2. 火箭军特色医学中心中医科, 北京 100088
2. Department of Chinese Medicine, Rocket Force Medical Center of PLA, Beijing 100088, China
非小细胞肺癌(non-small cell lung cancer,NSCLC)是肺癌最主要的病理类型[1-2],占所有肺癌的85%[3]。目前只有近30%的NSCLC患者能在诊断时即接受治愈性手术,大部分NSCLC患者只能接受酪氨酸激酶抑制剂(tyrosine kinase inhibitor,TKI)、放化疗和免疫疗法等辅助治疗[4]。与化疗相比,表皮生长因子受体(epidermal growth factor receptor,EGFR)突变NSCLC患者一线EGFR-TKI治疗的有效率更高、无进展生存期更长[5-6]。对于第一代和第二代EGFR-TKI药物,皮疹(包括干燥症、瘙痒、痤疮样皮疹及头发、指甲的变化)是最主要的不良反应,总体发生率为47%~100%,且其与患者预后密切相关[7];10%的皮疹被归类为严重不良反应(3、4级)[8],迫使患者减少TKI剂量甚至中断治疗[9]。目前针对TKI相关皮疹的药物主要为皮质类固醇和四环素[10],但不良反应较多,包括肠道菌群失调和免疫力下降,因此不适合长期使用[11]。
中医药作为中华民族文化传承积累的瑰宝,能有效改善肺癌患者的生活质量,缓解皮疹症状[12],延长生存时间[13]。《素问》言:“肺者,气之本,魄之处也,其华在毛,其充在皮”。传统中医理论认为肺主体表皮肤、汗腺和毫毛等,肺和皮毛在生理病理上存在密切的联系,肺与皮毛任意一方病变均可影响另一方的功能[14]。现代医学研究显示,EGFR不仅在NSCLC中表达且可能存在突变,而且广泛表达于皮肤组织,角质细胞分化表皮的过程也受EGFR信号通路调控[15];服用EGFR-TKI药物不仅能抑制肺肿瘤进展,亦会导致皮毛变化。种种迹象表明,EGFR突变NSCLC与TKI相关皮疹存在密切联系。
生物信息学是分子生物学和信息技术的交叉学科,基于高通量测序技术的生物信息学技术被广泛运用于探索疾病的相关基因。基因集富集分析(gene set enrichment analysis,GSEA)能识别特定治疗或疾病触发的特定基因集或分子信号通路[16]。本研究利用生物信息学方法对EGFR突变NSCLC及TKI相关皮疹之间的关联进行初探,并通过数据检索、分子对接预测相关药物,以期为EGFR突变NSCLC及TKI相关皮疹的临床诊疗和研究提供指导。
1 材料和方法 1.1 差异表达基因筛选利用基因表达汇编(Gene Expression Omnibus,GEO)数据库(https://www.ncbi.nlm.nih.gov/geo/)下载相关数据。(1)以“①EGFR突变NSCLC;②人类;③TKI药物”为筛选条件从GSE67051数据集中获得细胞实验基因芯片数据:人源EGFR突变NSCLC细胞系PC9和HCC827各6例,分别予厄洛替尼和空白处理(PC9、HCC827细胞各3例)。(2)以“①TKI相关皮疹;②人类”为筛选条件从GSE106151数据集中获得细胞实验基因芯片数据:人源正常成纤维细胞13例,其中5例给予厄洛替尼处理,8例未给予厄洛替尼处理。
利用R 4.3.2软件limma包对测序数据进行背景校正、归一化和表达值计算,将未使用TKI药物处理的细胞作为对照组,分析使用TKI药物处理和未使用TKI药物处理的EGFR突变NSCLC细胞的差异表达基因,以及使用TKI药物处理和未使用TKI药物处理的正常成纤维细胞的差异表达基因。设定调整后P<0.05、FC≥1.5(|log2(FC)|≥0.585,FC为差异倍数)为差异表达基因的筛选标准,其中log2(FC)≥0.585代表mRNA表达上调,log2(FC)≤-0.585代表mRNA表达下调。利用R 4.3.2软件heatmap包对筛选得到的差异表达基因进行热图及聚类分析,并对差异化处理后芯片数据的P值求常用对数的负值,将数据导入R 4.3.2软件绘制火山图。
1.2 交集靶基因预测使用TKI药物处理的EGFR突变NSCLC细胞及正常成纤维细胞同时出现部分基因的差异表达,此可作为两者的关联因素。利用R 4.3.2软件venn包筛选EGFR突变NSCLC细胞差异表达基因及TKI相关皮疹差异表达基因的交集靶基因,并绘制维恩图。
1.3 功能富集分析将EGFR突变NSCLC、TKI相关皮疹的差异表达基因和交集靶基因通过R 4.3.2软件org.Hs.eg.db包转换为基因ID,利用R 4.3.2软件ClusterProfiler包进行基因本体(Gene Ontology,GO)功能、京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)路径富集分析和GSEA。
1.4 交集靶基因蛋白质-蛋白质相互作用(protein-protein interaction,PPI)网络与核心网络的构建将筛选的交集靶基因输入String数据库(https://cn.string-db.org/)构建药物-疾病PPI网络,其中种属(organism)中选择智人(Homo sapiens),设定最低交互分数(minimum required interaction score)≥0.7,同时删去游离靶基因。将获取的PPI信息导入Cytoscape 3.8.0软件中,使用cytoNCA插件进行拓扑分析,并获取核心网络。
1.5 潜在中药预测将筛选的交集靶基因录入CoreMine Medical数据库(http://coremine.com/medical)中,以P<0.05为筛选条件筛选潜在中药,对频次排前5位的潜在中药进行靶基因预测并制作潜在中药靶基因表。根据《中华本草》《中药学》《中药大辞典》对中药的性味归经进行统计分析。选取频次最高的中药作为示例,通过查找文献及参考CoreMine Medical数据库筛选与靶基因支持数较多、有实验数据支持的中药化学成分。
1.6 分子对接分析将药物靶基因与其对应的药物活性成分作为研究对象,通过PubChem数据库(https://pubchem.ncbi.nlm.nih.gov/)查询有效活性成分的二维结构,使用ChemBio 3D 14.0.0软件转化为三维结构,并调节其结构以达到自由能最小。通过PDB数据库(https://www.rcsb.org/)下载靶蛋白的三维结构,运用Pymol软件移除核心蛋白的小分子配体及水分子。采用AutoDockTools 1.5.7软件将靶蛋白、配体转换为pdbqt格式并寻找活性口袋,利用AutoDock Vina 1.1.2软件进行分子结合能计算和分子对接,并采用Pymol软件进行可视化。
2 结果 2.1 差异表达基因根据筛选标准,在GSE67051数据集中筛选出1 702个差异表达基因,包括1 234个高表达基因及468个低表达基因。在GSE106151数据集中筛选出485个差异表达基因,包括117个高表达基因及368个低表达基因。显著性意义最大的100个差异表达基因的热图见图 1A、1B,差异化处理后芯片数据的火山图见图 1C、1D。
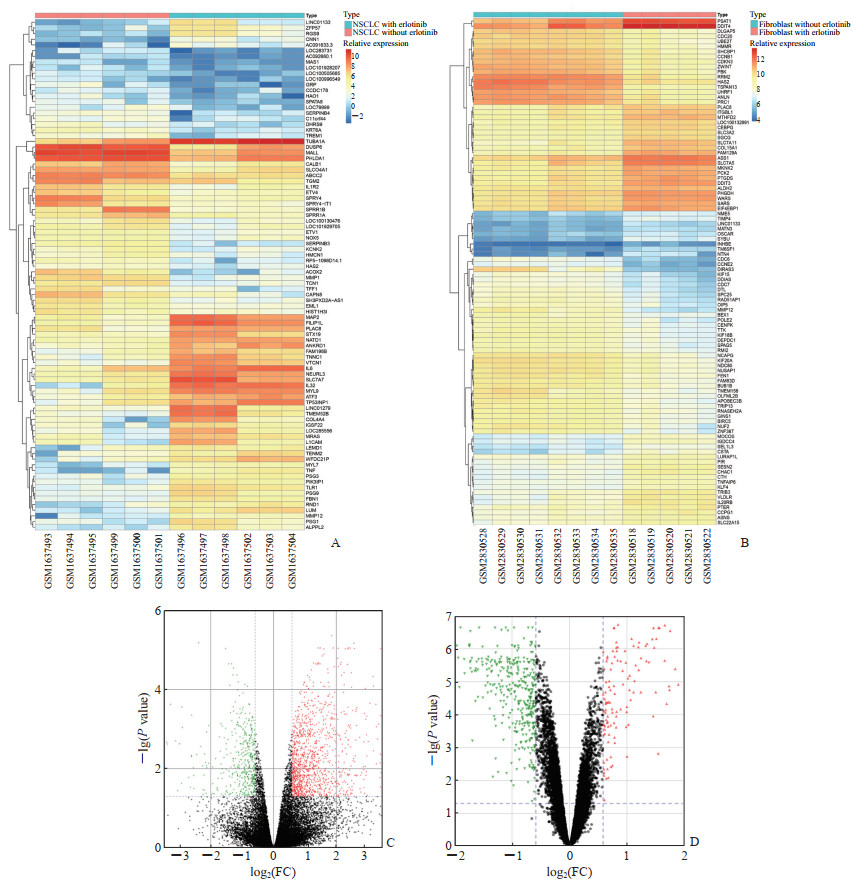
|
图 1 差异表达基因的热图和火山图 Fig 1 Heatmap and volcano map of differentially expressed genes A: Heatmap of cluster of the top 100 differentially expressed genes with the largest significance in EGFR mutant NSCLC; B: Heatmap of cluster of the top 100 differentially expressed genes with the largest significance in TKI-related rash; C: Volcano map of EGFR mutant NSCLC; D: Volcano map of TKI-related rash. EGFR: Epidermal growth factor receptor; NSCLC: Non-small cell lung cancer; TKI: Tyrosine kinase inhibitor; FC: Fold change. |
2.2 交集靶基因
将1 702个EGFR突变NSCLC细胞的差异表达基因与485个TKI相关皮疹的差异表达基因通过R 4.3.2软件venn包分析得到126个交集靶基因。
2.3 功能富集分析GO功能富集分析显示,厄洛替尼处理后EGFR突变NSCLC细胞和成纤维细胞的差异表达基因、交集靶基因显著富集的生物学过程均主要涉及细胞器分裂、核分裂、有丝分裂等(图 2)。KEGG富集分析显示,厄洛替尼处理后EGFR突变NSCLC细胞的差异表达基因主要涉及细胞循环、溶酶体、DNA复制、细胞外基质(extracellular matrix,ECM)受体相关作用等通路(图 3A);厄洛替尼处理后成纤维细胞的差异表达基因及其与EGFR突变NSCLC细胞的交集靶基因均主要涉及DNA复制、细胞循环、错配修复、碱基剪切修复、同源重组等通路(图 3B、3C)。
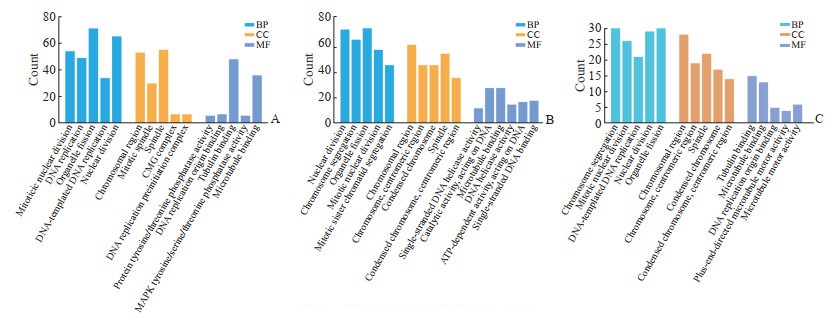
|
图 2 基因本体功能富集分析结果 Fig 2 Gene Ontology function enrichment analysis results A: Histogram of enrichment analysis of differentially expressed genes in EGFR mutant NSCLC; B: Histogram of enrichment analysis of differentially expressed genes in TKI-related rash; C: Histogram of enrichment analysis of cross-over target genes. EGFR: Epidermal growth factor receptor; NSCLC: Non-small cell lung cancer; TKI: Tyrosine kinase inhibitor; BP: Biological process; CC: Cellular component; MF: Molecular function; CMG: CDC45-MCM2-7-GINS; MAPK: Mitogen-activated protein kinase. |
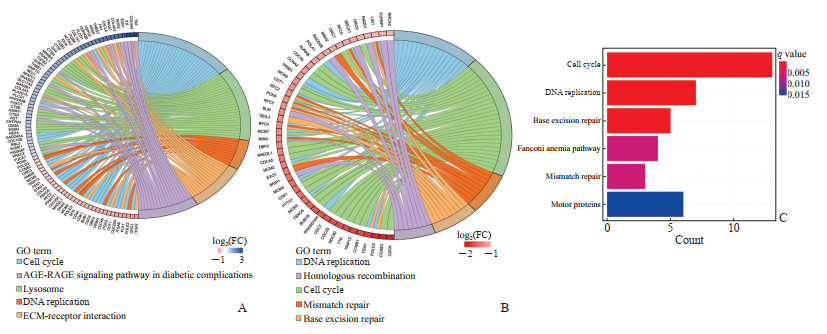
|
图 3 KEGG富集分析结果 Fig 3 KEGG enrichment analysis results A: Chord plot of enrichment analysis of differentially expressed genes in EGFR mutant NSCLC; B: Chord plot of enrichment analysis of differentially expressed genes in TKI-related rash; C: Histogram of enrichment analysis of cross-over target genes. KEGG: Kyoto Encyclopedia of Genes and Genomes; EGFR: Epidermal growth factor receptor; NSCLC: Non-small cell lung cancer; TKI: Tyrosine kinase inhibitor; GO: Gene Ontology; FC: Fold change; AGE: Advanced glycation end product; RAGE: Receptor of AGE; ECM: Extracellular matrix. |
GSEA显示,相较于未使用厄洛替尼处理的EGFR突变NSCLC细胞,厄洛替尼处理的EGFR突变NSCLC细胞在细胞循环、DNA修复、嘧啶代谢、RNA聚合酶、剪接体等通路上富集较少,在趋化因子信号通路、细胞因子-细胞因子受体相互作用、核苷酸结合寡聚域(nucleotide-binding oligomerization domain,NOD)样受体信号通路、溶酶体、TGF-β信号通路上富集较多;相较于未使用厄洛替尼处理的成纤维细胞,厄洛替尼处理的成纤维细胞在细胞循环、DNA修复、剪接体、嘌呤代谢、嘧啶代谢等通路上富集较少,在甘氨酸丝氨酸和苏氨酸代谢、哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)信号通路、氮代谢等通路上富集较多(图 4)。
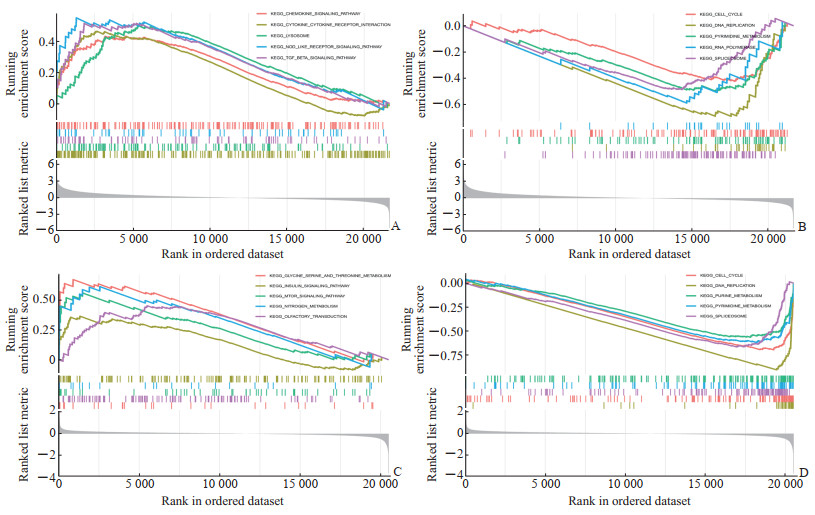
|
图 4 GSEA富集分析结果 Fig 4 GSEA enrichment analysis results A: EGFR mutant NSCLC treated with erlotinib; B: EGFR mutant NSCLC treated without erlotinib; C: TKI-related rash treated with erlotinib; D: TKI-related rash treated without erlotinib. GSEA: Gene set enrichment analysis; EGFR: Epidermal growth factor receptor; NSCLC: Non-small cell lung cancer; TKI: Tyrosine kinase inhibitor; NOD: Nucleotide-binding oligomerization domain; TGF: Transforming growth factor; mTOR: Mammalian target of rapamycin. |
2.4 交集靶基因PPI网络及核心网络
126个交集靶基因的PPI网络图见图 5A,经过2次拓扑分析得到的核心网络图见图 5B。如图所示,核心网络存在13个核心靶基因,包括甲状腺素受体结合因子13(thyroid hormone receptor interactor 13,TRIP13)、非染色体结构维护凝聚蛋白Ⅰ复合物亚基G(non-structural maintenance of chromosome proteins condensin Ⅰ complex subunit G,NCAPG)、驱动蛋白家族成员23(kinesin family member 23,KIF23)、驱动蛋白家族成员11(kinesin family member 11,KIF11)、增殖细胞核抗原钳夹相关因子(proliferating cell nuclear antigen clamp associated factor,KIAA0101)、细胞质分裂蛋白调控因子1(protein regulator of cytokinesis 1,PRC1)、PDZ结合激酶(PDZ-binding kinase,PBK)、细胞分裂周期相关因子8(cell division cycle-associated 8,CDCA8)、周期蛋白依赖性激酶1(cyclin-dependent protein kinase 1,CDK1)、有丝分裂阻滞缺陷2样蛋白1(mitotic arrest deficient 2 like 1,MAD2L1)、核酸外切酶1(exonuclease 1,EXO1)、细胞分裂周期基因45(cell division cycle 45,CDC45)、Opa相互作用蛋白5(Opa interacting protein 5,OIP5),核心靶基因两两之间均存在联系。
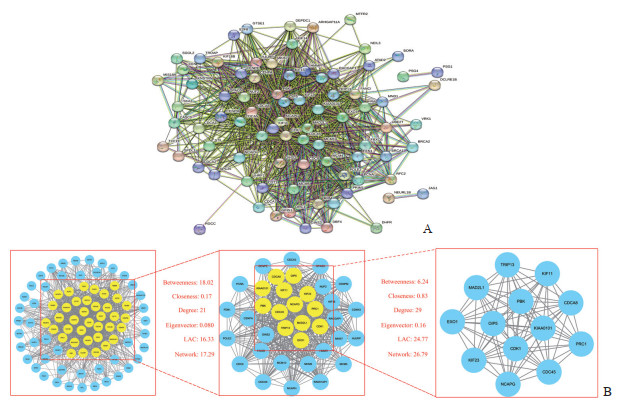
|
图 5 EGFR突变NSCLC与TKI相关皮疹交集靶基因的PPI网络图 Fig 5 PPI network diagram of cross-over target genes of EGFR mutant NSCLC and TKI-related rash A: PPI network of cross-over target genes; B: PPI core network of cross-over target genes. EGFR: Epidermal growth factor receptor; NSCLC: Non-small cell lung cancer; TKI: Tyrosine kinase inhibitor; PPI: Protein-protein interaction; LAC: Local average connectivity. |
2.5 潜在中药
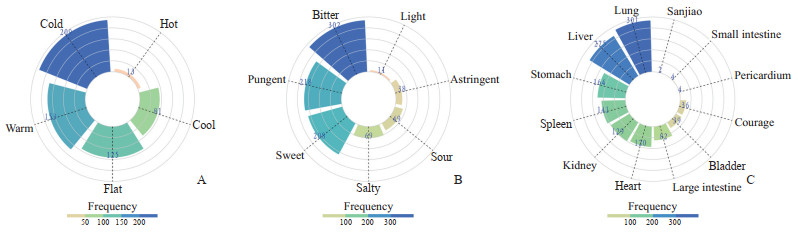
将交集靶基因映射到CoreMine Medical数据库中获取的潜在有效中药有354种,包括黄芩、水牛角、人参、郁金、僵蚕、七叶一枝花、紫草等,预测获得频次排前5位的中药的靶基因见表 1。对354种潜在中药进行性味归经分析,四气以寒、温、平居多,五味以苦、辛、甘居多,归经以归肺、肝、胃居多(图 6)。
|
|
表 1 频次排前5位潜在中药的靶基因预测结果 Tab 1 Prediction results of target genes of potential traditional Chinese medicine in top 5 of frequency |
|
图 6 354种潜在中药的性味归经分布 Fig 6 Nature, flavour, and channel tropism distribution of 354 kinds of potential traditional Chinese medicine A: Distribution map of four natures; B: Distribution map of five flavours; C: Distribution map of channel tropism. |
2.6 中药有效成分和分子对接
以频次最高的黄芩为例,通过分析CoreMine Medical数据库及文献中有实验支持的证据,筛选基因对应黄芩的有效活性成分,其中着丝粒相关蛋白E(centromere-associated protein E,CENPE)仅有生物信息学理论支撑,无实验数据支持故删除。将黄芩有效活性成分与对应靶蛋白进行分子对接,若分子结合能<0说明配体与受体可自发结合,当结合能≤-5 kcal/mol(1 kcal=4.184 kJ)说明两者形成稳定对接,黄芩有效活性成分与靶蛋白的结合能分析结果见表 2。黄芩有效活性成分黄芩素与靶蛋白编码双孔钾离子通道亚家族K成员2(potassium two pore domain channel subfamily K member 2,KCNK2)可自发结合,结合能<-10 kcal/mol,结合效果极佳;黄芩苷与靶蛋白DNA损伤诱导转录因子3(DNA-damage inducible transcription 3,DDIT3),千层纸素与靶蛋白CDK1,汉黄芩素与靶蛋白细胞分裂周期基因6(cell division cycle 6,CDC6)、KCNK2、基质金属蛋白酶-1(matrix metalloproteinase-1,MMP1),黄芩素与靶蛋白超长链脂肪酸延伸酶6(elongase of very long chain fatty acid 6,ELOVL6),野黄芩素与靶蛋白蛋白磷酸酶2A癌性抑制因子(cancerous inhibitor of protein phosphatase 2A,KIAA1524;又称CIP2A),以及紫茎芹醚与靶蛋白二氢叶酸还原酶(dihydrofolate reductase,DHFR)均可自发结合,结合能均<-5 kcal/mol,结合效果佳。黄芩有效活性成分的三维结构模拟图见图 7。通过实验和分子动力学等证据支持所筛选的中药可作为EGFR突变NSCLC伴TKI相关皮疹患者的治疗药物。
|
|
表 2 黄芩有效活性成分与靶蛋白的结合能 Tab 2 Binding energy of active components of Scutellaria baicalensis and target proteins |
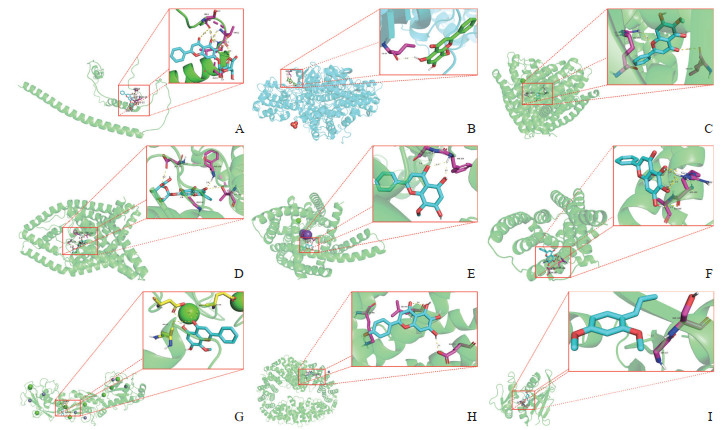
|
图 7 黄芩有效活性成分的三维结构模拟图 Fig 7 3D structure simulation diagram of active components of Scutellaria baicalensis A: DDIT3-baicalin; B: CDC6-wogonin; C: CDK1-oroxylin A; D: KCNK2-baicalein; E: KCNK2-wogonin; F: ELOVL6-baicalein; G: MMP1-wogonin; H: KIAA1524-scutellarein; I: DHFR-nothosmyrnol. DDIT3: DNA-damage inducible transcription 3; CDC6: Cell division cycle 6; CDK1: Cyclin-dependent protein kinase 1; KCNK2: Potassium two pore domain channel subfamily K member 2; ELOVL6: Elongase of very long chain fatty acid 6; MMP1: Matrix metalloproteinase-1; KIAA1524: Cancerous inhibitor of protein phosphatase 2A; DHFR: Dihydrofolate reductase. |
3 讨论
目前关于EGFR突变NSCLC与TKI相关皮疹间的关联研究较少,本研究利用生物信息学方法从转录和蛋白质水平探讨了两者的关联机制并预测了TKI相关皮疹的有效中药、用药规律,以期把握疾病之间的内在联系并为临床治疗提供指导。
本研究利用生物信息学方法预测获得EGFR突变NSCLC与TKI相关皮疹之间存在126个交集靶基因,提取了13个核心靶基因:TRIP13、NCAPG、KIF23、KIF11、KIAA0101、PRC1、PBK、CDCA8、CDK1、MAD2L1、EXO1、CDC45、OIP5,其中大部分与细胞周期、DNA修复相关。目前虽然没有直接证据支持EGFR突变NSCLC与TKI相关皮疹间存在核心基因表达的联系,但多项研究提示上述多种核心靶基因在肺癌发生及皮肤发育过程中起着重要作用,如PRC1是细胞的重要染色质调节剂,是细胞质分裂必需的蛋白质,且其核心亚基Ring1b在人类表皮中含量丰富,对表皮的完整性至关重要,而PRC1缺失可促进皮肤活性基因表达上调,导致皮肤发生水疱,甚至可能引起人类皮肤脆弱综合征[17-18];与此同时,PRC1在肺癌细胞系中过度表达,PRC1耗尽可导致细胞衰老,使肺肿瘤的形成减少[19-20]。PBK是一种细胞周期调节剂,在日光紫外线诱导的皮肤基底细胞癌中起重要作用[21],PBK的表达与突变影响肺腺癌细胞的增殖和活力[22]。CDCA8的转录表达在皮肤黑色素瘤样本中明显上调[23],其磷酸化可导致肺癌细胞的生长受到抑制[24]。这些证据将为研究NSCLC与TKI相关皮疹之间的相关性提供了方向。
为了进一步研究EGFR突变NSCLC、TKI相关皮疹在分子功能、生物学过程、基因富集等方面的特征,本研究通过对差异表达基因、交集靶基因进行功能富集分析。通过GO富集分析发现EGFR突变NSCLC细胞、正常成纤维细胞的差异表达基因、交集靶基因均富集在染色体、纺锤体等区域,具有调节微管运动等功能,并参与了有丝分裂、核分裂、细胞器分裂、染色体分离、DNA复制等生物学过程。通过KEGG富集分析发现EGFR突变NSCLC细胞、正常成纤维细胞的差异表达基因、交集靶基因均富集在DNA复制、溶酶体、细胞循环等通路,提示EGFR突变NSCLC、TKI相关皮疹在基因功能、信号通路上具有同源性。GSEA提示,使用EGFR-TKI药物厄洛替尼治疗后,NSCLC的趋化因子通路、NOD样受体信号通路、TGF-β信号通路、细胞因子/细胞因子受体信号通路、溶酶体信号通路被激活,这些通路与厄洛替尼的抗肿瘤作用及耐药性相关。EGFR在表皮生长因子的刺激下被内化并通过网格蛋白包被的囊泡转运到早期内体,EGFR募集及磷酸化信号分子激活下游信号通路调节细胞生长,在EGFR-TKI药物敏感的NSCLC细胞系中,内化的表皮生长因子-EGFR复合物在表皮生长因子刺激下被递送至溶酶体降解从而终止细胞内的EGFR信号转导[25-26];趋化因子、细胞因子激活可使免疫相关细胞发挥强大的细胞毒性及抗肿瘤作用[27-28],而部分细胞因子如IL-6上调也增加了EGFR突变NSCLC对EGFR-TKI药物不依赖癌基因的获得性耐药[29];TGF-β信号通路被EGFR激活亦可诱导上皮间质转化而导致原发性耐药[30]。EGFR被激活后可磷酸化下游信号通路,包括mTOR信号通路。mTOR是一种丝氨酸/苏氨酸激酶,存在2种具有不同结构和功能的复合物(mTORC1和mTORC2),mTORC1在表皮分化、组织生长和屏障形成中起着关键作用,mTORC2可能控制表皮分层、终末分化[31]。GESA分析提示经过厄洛替尼处理的成纤维细胞中mTOR信号通路异常上调,这可能是TKI相关皮疹的重要机制之一。同时,在厄洛替尼处理的成纤维细胞中氨基酸代谢通路如甘氨酸丝氨酸和苏氨酸代谢通路、氮代谢通路被激活。作为角质层中天然保湿因子的重要来源,表皮蛋白聚丝蛋白原主要由丝氨酸、甘氨酸、丙氨酸、瓜氨酸和苏氨酸构成,在维持人体皮肤屏障的完整性方面发挥重要作用[32];聚丝蛋白衍生的天然保湿因子水平的扰动与皮肤病中角质层完整性降低密切相关。氨基酸代谢通路的扰动也可能是TKI相关皮疹的重要机制之一。
研究表明TKI诱发的皮疹与NSCLC预后改善呈正相关[7],且TKI相关皮疹患者TKI治疗时间更长,无进展生存期更长[10, 33]。为了更好地改善TKI相关皮疹症状及患者的生活质量,延长TKI治疗时间,本研究针对交集靶基因预测获得了354种中药,其中黄芩、水牛角、高良姜、人参类药物出现频次高,中药中寒、苦、辛类药物居多,但也不乏温、平、甘类药物相佐,归经以归肺、肝、胃经为主。此结论与临床用药经验大部分相符:现代中医医家认为肺癌患者正气不足,EGFR-TKI药物多属温热,药邪内侵,于中焦化热,上输于肺以达其功,风、湿、热毒发于腠理而致皮疹[13]。现临床中药多以水牛角、黄芩、紫草、黄柏、喜树果等寒、苦、辛类药物清热燥湿化瘀为主。刘卫东[34]用含有黄芩等药物炮制的清热润燥膏治疗NSCLC患者的TKI相关皮疹,相较于醋酸曲安奈德乳膏,其能有效改善皮疹;张振华和花宝金[35]使用黄芩、黄柏等清热燥湿中药治疗肺癌中邪热较盛者,皮疹缓解,体重稳定,临床疗效佳。但研究显示潜在治疗中药中不乏人参类、高良姜、白术、藁本、黄芪等温、平、甘类药物,提示在临证时需关注NSCLC患者多患病日久、体质本虚的特点,用药时亦应兼顾温养肺卫。
综上所述,本研究通过生物信息学方法观察到TKI治疗后EGFR突变NSCLC患者抗肿瘤作用及耐药性相关信号通路激活,说明肿瘤生长受到抑制,但伴随着耐药性的出现;成纤维细胞受TKI影响后mTOR信号通路、氨基酸代谢通路出现扰动,导致皮肤屏障完整性受损,随即发生TKI相关皮疹;TKI影响核分裂、DNA复制等生物学过程的同时调控肺、皮毛功能;药物预测出临床在治疗EGFR突变NSCLC伴TKI相关皮疹时,可选用性属寒、温、平,味属苦、辛、甘,归胃、肺、肝经的中药。本研究初探了转录和蛋白质水平EGFR突变NSCLC、TKI相关皮疹间的潜在关联机制,为临证提供了用药指导,但各潜在基因在EGFR突变NSCLC、TKI相关皮疹中的具体作用及预测中药的干预效果目前尚未可知,需进一步研究。
| [1] |
MILLER K D, NOGUEIRA L, MARIOTTO A B, et al. Cancer treatment and survivorship statistics, 2019[J]. CA Cancer J Clin, 2019, 69(5): 363-385. DOI:10.3322/caac.21565 |
| [2] |
DUMA N, SANTANA-DAVILA R, MOLINA J R. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment[J]. Mayo Clin Proc, 2019, 94(8): 1623-1640. DOI:10.1016/j.mayocp.2019.01.013 |
| [3] |
SIEGEL R L, MILLER K D, FUCHS H E, et al. Cancer statistics, 2021[J]. CA A Cancer J Clin, 2021, 71(1): 7-33. DOI:10.3322/caac.21654 |
| [4] |
SHALATA W, JACOB B M, AGBARYA A. Adjuvant treatment with tyrosine kinase inhibitors in epidermal growth factor receptor mutated non-small-cell lung carcinoma patients, past, present and future[J]. Cancers, 2021, 13(16): 4119. DOI:10.3390/cancers13164119 |
| [5] |
LIU J F, SUN X S, YIN J H, et al. Adjuvant EGFR-TKI therapy in resected EGFR-mutation positive non-small cell lung cancer: a real-world study[J]. Front Oncol, 2023, 13: 1132854. DOI:10.3389/fonc.2023.1132854 |
| [6] |
WU Q, LUO W, LI W, et al. First-generation EGFR-TKI plus chemotherapy versus EGFR-TKI alone as first-line treatment in advanced NSCLC with EGFR activating mutation: a systematic review and meta-analysis of randomized controlled trials[J]. Front Oncol, 2021, 11: 598265. DOI:10.3389/fonc.2021.598265 |
| [7] |
LIAO D, YAO D, LIU N, et al. Correlation of plasma erlotinib trough concentration with skin rash in Chinese NSCLC patients harboring exon 19 deletion mutation[J]. Cancer Chemother Pharmacol, 2018, 82(3): 551-559. DOI:10.1007/s00280-018-3642-4 |
| [8] |
PENG Y, LI Q, ZHANG J, et al. Update review of skin adverse events during treatment of lung cancer and colorectal carcinoma with epidermal growth receptor factor inhibitors[J]. Biosci Trends, 2019, 12(6): 537-552. DOI:10.5582/bst.2018.01246 |
| [9] |
DING P N, LORD S J, GEBSKI V, et al. Risk of treatment-related toxicities from EGFR tyrosine kinase inhibitors: a meta-analysis of clinical trials of gefitinib, erlotinib, and afatinib in advanced EGFR-mutated non-small cell lung cancer[J]. J Thorac Oncol, 2017, 12(4): 633-643. DOI:10.1016/j.jtho.2016.11.2236 |
| [10] |
ALANEN V, IIVANAINEN S, ARFFMAN M, et al. Tetracyclines increase the survival of NSCLC patients treated with EGFR TKIs: a retrospective nationwide registry study[J]. ESMO Open, 2020, 5(5): e000864. DOI:10.1136/esmoopen-2020-000864 |
| [11] |
KOZUKI T. Skin problems and EGFR-tyrosine kinase inhibitor[J]. Jpn J Clin Oncol, 2016, 46(4): 291-298. DOI:10.1093/jjco/hyv207 |
| [12] |
马会龙. 基于数据挖掘的中医药治疗第一代EGFR-TKI相关性皮疹用药规律分析及消风散联合多西环素临床疗效观察[D]. 天津: 天津中医药大学, 2021.
|
| [13] |
蒙家泉, 焦丽静, 毕凌, 等. 中医药与表皮生长因子受体酪氨酸激酶抑制剂结合治疗肺癌的理论初探[J]. 中华中医药杂志, 2021, 36(6): 3703-3705. |
| [14] |
朱杨壮壮, 董文馨, 邹纯朴, 等. 基于"肺主皮毛" 理论探讨银屑病与肺癌共病免疫机制[J]. 中华中医药杂志, 2022, 37(5): 2813-2819. |
| [15] |
FUCHS E, RAGHAVAN S. Getting under the skin of epidermal morphogenesis[J]. Nat Rev Genet, 2002, 3(3): 199-209. DOI:10.1038/nrg758 |
| [16] |
CANZLER S, HACKERMÜLLER J. multiGSEA: a GSEA-based pathway enrichment analysis for multi-omics data[J]. BMC Bioinformatics, 2020, 21(1): 561. DOI:10.1186/s12859-020-03910-x |
| [17] |
COHEN I, ZHAO D, BAR C, et al. PRC1 fine-tunes gene repression and activation to safeguard skin development and stem cell specification[J]. Cell Stem Cell, 2018, 22(5): 726-739. DOI:10.1016/j.stem.2018.04.005 |
| [18] |
COHEN I, ZHAO D, MENON G, et al. PRC1 preserves epidermal tissue integrity independently of PRC2[J]. Genes Dev, 2019, 33(1/2): 55-60. DOI:10.1101/gad.319939.118 |
| [19] |
HANSELMANN S, WOLTER P, MALKMUS J, et al. The microtubule-associated protein PRC1 is a potential therapeutic target for lung cancer[J]. Oncotarget, 2018, 9(4): 4985-4997. DOI:10.18632/oncotarget.23577 |
| [20] |
HANSELMANN S, GERTZMANN D, SHIN W J, et al. Expression of the cytokinesis regulator PRC1 results in p53-pathway activation in A549 cells but does not directly regulate gene expression in the nucleus[J]. Cell Cycle, 2023, 22(4): 419-432. DOI:10.1080/15384101.2022.2122258 |
| [21] |
WANG L, ZHANG Z, GE R, et al. Gossypetin inhibits solar-UV induced cutaneous basal cell carcinoma through direct inhibiting PBK/TOPK protein kinase[J]. Anticancer Agents Med Chem, 2019, 19(8): 1029-1036. DOI:10.2174/1871520619666190301123131 |
| [22] |
LEI B, QI W, ZHAO Y, et al. PBK/TOPK expression correlates with mutant p53 and affects patients' prognosis and cell proliferation and viability in lung adenocarcinoma[J]. Hum Pathol, 2015, 46(2): 217-224. DOI:10.1016/j.humpath.2014.07.026 |
| [23] |
GUO R, YING J, JIA L, et al. Regulators CDCA8 as potential targets and biomarkers for the prognosis of human skin cutaneous melanoma[J]. J Cosmet Dermatol, 2022, 21(11): 6034-6048. DOI:10.1111/jocd.15091 |
| [24] |
HAYAMA S, DAIGO Y, YAMABUKI T, et al. Phosphorylation and activation of cell division cycle associated 8 by aurora kinase B plays a significant role in human lung carcinogenesis[J]. Cancer Res, 2007, 67(9): 4113-4122. DOI:10.1158/0008-5472.can-06-4705 |
| [25] |
NISHIMURA Y, ITOH K. Involvement of SNX1 in regulating EGFR endocytosis in a gefitinib-resistant NSCLC cell lines[J]. Cancer Drug Resist, 2019, 2(3): 539-549. DOI:10.20517/cdr.2019.15 |
| [26] |
NISHIMURA Y, YOSHIOKA K, BERECZKY B, et al. Evidence for efficient phosphorylation of EGFR and rapid endocytosis of phosphorylated EGFR via the early/late endocytic pathway in a gefitinib-sensitive non-small cell lung cancer cell line[J]. Mol Cancer, 2008, 7: 42. DOI:10.1186/1476-4598-7-42 |
| [27] |
YANG F, ZHANG S, MENG Q, et al. CXCR1 correlates to poor outcomes of EGFR-TKI against advanced non-small cell lung cancer by activating chemokine and JAK/STAT pathway[J]. Pulm Pharmacol Ther, 2021, 67: 102001. DOI:10.1016/j.pupt.2021.102001 |
| [28] |
HUNG W Y, CHANG J H, CHENG Y, et al. Leukocyte cell-derived chemotaxin 2 retards non-small cell lung cancer progression through antagonizing MET and EGFR activities[J]. Cell Physiol Biochem, 2018, 51(1): 337-355. DOI:10.1159/000495233 |
| [29] |
PATEL S A, NILSSON M B, YANG Y, et al. IL6 mediates suppression of T- and NK-cell function in EMT-associated TKI-resistant EGFR-mutant NSCLC[J]. Clin Cancer Res, 2023, 29(7): 1292-1304. DOI:10.1158/1078-0432.CCR-22-3379 |
| [30] |
ZHANG Y, ZENG Y, LIU T, et al. The canonical TGF-β/Smad signalling pathway is involved in PD-L1-induced primary resistance to EGFR-TKIs in EGFR-mutant non-small-cell lung cancer[J]. Respir Res, 2019, 20(1): 164. DOI:10.1186/s12931-019-1137-4 |
| [31] |
WANG J, CUI B, CHEN Z, et al. The regulation of skin homeostasis, repair and the pathogenesis of skin diseases by spatiotemporal activation of epidermal mTOR signaling[J]. Front Cell Dev Biol, 2022, 10: 950973. DOI:10.3389/fcell.2022.950973 |
| [32] |
MARK H, HARDING C R. Amino acid composition, including key derivatives of eccrine sweat: potential biomarkers of certain atopic skin conditions[J]. Int J Cosmet Sci, 2013, 35(2): 163-168. DOI:10.1111/ics.12019 |
| [33] |
ALANEN V, IIVANAINEN S, ARFFMAN M, et al. Purchase of prophylactic topical corticosteroids is associated with improved survival in NSCLCs treated with EGFR TKI: real-world cohort study[J]. Acta Oncol, 2021, 60(9): 1100-1105. DOI:10.1080/0284186X.2021.1937309 |
| [34] |
刘卫东. 清热润燥膏治疗非小细胞肺癌靶向药物相关性皮疹的临床观察[D]. 长沙: 湖南中医药大学, 2021.
|
| [35] |
张振华, 花宝金. 花宝金治疗表皮生长因子受体酪氨酸激酶抑制剂所致皮疹经验[J]. 中华中医药杂志, 2020, 35(5): 2497-2500. |
 2024, Vol. 45
2024, Vol. 45


