变量为编码水平时, 模型拟合方程如式(3) 所示.其中, 二次项系数均为负值, 可知方程有极大值, 拟合曲面开口朝下.预测葡萄糖浓度(X1)为15.4 g·L-1、蛋白胨浓度(X2)为43.3 g·L-1、初始pH值(X3)为6.68时, 漆酶活性达到最大值25.43 U·mL-1.
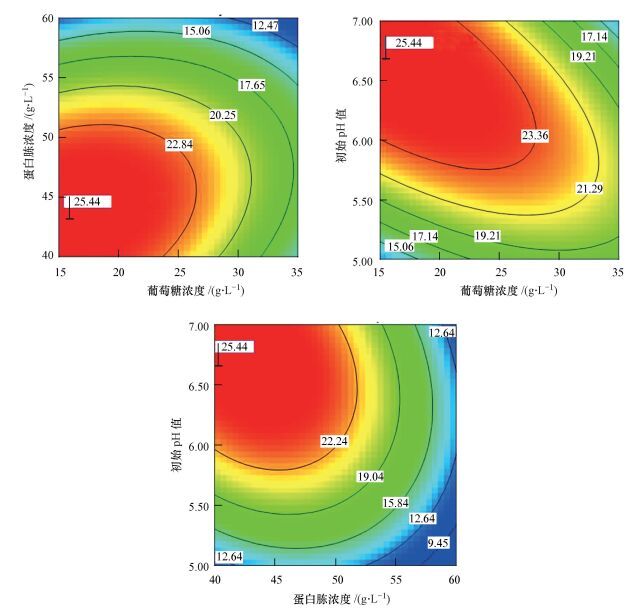 |
| 图 1 显著影响M.verrucaria NF-08产漆酶的各因素交互作用等高线图 (旗标表示响应面模型预测的漆酶活性最大值, U·mL-1) Fig. 1 Contour plotted in interaction of factors significantly influcing the laccase production by M.verrucaria NF-08 |
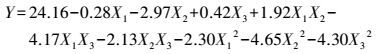
|
(3) |
选取中心组合试验中模型极大值时各因素对应的水平取值进行六处理重复验证试验, 酶活为(25.58±1.60) U·mL-1, 与预测值25.43 U·mL-1无显著差异(统计结果未列), 即中心组合试验优化模型拟合性良好, 结果可靠. 本研究中响应面法优化后, M.verrucaria NF-08产酶水平是单因素试验后(16.82 U·mL-1)的1.52倍, 提高了52.1%.
3.2 供试菌M.verrucaria NF-08染料脱色作用 3.2.1 酶浓度对脱色率的影响如图 2a所示, 就染料结构类型而言, M.verrucaria NF-08漆酶粗酶对偶氮类染料橙黄Ⅰ和铬黑T的脱色效果优于芳甲烷类染料碱性品红和结晶紫, 而对蒽醌类染料茜素红和酸性媒介红脱色效果最差.6种供试染料的脱色率均随粗酶浓度增加而升高.在本研究选取的3个粗酶浓度条件下, 橙黄Ⅰ、酸性媒介红和结晶紫脱色率升高幅度显著.当粗酶浓度达到5 U·mL-1时, 铬黑T和碱性品红脱色率已接近完全脱色(脱色率分别为99.2%和94.6%), 茜素红脱色率为12.6%, 当粗酶浓度增加至8 U·mL-1时, 三者脱色率不再显著升高.
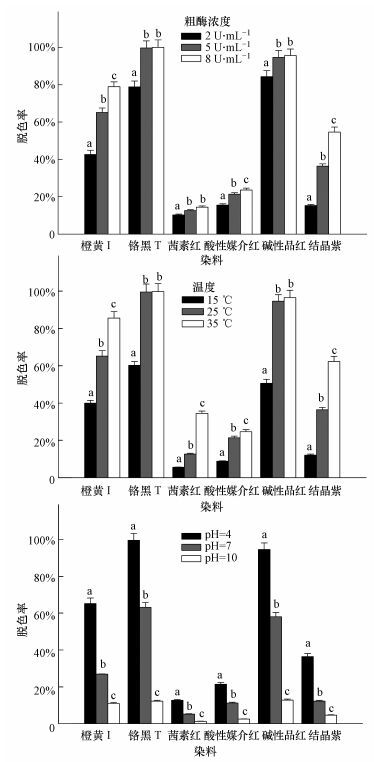 |
| 图 2 M.verrucaria NF-08漆酶粗酶浓度(a)、温度(b)和pH值(c)对染料脱色的影响 (不同字母代表不同因素值对同一染料脱色率有显著差异) Fig. 2 The effects of concentration of crude laccase from M.verrucaria NF-08(a), temperatures (b) and pH values (c) on dye decolorization by crude laccase from M.verrucaria NF-08(Different letters indicate the significant differences among different factor values) |
温度对M.verrucaria NF-08漆酶粗酶染料脱色的影响研究表明(图 2b), 6种供试染料脱色率均随温度升高而上升.反应温度为15 ℃时, 6种供试染料脱色率均低于60%.温度上升至25 ℃时, 所有供试染料脱色率均有显著提升, 其中, 铬黑T和碱性品红分别达到99.2%和94.6%, 且不再随温度升高而上升.当反应温度升高至35 ℃时, 另外4种染料脱色率均进一步显著增高, 幅度较大的是橙黄Ⅰ和结晶紫, 分别达到85.6%和62.3%.
3.2.3 pH值对脱色率的影响如图 2c所示, 6种供试染料脱色率均随反应体系pH值升高而显著降低. 在酸性条件下即pH值为4时, 铬黑T和碱性品红达到最大脱色率, 分别为99.2%和94.6%;在碱性条件即pH值为10时, 铬黑T和碱性品红脱色率分别降至12.2%和12.8%.对于其他4种染料橙黄Ⅰ、茜素红、酸性媒介红和结晶紫, 当反应体系从中性条件(pH=7) 转换至碱性条件, 脱色率均显著降至30%以下, 碱性条件pH为10时, 均降至10%以下.
4 讨论(Discussion)响应面法是微生物发酵研究中常用的试验设计(Design of Experiment, DOE)方法, 通过多元二次回归构建曲面模型, 获得最优工艺参数.相比传统的单因素及正交试验等发酵条件研究方法, 响应面法具有规模小、周期短、成本低等优越性, 近年来也见于漆酶微生物发酵优化的报道中.例如, Jegatheesan等(2015) 以分离自土壤样品的漏斗稜孔菌(Polyporus arcularius)为供试菌, 通过PB (Plackett-Burman)设计和中心组合设计, 获得最优酵母提取物和铜离子浓度, 在发酵15 d时漆酶产量从9.3 U·mL-1提高至28.3 U·mL-1. Aslam等(2016) 报道了P.nebrodensis WC850发酵木质纤维素类固体底物产漆酶的响应面优化过程, 在最优pH值、温度、接种量、发酵时间和湿度条件下, 酶活提高了18.3%.埃及学者通过响应面法研究来自海洋朽木的细极链格孢菌(Alternaria tenuissima) KM651985产漆酶条件, 优化培养基组分和诱导物, 产酶水平比初始条件提高了6.33倍(El Aty et al., 2016) . 本研究结果表明, 响应面法也是优化M.verrucaria NF-08产酶的有效方法, 漆酶活性从单因素试验后的16.82 U·mL-1增加到25.58 U·mL-1, 提高幅度显著, 高于目前文献报道的半知真菌产酶水平, 也高于本课题组同期分离的同种不同株的M.verrucaria NF-05的产酶水平19.94 U·mL-1 (Zhao et al., 2015) .
染料按化学结构可分为硝基、偶氮、蒽醌、靛族、芳甲烷等多种类型, 多为难降解的芳香族杂环类化合物, 造成了巨大的环境尤其是水体污染.漆酶底物宽泛, 通过氧化染料生色基团使其脱色.产酶微生物全细胞脱色虽然成本低(郑楠等, 2010;Jin et al., 2013), 但易带来微生物二次污染, 而纯化或商品漆酶脱色虽然效果好(Campos et al., 2016; Dai et al., 2016), 但成本过高.因此, 很多学者尝试无细胞发酵液上清即粗酶液染料脱色研究.隔孢伏革菌属真菌Peniophora sp.NFCCI-2131漆酶粗酶液能够在氧化还原介体ABTS和HBT (1-羟基苯并三唑, 1-hydroxybenzotrizole)存在时, 对5种染料(偶氮类氨基黑和甲基橙、芳甲烷类结晶紫和亮绿、噻嗪类亚甲基蓝)有效脱色(Shankar et al., 2015) .Jin等(2013) 发现, 烟曲霉(A. fumigatus) AF1粗酶液对10种染料尤其是偶氮类染料(脱色率74.2%~98.5%)有很好的脱色效果.上述研究表明, 染料结构类型(及具体种类)与脱色率之间并无直接相关性, 与本研究结果一致.
温度与pH值直接影响蛋白活性, 因而也是漆酶染料脱色效果中重点考察的2个因素.漆酶对染料脱色是酶促反应过程, 因此, 最大脱色率的获得条件往往与蛋白最适作用条件一致, 且当染料浓度与酶浓度达到相对饱和后, 脱色率不再随酶浓度的增加而增加.Sridhar等(2013) 进行硬孔菌属真菌(Rigidoporus sp.)漆酶粗酶对8种不同结构类型染料脱色时发现, 在该酶最适作用pH值下, 温度是最显著的影响因素, 高温引起的蛋白变性失活是导致脱色率下降的直接原因, 其次是染料浓度和反应时间.Yesilada等(2014) 的研究表明, 在温度30~50 ℃、pH值4.5~6.0的条件下, 硬毛粗毛盖菌(Funalia trogii) ATCC 200800漆酶粗酶对偶氮类染料活性黑5和蒽醌类染料活性蓝171的脱色率达到65%以上, 且随酶浓度增大而增大.本研究从工业应用角度出发, 选取较为温和的研究条件, M.verrucaria NF-08在中温(25~35 ℃)、酸性至中性(pH为4~7) 条件下能够有效脱色的染料结构多样, 种类丰富.课题组前期获得的同种不同株M.verrucaria NF-05纯化漆酶, 最适温度和pH值分别为40 ℃和4.0, 在本研究条件即25~35 ℃和pH值为4~7条件下温浴1 h后, 蛋白活性保持在50%以上(Zhao et al., 2012) .M.verrucaria NF-08胞外漆酶的纯化与性质研究工作正在进行中, 下一步将验证蛋白最适作用及稳定条件与获得最高染料脱色率的温度和pH值条件是否具有一致性, 并拓宽参数范围以期获得更高的染料脱色率. M.verrucaria NF-08漆酶粗酶制备周期短、作用范围广、脱色反应成本低且条件温和, 在染料污染的水体及环境减毒修复领域展现出良好的工业应用潜力.
5 结论(Conclusions)1) 以M.verrucaria NF-08为供试菌株, 采用响应面方法通过三步试验设计优化菌株产漆酶条件.两水平析因试验设计结果表明, 葡萄糖浓度、蛋白胨浓度和培养基初始pH值对M. verrucaria NF-08产漆酶活性有显著影响.中心组合设计预测培养基中葡萄糖为15.4 g·L-1、蛋白胨为43.3 g·L-1、初始pH值为6.68时, 漆酶活性达到最大值25.43 U·mL-1.六处理重复验证试验得出供试菌发酵液漆酶活性为(25.58±1.60) U·mL-1. M.verrucaria NF-08产酶水平是单因素试验后(16.82 U·mL-1)的1.52倍, 提高了52.1%.
2) M.verrucaria NF-08漆酶粗酶对3种结构类型(偶氮类、蒽醌类和芳甲烷类)6种染料均有脱色效果, 且为偶氮类优于芳甲烷类优于蒽醌类. 脱色率随粗酶浓度增大(2~8 U·mL-1)、反应温度升高(15~35 ℃)而上升, 随pH值上升(4~10) 而下降.粗酶浓度为5 U·mL-1, 反应温度为25 ℃, pH值为4时, 偶氮类染料铬黑T和芳甲烷类染料碱性品红即可几乎完全脱色, 脱色率分别为99.2%和94.6%.
| [${referVo.labelOrder}] | Adnan L A, Sathishkumar P, Yusoff A R M, et al. 2015. Metabolites characterisation of laccase mediated Reactive Black 5 biodegradation by fast growing ascomycete fungus Trichoderma atroviride F03[J]. International Biodeterioration & Biodegradation, 104: 274–282. |
| [${referVo.labelOrder}] | Aslam S, Asgher M, Hussain F, et al. 2016. Exploration of optimum operating conditions for enhanced laccase enzyme production by Pleurotus nevrodensis WC 850 through response surface methodology[J]. Journal of Animal and Plant Sciences, 26(3): 794–804. |
| [${referVo.labelOrder}] | Battista E, Lettera V, Villani M, et al. 2017. Enzymatic sensing with laccase-functionalized textile organic biosensors[J]. Organic Electronics, 40: 51–57. DOI:10.1016/j.orgel.2016.10.037 |
| [${referVo.labelOrder}] | 陈今朝, 王剑锋, 李江, 等. 2010. 煤附生真菌产漆酶菌株的分离鉴定及产酶特性研究[J]. 菌物学报, 2010, 29(3): 389–396. |
| [${referVo.labelOrder}] | Campos P A, Levin L N, Wirth S A. 2016. Heterologous production,characterization and dye decolorization ability of a novel thermostable laccase isoenzyme from Trametes trogii BAFC 463[J]. Process Biochemistry, 51(7): 895–903. DOI:10.1016/j.procbio.2016.03.015 |
| [${referVo.labelOrder}] | Dai J A, Wang H X, Chi H, et al. 2016. Immobilization of laccase from Pleurotus ostreatus on magnetic separable SiO2 support and excellent activity towards azo dye decolorization[J]. Journal of Environmental Chemical Engineering, 4(2): 2585–2591. DOI:10.1016/j.jece.2016.04.037 |
| [${referVo.labelOrder}] | El Aty A A A, Hamed E R, Ahmed A, et al. 2016. Induction and enhancement of the novel marine-derived Alternaria tenuissima KM651985 laccase enzyme using response surface methodology:application to azo and triphenylmethane dyes decolorization[J]. Journal of Applied Pharmaceutical Science, 6(4): 6–14. |
| [${referVo.labelOrder}] | Feng X Y, Chen H Y, Xue D S, et al. 2013. Enhancement of laccase activity by marine-derived deuteromycete Pestalotiopsis sp.J63 with agricultural residues and inducers[J]. Chinese Journal of Chemical Engineering, 21(10): 1182–1189. DOI:10.1016/S1004-9541(13)60567-4 |
| [${referVo.labelOrder}] | Gupta V, Garg S, Capalash N, et al. 2015. Production of thermo-alkali-stable laccase and xylanase by co-culturing of Bacillus sp.and B.halodurans for biobleaching of kraft pulp and deinking of waste paper[J]. Bioprocess and Biosystems Engineering, 38(5): 947–956. DOI:10.1007/s00449-014-1340-0 |
| [${referVo.labelOrder}] | 高冬妮, 范晓旭, 赵丹. 2015. 产漆酶半知真菌Myrothecium verrucaria NF-08菌株的分离及产酶研究[J]. 林业科学, 2015, 51(1): 80–87. |
| [${referVo.labelOrder}] | Jegatheesan M, Eyini M. 2015. Response surface methodology mediated modulation of laccase production by Polyporus arcularius[J]. Arabian Journal for Science and Engineering, 40(7): 1809–1818. DOI:10.1007/s13369-014-1499-3 |
| [${referVo.labelOrder}] | Jin X C, Ning Y. 2013. Laccase production optimization by response surface methodology with Aspergillus fumigatus AF1 in unique inexpensive medium and decolorization of different dyes with the crude enzyme or fungal pellets[J]. Journal of Hazardous Materials, 262: 870–877. DOI:10.1016/j.jhazmat.2013.09.024 |
| [${referVo.labelOrder}] | Kandasamy S, Muniraj I K, Purushothaman N, et al. 2016. High level secretion of laccase (LccH) from a newly isolated white-rot Basidiomycete,Hexagonia hirta MSF2[J]. Frontiers in Microbiology, 7(38): 1–12. |
| [${referVo.labelOrder}] | Lettera V, Pezzella C, Cicatiello P, et al. 2016. Efficient immobilization of a fungal laccase and its exploitation in fruit juice clarification[J]. Food Chemistry, 196: 1272–1278. DOI:10.1016/j.foodchem.2015.10.074 |
| [${referVo.labelOrder}] | Murugesan K, Chang Y Y, Kim Y M, et al. 2010. Enhanced transformation of triclosan by laccase in the presence of redox mediators[J]. Water Research, 44(1): 298–308. DOI:10.1016/j.watres.2009.09.058 |
| [${referVo.labelOrder}] | Niladevi K N, Sukumaran R K, Jacob N, et al. 2009. Optimization of laccase production from a novel strain-Streptomyces psammoticus using response surface methodology[J]. Microbiological Research, 164(1): 105–113. DOI:10.1016/j.micres.2006.10.006 |
| [${referVo.labelOrder}] | Nogueira S D C P, de Almeida Batini J H, Michelin M, et al. 2015. Laccase production by Aspergillus niveus on SSF using wheat bran as alternative carbon source and its synergistic effect on pulp biobleaching using a mix of laccase/xylanase from the same microorganism[J]. Journal of Biochemical Technology, 6(2): 929–937. |
| [${referVo.labelOrder}] | Shankar S, Nill S. 2015. Effect of metal ions and redox mediators on decolorization of synthetic dyes by crude laccase from a novel white rot fungus Peniophora sp.(NFCCI-2131)[J]. Applied Biochemistry and Biotechnology, 175(1): 635–647. DOI:10.1007/s12010-014-1279-2 |
| [${referVo.labelOrder}] | Sridhar S, Chinnathambi V, Arumugam P, et al. 2013. In silico and in vitro physicochemical screening of Rigidoporus sp.crude laccase-assisted decolorization of synthetic dyes-approaches for a cost-effective enzyme-based remediation methodology[J]. Applied Biochemistry and Biotechnology, 169(3): 911–922. DOI:10.1007/s12010-012-0041-x |
| [${referVo.labelOrder}] | Tominaga M, Sasaki A, Togami M. 2016. Bioelectrocatalytic oxygen reaction and chloride inhibition resistance of laccase immobilized on single-walled carbon nanotube and carbon paper electrodes[J]. Electrochemistry, 84(5): 315–318. DOI:10.5796/electrochemistry.84.315 |
| [${referVo.labelOrder}] | Wu Z, Si W, Xue H, et al. 2015. Molecular characterization and expression profiling of novel laccase isoenzyme genes from Fusarium solani X701,a wood-rotting ascomycete[J]. International Biodeterioration & Biodegradation, 104: 123–128. |
| [${referVo.labelOrder}] | Yesilada O, Birhanli E, Ercan S, et al. 2014. Reactive dye decolorization activity of crude laccase enzyme from repeated-batch culture of Funalia trogii[J]. Turkish Journal of Biology, 38(1): 103–110. |
| [${referVo.labelOrder}] | Yoshida H. 1883. Chemistry of lacquer (urushi)[J]. Journal of Chemical Society, 43: 472–486. DOI:10.1039/CT8834300472 |
| [${referVo.labelOrder}] | Yuan X H, Tian G T, Zhao Y C, et al. 2016. Biochemical characteristics of three laccase isoforms from the Basidiomycete Pleurotus nebrodensis[J]. Molecules, 21(2): 294–297. |
| [${referVo.labelOrder}] | Zhao D,Sun Y Y,Du R P,et al.2015.Optimization of fermentation parameters for laccase production by a novel deuteromycete fungus Myrothecium verrucaria NF-05 using response surface methodology[C].International Conference on Civil,Materials and Environmental Sciences.London.425-427 |
| [${referVo.labelOrder}] | Zhao D, Zhang X, Cui D Z, et al. 2012. Characterisation of a novel white laccase from the deuteromycete fungus Myrothecium verrucaria NF-05 and its decolourisation of dyes[J]. PloS One, 7(6). |
| [${referVo.labelOrder}] | 郑楠, 赵敏, 梅丽艳, 等. 2010. 新月弯孢霉菌丝球对染料脱色作用的研究[J]. 菌物学报, 2010, 29(5): 745–752. |
 2017, Vol. 37
2017, Vol. 37