每年纺织、造纸、制革等行业会产生大量染料废水,若不经处理直接排放,将对环境造成严重危害 (Eren, 2012).其中,偶氮染料 (—N=N—) 是使用最多的商业染料,占染料总量的50%,具有毒性强、含盐量高、致突变、致癌、难降解等特点 (Ji et al., 2009;Xu et al., 2010).偶氮染料废水的常用处理方法有吸附 (Gupta et al., 2011)、膜过滤 (Anipsitakis et al., 2003)、光催化 (Saleh et al., 2012; Khataee et al., 2009)、臭氧化 (Bai et al., 2011; Faria et al., 2009) 等.其中,吸附和膜过滤虽然可以使偶氮染料废水脱色,但无法使偶氮染料降解和矿化,光催化和臭氧化能够氧化降解偶氮染料,但操作过程中都存在一些不足.
近年来,基于硫酸根自由基 (SO4-·) 的高级氧化技术对偶氮染料氧化降解作用明显 (Anipsitakis et al., 2003; Rastogi et al., 2009).硫酸根自由基 (SO4-·) 具有较高的氧化还原电位 (E0=2.5~3.1 V),可以氧化降解大部分有机污染物 (Yang et al., 2011),相比于羟基自由基 (HO·) 对溶液具有更宽的pH适用范围,且半衰期较长,有利于水中污染物的去除.过一硫酸盐是产生SO4-·的常用氧化剂,常温下比较稳定不易分解,在加热 (Waldemer et al., 2007; Liang et al., 2008; Ghauch et al., 2012)、紫外光照射 (Gao et al., 2012; He et al., 2013)、过渡金属离子 (Kusic et al., 2011; Rodriguez et al., 2014; Yang et al., 2015; Anipsitakis et al., 2003)、活性炭 (Oh et al., 2015; Lee et al., 2013) 等条件下,能够被活化产生SO4-·.但这些方法都存在一些缺点,如耗能和经济成本高、操作过于复杂、会造成二次污染等,从而限制了它们的应用.有学者发现,用活性碳纤维 (ACF) 能加速活化PMS产生SO4-·降解偶氮染料废水 (Yang et al., 2015),由于ACF经济成本低,因而具有很大优势.同时,有研究证明,超声波能促进活化PMS产生SO4-·(Cai et al., 2014; Gayathri et al., 2010),但由于超声波作用较小,因此,催化降解效果不明显.
本实验通过ACF/US协同活化PMS降解AO7,研究AO7降解的主要影响因素 (ACF投加量、PMS浓度、US功率、初始pH、Cl-浓度),并对各反应条件对降解反应的影响进行分析.
2 材料与方法 (Materials and methods) 2.1 材料试剂与实验设备活性碳纤维 (ACF) 购于江苏苏通碳纤维有限公司,自行处理成5 mm的块状备用;过一硫酸盐 (HKSO5·0.5KHSO4·0.5K2SO4,PMS) 购于Sigma-Aldrich;酸性橙7(AO7) 购于国药集团化学试剂有限公司;盐酸 (HCl)、硫酸 (H2SO4)、苯酚 (Phenol)、氯化钠 (NaCl) 均为分析纯,购于国药集团化学试剂有限公司;实验室用水为去离子水.实验用超声波仪购于昆山市超声仪器有限公司 (KQ-200KDB型),实验用搅拌装置购于上海标本模型厂 (JB50-D型).
2.2 降解实验在一定温度下,将250 mL AO7溶液注入烧杯,并保持AO7浓度为20 mg·L-1.加入一定量的PMS,用稀H2SO4或NaOH调节pH值,随后将一定量的ACF迅速加入到反应烧杯中并启动搅拌器 (转速300 r·min-1),同时立即启动超声波仪,记为反应开始时间.在预定反应时间间隔快速取样5 mL,用0.45 μm滤膜过滤留待后续测量.
2.3 分析方法AO7浓度利用TU-1810紫外可见分光光度计测定,于AO7最大吸收波长484 nm处测量其吸光度,代入标准曲线求得对应染料浓度.AO7矿化率采用总有机碳分析仪 (TOC-LCPH,岛津) 测定.反应体系内自由基检测利用Perkinelmer LS55荧光分光光度计测定,荧光吸收波长为425 nm,激波长为315 nm.降解产物采用GC/MS测试分析 (Agilent 7890A/C),测试前现对反应样品预处理,预处理过程如下:取50 mL反应液并加入抑制剂淬灭后,加入30 mL二氯甲烷萃取3次,萃取液用无水硫酸钠脱水,旋转蒸发至1 mL后,利用GC/MS分析测定.
3 结果与讨论 (Results and discussion) 3.1 FT-IR分析活性炭纤维在催化反应前后的FT-IR谱图如图 1所示,3430 cm-1处为O—H伸缩振动吸收峰 (O′Reilly et al., 1983),1730 cm-1附近的吸收峰是ACF表面羧基及内酯基中的C=O特征伸缩振动峰 (Demir Cakan et al., 2009; El Hendawy, 2006),1170 cm-1处可归于CH2—O—CH2中的C—O对称伸缩振动峰 (Sun et al., 2004; Fierro et al., 2007).从图 1可以看出,反应后,ACF表面官能团在3430 cm-1处有所减小,证明反应前后ACF表面O—H振动吸收峰可能活化PMS降解AO7.
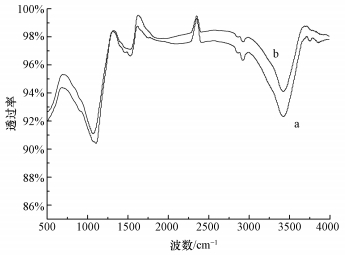 |
| 图 1 ACF反应前后的FT-IR图谱 (a.反应前; b.反应后) Fig. 1 FT-IR spectra of the ACF (a.before the reaction; b.after the reaction) |
图 2显示了不同体系下AO7的脱色效果,结果表明,PMS单独存在时,AO7几乎不降解;加入超声波后,AO7在30 min内降解了9.8%,说明超声波对PMS有一定的活化作用;反应体系中仅有ACF进行吸附时,30 min内AO7浓度减小了62.4%;用ACF或GAC (颗粒活性炭) 活化PMS时,30 min内AO7分别降解了80.5%和25.0%;而加入US后,与ACF或GAC共同活化PMS时,AO7的脱色率分别为100%和51.7%,表明在有无US的条件下,ACF活化PMS的效果都要高于GAC;在加入US后,ACF活化PMS的效果最好,远超US和ACF单独活化PMS体系,说明US协同ACF活化PMS降解AO7效果显著.分析原因可能是,US在水中作用产生超声空化,形成瞬间的高温高压作用于ACF的微孔和表面,使得ACF微孔结构改变,导致ACF比表面积变大,其表面活性位点与AO7接触的机会变多,速度变快,同时其表面活性位点数量也增多,更有利于活化PMS产生SO4-·.而与GAC相比,ACF对PMS的活化效果更好,原因可能是ACF是一个高度多孔碳质材料,具有更大的比表面积和更多的活性点位 (Yao et al., 2013).
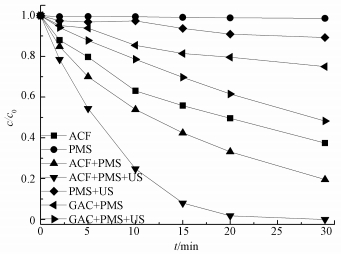 |
| 图 2 不同体系中AO7的降解效果 ([AO7]=20 mg·L-1,pH=7,[ACF]=0.3 g·L-1,[GAC]=0.3 g·L-1,n(PMS)/n(AO7)=20/1,US功率10 W·cm-2) Fig. 2 Degradation of AO7 in different systems |
ACF投加量对AO7降解效果的影响如图 3所示.从图中可知,在US/ACF/PMS体系中,随着ACF投加量的增大,AO7的降解速率加快.投加量为0.05 g·L-1时,30 min时AO7的降解率为50%;投加量为0.3 g·L-1时,AO7在30 min时完全降解;继续增大投加量为0.6 g·L-1时,AO7在10 min就可完全降解.这主要是因为随着ACF投加量增大,有更多的活化点位活化PMS,产生自由基降解偶氮染料.
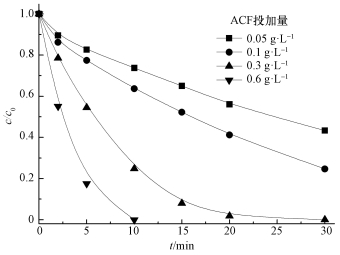 |
| 图 3 ACF投加量对AO7降解的影响 ([AO7]=20 mg·L-1,pH=7,n(PMS)/n(AO7)=20/1,US功率10 W·cm-2) Fig. 3 Effect of ACF dosage on the removal of AO7 |
不同PMS浓度对AO7降解的影响如图 4所示.相比于US/ACF体系,US/ACF/PMS体系氧化降解AO7的速率明显提高,当n(PMS)/n(AO7)=1/1~100/1时,30 min后AO7脱色率均达到100%,这说明反应过程中PMS浓度对AO7的降解反应有明显的作用.同时,当n(PMS)/n(AO7) 由1/1增加到40/1时,AO7降解速度显著加快.由此得出,在一定PMS浓度范围内,随着n(PMS)/n(AO7) 增大,活化降解AO7速率越快.但当PMS浓度过高时 (本实验中取n(PMS)/n(AO7)=100/1),反应速率没有进一步增大,且与n(PMS)/n(AO7)=40/1相比有稍许的抑制作用,原因可能是当PMS浓度过高时,会产生大量的自由基相互猝灭,使AO7降解速率减缓 (Yang et al., 2011).
 |
| 图 4 PMS浓度对AO7降解的影响 ([AO7]=20 mg·L-1,pH=7,[ACF]=0.3 g·L-1,US功率10 W·cm-2) Fig. 4 Effect of PMS concentration on the removal of AO7 |
为了研究初始pH对US/ACF/PMS体系氧化降解AO7的影响,通过0.1 mol·L-1的NaOH和稀硫酸调节反应初始pH,结果如图 5所示.从图中可知,在pH为2时反应速率最快,且随着pH的增大,在pH分别为4、6、8时,反应速率逐渐降低;然而当pH=10时,反应速率又有所升高.这一现象可能与ACF的表面零电荷点有关,即水溶液中固体表面静电荷为0时的pH值.当溶液pH < pHpzc时,ACF表面带正电荷,溶液pH > pHpzc时,ACF表面带负电荷.经测得ACF的pHpzc为2.3,因此,当溶液pH分别为4、6、8时,ACF表面带负电荷.由于AO7为阴性染料,导致AO7与ACF的表面产生相互排斥的效果,使得表面反应不容易进行.Zhou等 (2015)证明,当溶液pH为强碱性的时候,对PMS的分解有一定促进作用,因此,当pH为10时,反应速率比pH为6和8时更快.
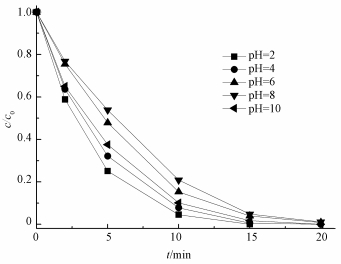 |
| 图 5 初始pH对AO7降解的影响 ([AO7]=20 mg·L-1,[ACF]=0.3 g·L-1,n(PMS)/n(AO7)=20/1,US功率10 W·cm-2) Fig. 5 Effect of initial pH on the removal of AO7 |
不同超声波功率对AO7降解的影响如图 6所示,在无超声波作用情况下,20 min时AO7仅可以被降解80%左右;然而当超声波功率密度为4 W· cm-2时,AO7在20 min内降解率高达98.2%;继续增大超声波功率到10 W· cm-2,AO7脱色率有一定提高但不明显.这说明加入超声波能促进ACF/PMS体系降解AO7,且超声波功率对反应降解速率影响较小.这主要是因为在超声波的作用下产生的声空化现象 (Gayathri et al., 2010; Chen et al., 2012).即溶液内部溶解的一些微型气泡,这些气泡在超声波作用下震动,当累积的压力脉冲达到一定值时,气泡由于定向扩散增大,形成共振腔,然后突然闭合,闭合时会在周围产生几千个大气压的压力,形成微激波,促进PMS产生SO4-·.
 |
| 图 6 超声波功率对AO7降解的影响 ([AO7]=20 mg· L-1,pH=7,[ACF]=0.3 g· L-1,n(PMS)/n(AO7)=20/1) Fig. 6 Effect of ultrasonic power on AO7 removal |
印染工艺中往往通过投加NaCl加速染色,导致其产生的废水中通常含有大量的NaCl,然而Cl-对高级氧化过程有较大影响 (Chan et al., 2009).图 7显示了不同浓度NaCl对活化降解AO7的影响,可以看出,加入Cl-会促进AO7的降解,且随着Cl-浓度的增加,AO7降解速率增大.当Cl-浓度为1 mmol·L-1时,反应20 min时降解率可达98.7%;当Cl-浓度达到300 mmol·L-1时,AO7在10 min时已经降解完全.
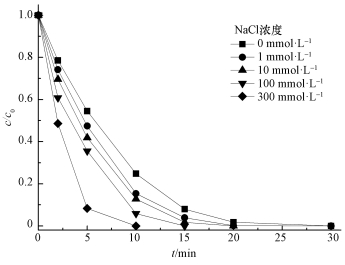 |
| 图 7 NaCl浓度对AO7降解的影响 ([AO7]=20 mg·L-1,pH=7,[ACF]=0.3 g·L-1,n(PMS)/n(AO7)=20/1,US功率10 W·cm-2) Fig. 7 Effect of NaCl amounts on AO7 removal |
产生以上实验结果的原因可能是,当NaCl存在时,Cl-与HSO5-反应生成具有强氧化性的HClO (式 (1)~(5))(Ji et al., 2015).在SO4-·和ClO-共同作用下,AO7降解速率明显提高.这与Yuan等 (2011)和Zhou等 (2015)的研究结论一致.
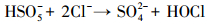
|
(1) |
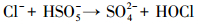
|
(2) |
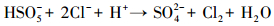
|
(3) |
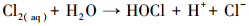
|
(4) |
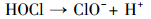
|
(5) |
研究表明,在活化PMS体系中,通常产生HO·、SO4-·和少量SO5-·,其中,SO5-·氧化能力相对较弱 (Xu et al., 2015).目前已有研究表明,叔丁醇 (TBA) 对HO·猝灭效果较好,而对SO4-·猝灭效果较弱;而甲醇 (MA) 含有α—H,对HO·和SO4-·均可以猝灭.因此,本实验先采用甲醇和叔丁醇对活化体系进行自由基鉴定,结果如图 8a所示.可以看出,在投加甲醇和叔丁醇体系中,在30 min时AO7依然可降解99%以上,相比未投加淬灭剂体系几乎没有变化.原因可能是MA和TBA是亲水性化合物,不容易靠近固体表面,由此可以猜测降解反应可能发生在ACF表面,因此,抑制剂对降解反应作用不明显.Liang等 (2013)和Guan等 (2013)在研究抑制剂猝灭过程中同样发现,TBA和MA对反应降解抑制作用不明显.为了进一步证明体系是通过产生HO·或SO4-·氧化降解AO7,本文补充了体系中HO·的荧光光谱分析 (图 9),US/ACF/PMS体系中成功检测出HO·的吸收峰,且随着反应时间增加HO·的吸收峰越高,由于SO4-·产生后能够变成HO·(式 (6))(Li et al., 2013),因此,US/ACF/PMS体系氧化降解AO7一定是产生了HO·或SO4-·.
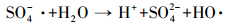
|
(6) |
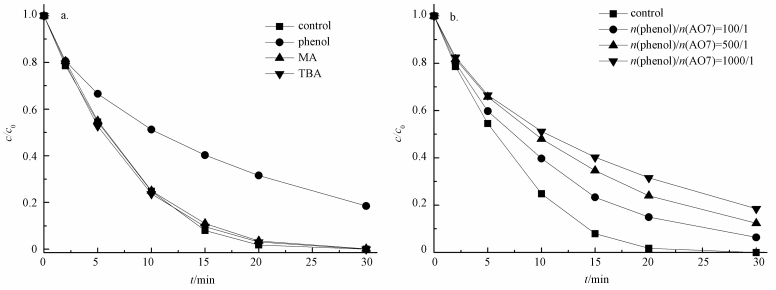 |
| 图 8 抑制剂 (苯酚) 浓度对AO7降解的影响 ([AO7]=20 mg·L-1,pH=7,[ACF]=0.3 g·L-1,n(PMS)/n(AO7)=20/1,US功率10 W·cm-2,n(MA)/n(AO7)=1000/1,n(TBA)/n(AO7)=1000/1) Fig. 8 Effect of radical scavengers on AO7 removal |
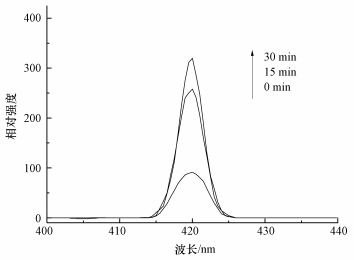 |
| 图 9 HO·的荧光光谱分析 Fig. 9 The fluorescence spectra analysis of HO· |
此外,苯酚被认为能够更有效地淬灭SO4-·和HO·(Yang et al., 2011),同时,苯酚具有疏水性,易于吸附于固相物质 (GAC) 表面,从而阻止PMS与GAC的活性点位接触,导致降解结果下降 (Zhang et al., 2013).当溶液中PMS与ACF接触后,反应产生的SO4-·会被苯酚淬灭,从而导致降解效果下降.如图 8b所示,在投加苯酚体系中,当n(苯酚)/n(AO7)=100/1时,30 min时AO降解了93.6%;增大苯酚浓度为n(苯酚)/n(AO7)=1000/1时,30 min时AO7降解了81.5%,这与ACF在超声波条件下吸附效果相近 (去除率75%).可见苯酚对ACF活化PMS降解AO7的抑制作用很强.且从ACF的红外图 (图 1) 可以看出,其表面含有大量官能团,如O—H、C=O等,这些官能团可以活化PMS产生自由基 (Yang et al., 2015).由此可以推断ACF活化PMS产生SO4-·发生在ACF表面.
3.9 ACF的重复使用性本文研究了ACF在超声条件下催化PMS降解AO7的重复使用性,结果如图 10所示.实验结果表明,在ACF重复使用3次时,AO7的降解率在反应30 min时仍会达到71.4%以上,在ACF重复使用4次后,30 min内对AO7的去除率降低到51.1%.原因可能是在降解AO7过程中,ACF首先将染料吸附,然后再将吸附在纤维中的染料原位催化降解.因此,催化过程中可能有部分染料及其中间产物吸附在ACF表面,从而影响其吸附性能.其次,在超声波存在条件下随着降解反应进行,ACF会由于超声波的作用而变碎,之后在用膜分离回收ACF的过程中,有部分ACF损失,也会导致AO7的降解率变小.
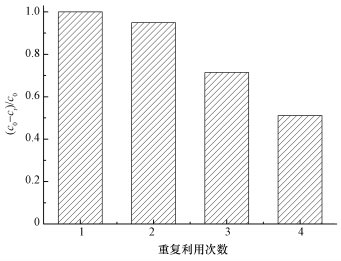 |
| 图 10 超声条件下ACF催化降解AO7的重复使用性 ([AO7]=20 mg·L-1,pH=7,[ACF]=0.3 g·L-1,n(PMS)/n(AO7)=20/1,US功率10 W·cm-2) Fig. 10 Recycling of ACF for catalytic removal of AO7 |
图 11a所示为US/ACF/PMS体系降解AO7过程中紫外可见光谱.可以看出,AO7主要有484 nm和310 nm处的特征吸收峰,分别代表AO7的发色基团偶氮键和萘环结构.随活化反应的进行,位于484 nm和310 nm处的AO7特征峰强度不断下降,表明AO7的偶氮键和萘环结构不断被SO4-·氧化;30 min后,偶氮键和萘环的特征峰近消失.
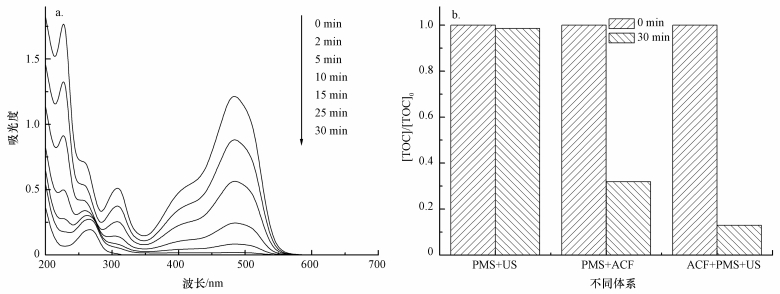 |
| 图 11 AO7降解紫外可见光谱 (a) 及TOC去除率 (b)([AO7]=20 mg·L-1,pH=7,[ACF]=0.3 g·L-1,n(PMS)/n(AO7)=20/1,US功率10 W·cm-2) Fig. 11 UV-Vis spectra for degradation of AO7(a) and TOC removal in US /ACF/PMS systems (b) |
为了进一步研究不同体系降解AO7的情况,本文还对反应过程中的TOC进行了测试,结果如图 11b所示,PMS/US、PMS/ACF和ACF/PMS/US体系30 min时对TOC的去除率分别为1.5%、73.1%和87.0%.结果表明,ACF/PMS/US体系对AO7不仅有良好的降解效果,而且具有一定的矿化能力.
3.11 AO7降解途径及产物分析为了进一步推测ACF/PMS/US体系降解AO7过程,利用GC/MS对AO7降解过程中的产物进行了鉴定.检测到主要产物为苯醌、1, 2-萘醌和香豆素,其化合物产物结构式如表 1所示.结合紫外可见光谱及文献 (王忠明等, 2016) 推测其降解过程为:AO7首先偶氮键断裂分为芳香族化合物,再氧化开环为有机酸,进而降解为小分子酸,最后矿化为CO2和H2O,其历程如图 12所示.
| 表 1 GC/MS鉴定的3种主要产物图 Table 1 3 main intermediates of AO7 removal obtained with GC/MS |
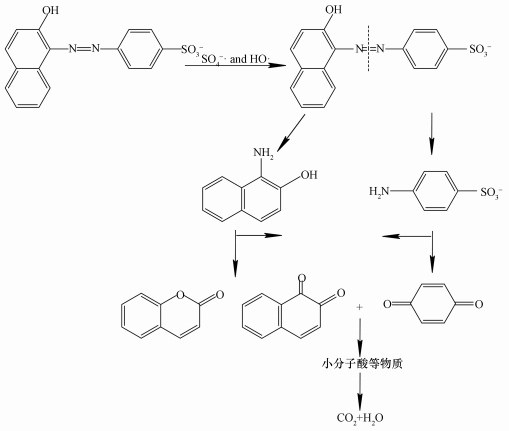 |
| 图 12 AO7可能的降解途径 Fig. 12 A possible pathway for degradation of AO7 |
1) 采用US和ACF协同活化PMS降解AO7效果明显,而且可以证实ACF活化PMS产生的自由基主要是SO4-·,PMS被活化的场所在ACF表面.
2) AO7降解效果随超声波功率、PMS浓度、ACF投加量、Cl-浓度的增大而得到提高.初始pH对降解有较大的影响,在强酸及强碱性条件下有利于反应进行.
3) ACF催化降解AO7具有一定的重复利用性;AO7在降解过程中偶氮键和萘环结构被破坏,并逐渐矿化成CO2和H2O.
| [${referVo.labelOrder}] | Anipsitakis G P, Dionysiou D D. 2003. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt[J]. Environmental Science & Technology, 37(20) : 4790–4797. |
| [${referVo.labelOrder}] | Bai C P, Xiong X F, Gong W Q, et al. 2011. Removal of rhodamine B by ozone-based advanced oxidation process[J]. Desalination, 278(1/3) : 84–90. |
| [${referVo.labelOrder}] | Cai C, Wang L, Gao H, et al. 2014. Ultrasound enhanced heterogeneous activation of peroxydisulfate by bimetallic Fe-Co/GAC catalyst for the degradation of Acid Orange 7 in water[J]. Journal of Environmental Sciences, 26(6) : 1267–1273. DOI:10.1016/S1001-0742(13)60598-7 |
| [${referVo.labelOrder}] | Chan K H, Chu W. 2009. Degradation of atrazine by cobalt-mediated activation of peroxymonosulfate:Different cobalt counteranions in homogenous process and cobalt oxide catalysts in photolytic heterogeneous process[J]. Water Research, 43(9) : 2513–2521. DOI:10.1016/j.watres.2009.02.029 |
| [${referVo.labelOrder}] | Chen W, Su Y. 2012. Removal of dinitrotoluenes in wastewater by sono-activated persulfate[J]. Ultrasonics Sonochemistry, 19(4) : 921–927. DOI:10.1016/j.ultsonch.2011.12.012 |
| [${referVo.labelOrder}] | Demir Cakan R, Baccile N, Antonietti M, et al. 2009. Carboxylate-rich carbonaceous materials via one-step hydrothermal carbonization of glucose in the presence of acrylic acid[J]. Chemistry of Materials, 21(3) : 484–490. DOI:10.1021/cm802141h |
| [${referVo.labelOrder}] | El Hendawy A A. 2006. Variation in the FTIR spectra of a biomass under impregnation, carbonization and oxidation conditions[J]. Journal of Analytical and Applied Pyrolysis, 75(2) : 159–166. DOI:10.1016/j.jaap.2005.05.004 |
| [${referVo.labelOrder}] | Eren Z. 2012. Ultrasound as a basic and auxiliary process for dye remediation:A review[J]. Journal of Environmental Management, 104 : 127–141. DOI:10.1016/j.jenvman.2012.03.028 |
| [${referVo.labelOrder}] | Faria P C C, Órfão J J M, Pereira M F R. 2009. Activated carbon and ceria catalysts applied to the catalytic ozonation of dyes and textile effluents[J]. Applied Catalysis B:Environmental, 88(3/4) : 341–350. |
| [${referVo.labelOrder}] | Fierro V, Torné Fernández V, Celzard A, et al. 2007. Influence of the demineralisation on the chemical activation of Kraft lignin with orthophosphoric acid[J]. Journal of Hazardous Materials, 149(1) : 126–133. DOI:10.1016/j.jhazmat.2007.03.056 |
| [${referVo.labelOrder}] | Gao Y, Gao N, Deng Y, et al. 2012. Ultraviolet (UV) light-activated persulfate oxidation of sulfamethazine in water[J]. Chemical Engineering Journal, 195-196 : 248–253. DOI:10.1016/j.cej.2012.04.084 |
| [${referVo.labelOrder}] | Gayathri P, Praveena Juliya Dorathi R, Palanivelu K. 2010. Sonochemical degradation of textile dyes in aqueous solution using sulphate radicals activated by immobilized cobalt ions[J]. Ultrasonics Sonochemistry, 17(3) : 566–571. DOI:10.1016/j.ultsonch.2009.11.019 |
| [${referVo.labelOrder}] | Ghauch A, Tuqan A M, Kibbi N, et al. 2012. Methylene blue discoloration by heated persulfate in aqueous solution[J]. Chemical Engineering Journal, 213 : 259–271. DOI:10.1016/j.cej.2012.09.122 |
| [${referVo.labelOrder}] | Guan Y, Ma J, Ren Y, et al. 2013. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals[J]. Water Research, 47(14) : 5431–5438. DOI:10.1016/j.watres.2013.06.023 |
| [${referVo.labelOrder}] | Gupta V K, Gupta B, Rastogi A, et al. 2011. A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye—Acid Blue 113[J]. Journal of Hazardous Materials, 186(1) : 891–901. DOI:10.1016/j.jhazmat.2010.11.091 |
| [${referVo.labelOrder}] | He X, de la Cruz A A, Dionysiou D D. 2013. Destruction of cyanobacterial toxin cylindrospermopsin by hydroxyl radicals and sulfate radicals using UV-254nm activation of hydrogen peroxide, persulfate and peroxymonosulfate[J]. Journal of Photochemistry and Photobiology A:Chemistry, 251 : 160–166. DOI:10.1016/j.jphotochem.2012.09.017 |
| [${referVo.labelOrder}] | Ji P, Zhang J, Chen F, et al. 2009. Study of adsorption and degradation of acid orange 7 on the surface of CeO2 under visible light irradiation[J]. Applied Catalysis B:Environmental, 85(3/4) : 148–154. |
| [${referVo.labelOrder}] | Ji Y, Dong C, Kong D, et al. 2015. New insights into atrazine degradation by cobalt catalyzed peroxymonosulfate oxidation:Kinetics, reaction products and transformation mechanisms[J]. Journal of Hazardous Materials, 285 : 491–500. DOI:10.1016/j.jhazmat.2014.12.026 |
| [${referVo.labelOrder}] | Khataee A R, Pons M N, Zahraa O. 2009. Photocatalytic degradation of three azo dyes using immobilized TiO2 nanoparticles on glass plates activated by UV light irradiation:Influence of dye molecular structure[J]. Journal of Hazardous Materials, 168(1) : 451–457. DOI:10.1016/j.jhazmat.2009.02.052 |
| [${referVo.labelOrder}] | Kusic H, Peternel I, Ukic S, et al. 2011. Modeling of iron activated persulfate oxidation treating reactive azo dye in water matrix[J]. Chemical Engineering Journal, 172(1) : 109–121. DOI:10.1016/j.cej.2011.05.076 |
| [${referVo.labelOrder}] | Lee Y, Lo S, Kuo J, et al. 2013. Promoted degradation of perfluorooctanic acid by persulfate when adding activated carbon[J]. Journal of Hazardous Materials, 261 : 463–469. DOI:10.1016/j.jhazmat.2013.07.054 |
| [${referVo.labelOrder}] | Li B, Li L, Lin K, et al. 2013. Removal of 1, 1, 1-trichloroethane from aqueous solution by a sono-activated persulfate process[J]. Ultrasonics Sonochemistry, 20(3) : 855–863. DOI:10.1016/j.ultsonch.2012.11.014 |
| [${referVo.labelOrder}] | Liang C, Bruell C J. 2008. Thermally activated persulfate oxidation of trichloroethylene: experimental investigation of reaction orders[J]. Industrial & Engineering Chemistry Research, 47(9) : 2912–2918. |
| [${referVo.labelOrder}] | Liang H, Zhang Y, Huang S, et al. 2013. Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate[J]. Chemical Engineering Journal, 218 : 384–391. DOI:10.1016/j.cej.2012.11.093 |
| [${referVo.labelOrder}] | 刘金生. 2007. 活性炭纤维脱硫与超声改性研究[D]. 上海: 上海交通大学 |
| [${referVo.labelOrder}] | Oh W, Lua S, Dong Z, et al. 2015. Performance of magnetic activated carbon composite as peroxymonosulfate activator and regenerable adsorbent via sulfate radical-mediated oxidation processes[J]. Journal of Hazardous Materials, 284 : 1–9. DOI:10.1016/j.jhazmat.2014.10.042 |
| [${referVo.labelOrder}] | O'Reilly J M, Mosher R A. 1983. Functional groups in carbon black by FTIR spectroscopy[J]. Carbon, 21(1) : 47–51. DOI:10.1016/0008-6223(83)90155-0 |
| [${referVo.labelOrder}] | Rastogi A, Al-Abed S R, Dionysiou D D. 2009. Effect of inorganic, synthetic and naturally occurring chelating agents on Fe (Ⅱ) mediated advanced oxidation of chlorophenols[J]. Water Research, 43(3) : 684–694. DOI:10.1016/j.watres.2008.10.045 |
| [${referVo.labelOrder}] | Rodriguez S, Vasquez L, Costa D, et al. 2014. Oxidation of Orange G by persulfate activated by Fe (Ⅱ), Fe (Ⅲ) and zero valent iron (ZVI)[J]. Chemosphere, 101 : 86–92. DOI:10.1016/j.chemosphere.2013.12.037 |
| [${referVo.labelOrder}] | Saleh T A, Gupta V K. 2012. Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide[J]. Journal of Colloid and Interface Science, 371(1) : 101–106. DOI:10.1016/j.jcis.2011.12.038 |
| [${referVo.labelOrder}] | Sun X, Li Y. 2004. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles[J]. Angewandte Chemie International Edition, 43(5) : 597–601. DOI:10.1002/(ISSN)1521-3773 |
| [${referVo.labelOrder}] | Waldemer R H, Tratnyek P G, Johnson R L, et al. 2007. Oxidation of chlorinated ethenes by heat-activated persulfate: Kinetics and products[J]. Environmental Science & Technology, 41(3) : 1010–1015. |
| [${referVo.labelOrder}] | 王忠明, 陈家斌, 张黎明, 等. 2016. 活性炭负载Co3O4活化过一硫酸盐降解金橙G[J]. 环境科学, 2016(37) : 2591–2600. |
| [${referVo.labelOrder}] | Xu L J, Chu W, Gan L. 2015. Environmental application of graphene-based CoFe2O4 as an activator of peroxymonosulfate for the degradation of a plasticizer[J]. Chemical Engineering Journal, 263 : 435–443. DOI:10.1016/j.cej.2014.11.065 |
| [${referVo.labelOrder}] | Xu X, Li X. 2010. Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion[J]. Separation and Purification Technology, 72(1) : 105–111. DOI:10.1016/j.seppur.2010.01.012 |
| [${referVo.labelOrder}] | Yang S, Xiao T, Zhang J, et al. 2015. Activated carbon fiber as heterogeneous catalyst of peroxymonosulfate activation for efficient degradation of Acid Orange 7 in aqueous solution[J]. Separation and Purification Technology, 143 : 19–26. DOI:10.1016/j.seppur.2015.01.022 |
| [${referVo.labelOrder}] | Yang S, Yang X, Shao X, et al. 2011. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature[J]. Journal of Hazardous Materials, 186(1) : 659–666. DOI:10.1016/j.jhazmat.2010.11.057 |
| [${referVo.labelOrder}] | Yao Y, Wang L, Sun L, et al. 2013. Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity[J]. Chemical Engineering Science, 101 : 424–431. DOI:10.1016/j.ces.2013.06.009 |
| [${referVo.labelOrder}] | Yuan R, Ramjaun S N, Wang Z, et al. 2011. Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process:Implications for formation of chlorinated aromatic compounds[J]. Journal of Hazardous Materials, 196 : 173–179. DOI:10.1016/j.jhazmat.2011.09.007 |
| [${referVo.labelOrder}] | Zhang J, Shao X, Shi C, et al. 2013. Decolorization of Acid Orange 7 with peroxymonosulfate oxidation catalyzed by granular activated carbon[J]. Chemical Engineering Journal, 232 : 259–265. DOI:10.1016/j.cej.2013.07.108 |
| [${referVo.labelOrder}] | Zhou J, Xiao J, Xiao D, et al. 2015. Transformations of chloro and nitro groups during the peroxymonosulfate-based oxidation of 4-chloro-2-nitrophenol[J]. Chemosphere, 134 : 446–451. DOI:10.1016/j.chemosphere.2015.05.027 |
| [${referVo.labelOrder}] | Zhou Y, Jiang J, Gao Y, et al. 2015. Activation of peroxymonosulfate by benzoquinone:A novel nonradical oxidation process[J]. Environ Sci Technol, 49(21) : 12941–12950. DOI:10.1021/acs.est.5b03595 |
 2017, Vol. 37
2017, Vol. 37