引用本文

纵瑞清, 张红艳, 武卉淇, 陈影. 原发性肝癌肝切除术后预防性抗凝对术后并发症的影响[J]. 海军军医大学学报, 2024, 45(8): 964-972

ZONG Ruiqing, ZHANG Hongyan, WU Huiqi, CHEN Ying. Effects of prophylactic anticoagulation on postoperative complications after hepatectomy for primary liver cancer[J]. Academic Journal of Naval Medical University, 2024, 45(8): 964-972 (in Chinese with English abstract)

原发性肝癌肝切除术后预防性抗凝对术后并发症的影响
1. 上海交通大学医学院附属瑞金医院急诊科, 上海 200025;
2. 海军军医大学(第二军医大学)第三附属医院重症医学科, 上海 201805
收稿日期: 2023-11-06 接受日期: 2024-01-18
基金项目: 海军军医大学(第二军医大学)校级课题(2023QN098).
摘要: 目的 探究原发性肝癌(PLC)患者术后预防性抗凝治疗能否降低术后并发症的发生风险,并探讨术后并发症的影响因素。方法 收集2019年2月至2021年5月在海军军医大学(第二军医大学)第三附属医院接受PLC肝切除手术治疗的495例患者的临床资料,根据术后是否进行预防性抗凝将患者分为抗凝组(287例,术后接受预防性低分子肝素抗凝治疗)和常规治疗组(208例)。对比两组患者术后并发症发生情况,并采用logistic回归模型分析并发症发生的影响因素。结果 495例患者肝切除术后总体并发症发生率为30.7%(152/495),按照发生率由高到低依次为感染(9.1%,45/495)、急性呼吸窘迫综合征(ARDS;6.5%,32/495)、出血(6.3%,31/495)、肝切除术后肝功能衰竭(PHLF;6.1%,30/495)、静脉血栓栓塞症(VTE;2.8%,14/495)。抗凝组术后VTE、ARDS、PHLF发生率均低于常规治疗组[1.4%(4/287)vs 4.8%(10/208)、3.8%(11/287)vs 10.1%(21/208)、3.8%(11/287)vs 9.1%(19/208),均P<0.05],但两组间术后出血的发生率差异无统计学意义(P>0.05)。多因素logistic回归分析显示,年龄、门静脉高压、肿瘤数量是VTE的独立危险因素,门静脉高压、术中出血、术中输血、术前降钙素原是PHLF的独立危险因素,腹水、术前胆红素是ARDS的独立危险因素,而术后预防性抗凝是VTE、ARDS的独立保护因素(均P<0.05)。结论 PLC患者肝切除术后预防性抗凝可以降低VTE、PHLF、ARDS的发生风险,且不会增加术后出血风险。年龄、门静脉高压、肿瘤数量、术中出血、术中输血、腹水、术前降钙素原、术前胆红素是PLC患者肝切除术后并发症发生的危险因素。
关键词:
肝肿瘤 肝切除术 原发性肝癌 预防性抗凝 静脉血栓栓塞症 急性呼吸窘迫综合征 肝功能衰竭 出血
Effects of prophylactic anticoagulation on postoperative complications after hepatectomy for primary liver cancer
ZONG Ruiqing
1,2
, ZHANG Hongyan
2, WU Huiqi
2, CHEN Ying
1

1. Department of Emergency, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China;
2. Department of Critical Care Medicine, The Third Affiliated Hospital of Naval Medical University (Second Military Medical University), Shanghai 201805, China
Supported by Project of Naval Medical University (Second Military Medical University) (2023QN098).
Abstract: Objective To investigate whether prophylactic anticoagulation therapy can reduce the risk of postoperative complications in patients with primary liver cancer (PLC) after hepatectomy, and to explore the influencing factors of postoperative complications. Methods The clinical data of 495 patients undergoing hepatectomy for PLC in The Third Affiliated Hospital of Naval Medical University (Second Military Medical University) from Feb. 2019 to May 2021 were collected. The patients were divided into anticoagulation group (n=287, receiving prophylactic low-molecular-weight heparin after surgery) and conventional treatment group (n=208). The postoperative complications were compared between the 2 groups, and the influencing factors were analyzed using logistic regression model. Results The postoperative overall complication incidence of the 495 patients after hepatectomy was 30.7% (152/495), ranking as infection (9.1%, 45/495), acute respiratory distress syndrome (ARDS; 6.5%, 32/495), bleeding (6.3%, 31/495), post-hepatectomy liver failure (PHLF; 6.1%, 30/495), and venous thromboembolism (VTE; 2.8%, 14/495). The incidence rates of postoperative VTE, ARDS, and PHLF were significantly lower in the anticoagulation group than those in the conventional treatment group (1.4% [4/287] vs 4.8% [10/208], 3.8% [11/287] vs 10.1% [21/208], and 3.8% [11/287] vs 9.1% [19/208]; all P < 0.05), but there was no significant difference in the incidence of postoperative bleeding between the 2 groups (P > 0.05). Multivariate logistic regression analysis showed that age, portal hypertension, and tumor number were independent risk factors for postoperative VTE; portal hypertension, intraoperative blood loss, intraoperative blood transfusion, and preoperative procalcitonin (PCT) were independent risk factors for PHLF; ascites and preoperative bilirubin were independent risk factors for ARDS; and postoperative prophylactic anticoagulation was an independent protective factor for VTE and ARDS (all P < 0.05). Conclusion Prophylactic anticoagulation can reduce the risks of VTE, PHLF, and ARDS in PLC patients after hepatectomy, without increasing the risk of postoperative bleeding. Age, portal hypertension, number of tumors, intraoperative blood loss, intraoperative blood transfusion, ascites, preoperative PCT, and preoperative bilirubin are risk factors for postoperative complications of PLC patients after hepatectomy.
Key words:
liver neoplasms hepatectomy primary liver cancer prophylactic anticoagulation venous thromboembolism acute respiratory distress syndrome liver failure hemorrhage
全世界范围内超过54%的原发性肝癌(primary liver cancer,PLC)发生在我国,严重威胁我国人民的健康和生命[1]。在我国PLC患者中大多数有慢性肝病史,HBV感染是其主要原因,合并肝硬化者占比更是高达85%以上[2],由此可见未来我国原发性肝细胞癌(hepatocellular carcinoma,HCC)仍是主要的卫生问题。肝切除和肝移植是目前HCC主要的、最有效的治疗手段,但是由于器官短缺,肝移植受到限制,因此肝切除手术仍然是HCC患者的首选治疗方案。尽管现代手术的安全性得以提升,但是肝切除手术依然是所有腹部外科手术中风险较高且并发症最多的手术之一,文献报道其并发症发生率为40.2%~55.5%[3-4]。即便肝切除手术死亡率已经从历史最高点的32%下降至目前的1%~10%[4-6],肝切除术后的高并发症及高死亡率还困扰着许多临床医师,不仅导致住院时间长、住院费用居高不下,也严重影响着患者的生命安全[7]。
既往已经有研究探讨,肝脏外科医师由于意识到术后出血风险而拒绝开具抗凝剂的化学预防处方[8]。然而有研究表明,术后抗凝血剂能有效降低门静脉血栓形成的发生率,且术后静脉血栓栓塞症(venous thromboembolism,VTE)的发生率远高于出血的发生率,因而支持肝切除术后常规进行VTE化学预防[9-10]。同时抗凝剂也具有抗炎、抗病毒、促进伤口愈合、预防贫血等重要的药理作用[11]。目前我国临床上肝切除术后预防性使用抗凝药物的病例相对较少,其原因之一就是顾虑术后出血的风险,而且目前肝切除术后预防性应用抗凝药物的结论大多基于国外临床研究。研究肝切除术后预防性抗凝对于临床医师提高VTE防治意识、规范诊疗行为、减少术后并发症、降低病死率、提高患者生活质量具有重要意义。本研究回顾性分析了肝切除术后预防性抗凝和非抗凝治疗对术后并发症的影响,并分析术后发生并发症的危险因素,旨在为临床降低术后并发症的发生率提供依据。
1 对象和方法
1.1 研究对象与分组
选择2019年2月至2021年5月在海军军医大学(第二军医大学)第三附属医院接受PLC肝切除手术的495例患者为研究对象。纳入标准:(1)不存在抗凝治疗的禁忌证;(2)知情同意病例数据用于研究;(3)能严格遵医嘱接受治疗;(4)入组前1个月内未进行过溶栓、抗凝、抗血小板等治疗;(5)术前检查无静脉血栓;(6)Child分级A级,且一般状况良好,美国麻醉师协会分级为Ⅰ级或Ⅱ级。排除标准:(1)治疗依从性低者;(2)合并血液、颅脑、肾脏、心脏等其他系统疾病者;(3)由于无法切除或其他原因未行肝切除术,仅行剖腹探查+活检术者。本研究通过海军军医大学(第二军医大学)第三附属医院医学伦理委员会审批(EHBHKY2021-K-011)。
以术后预防性抗凝与否将患者分为两组:(1)抗凝组287例,术后24 h内接受低分子肝素抗凝治疗(低分子肝素钙注射液,商品名为速碧林,0.4 mL、1次/12 h皮下注射),并予广谱抗生素预防感染、抑酸、化痰、肠外营养(包括氨基酸、中长链脂肪乳、葡萄糖溶液、多种维生素)、白蛋白、利尿、控制血压、维持内环境稳定及水、电解质平衡等常规术后处理;(2)常规治疗组208例,仅给予广谱抗生素预防感染、抑酸、化痰、肠外营养(包括氨基酸、中长链脂肪乳、葡萄糖溶液、多种维生素)、白蛋白、利尿、控制血压、维持内环境稳定及水、电解质平衡等常规术后处理。
1.2 观察指标
(1)凝血功能指标:分别在治疗前、后抽取患者3 mL静脉血,以3 500 r/min(离心半径为5 cm)离心15 min获得血浆,检测国际标准化比值、凝血酶原时间等凝血功能指标。(2)急性呼吸窘迫综合征(acute respiratory distress syndrome,ARDS)发生情况:ARDS的诊断需满足以下条件。①在已知临床损害发生的1周内出现新的呼吸系统症状;②X线片或CT检查示双肺致密影,并且用胸腔积液、肺叶/肺塌陷或结节不能完全解释;③无法用心力衰竭或体液超负荷完全解释,对于不存在危险因素的患者需要排除静水压相关性肺水肿;④存在中到重度氧合指数下降,其中氧合指数定义为PaO2/FiO2(PaO2为动脉氧分压,FiO2为吸入氧浓度)。(3)肝切除术后肝功能衰竭(post-hepatectomy liver failure,PHLF)发生情况:PHLF是指各种因素引起的严重肝脏损害,导致肝脏合成、解毒、代谢和生物转化功能严重障碍或失代偿,从而出现以黄疸、凝血功能障碍、肝肾综合征、肝性脑病、腹水等为主要表现的一组临床症候群。(4)VTE发生情况:VTE包括深静脉血栓形成、肺动脉栓塞、门静脉血栓,其中深静脉血栓形成的诊断采用二维超声检查,肺动脉栓塞的诊断采用CT血管成像,门静脉血栓的诊断采用超声、CT和MRI检查。(5)收集患者治疗过程中的白细胞计数、中性粒细胞比例、CRP、降钙素原、血红蛋白、血细胞比容及术中出血、术中输血、术后出血等情况。
1.3 统计学处理
采用SPSS 25.0软件进行统计学分析。对计量资料行正态性检验,符合正态分布的计量资料以x±s表示,组间比较采用Z检验;不符合正态分布的计量资料以中位数(下四分位数,上四分位数)表示,组间比较采用秩和检验。计数资料以例数和百分数表示,组间比较采用χ2检验。将单因素logistic回归分析中P<0.05的变量纳入多因素logistic回归模型,分析并发症发生的独立影响因素。采用ROC曲线和AUC描述和比较不同指标对术后并发症预测的准确性。检验水准(α)为0.05。
2 结果
2.1 两组患者一般资料的比较
抗凝组287例,男236例、女51例,年龄为18~90(56.00±12.43)岁。常规治疗组208例,男166例、女42例,年龄为20~79(57.00±11.02)岁。两组患者性别、年龄、高血压病史、糖尿病史、吸烟史、既往肝脏手术史、肝硬化病史、门静脉高压病史等差异均无统计学意义(均P>0.05)。抗凝组有腹水、术中输血的患者比例均低于常规治疗组(均P<0.05),术中肝门阻断时间长于常规治疗组(P<0.001)。见表 1。
表 1
(Tab 1)
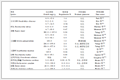
表 1 两组PLC患者一般临床资料的比较
Tab 1 Comparison of general clinical data of PLC patients between 2 groups
| Index |
Total N=495 |
Anticoagulation group N=287 |
Conventional treatment group N=208 |
P value |
| Gender, n (%) |
|
|
|
0.496 |
| Female |
93 (18.8) |
51 (17.8) |
42 (20.2) |
|
| Male |
402 (81.2) |
236 (82.2) |
166 (79.8) |
|
| Age/year, x±s |
56.00±11.86 |
56.00±12.43 |
57.00±11.02 |
0.296 |
| History of hypertension, n (%) |
113 (22.8) |
66 (23.0) |
47 (22.6) |
0.917 |
| History of diabetes mellitus, n (%) |
54 (10.9) |
31 (10.8) |
23 (11.1) |
0.928 |
| Smoking history, n (%) |
198 (40.0) |
115 (40.1) |
83 (39.9) |
0.970 |
| Drinking history, n (%) |
150 (30.3) |
85 (29.6) |
65 (31.2) |
0.696 |
| History of hepatitis B, n (%) |
229 (46.3) |
130 (45.3) |
99 (47.6) |
0.612 |
| Previous history of liver surgery, n (%) |
52 (10.5) |
34 (11.8) |
18 (8.7) |
0.253 |
| Cirrhosis, n (%) |
224 (45.3) |
133 (46.3) |
91 (43.8) |
0.567 |
| Ascites, n (%) |
56 (11.3) |
25 (8.7) |
31 (14.9) |
0.032 |
| Intraoperative blood loss, n (%) |
31 (6.3) |
14 (4.9) |
17 (8.2) |
0.136 |
| Intraoperative blood transfusion, n (%) |
115 (23.2) |
47 (16.4) |
68 (32.7) |
<0.001 |
| Surgical method, n (%) |
|
|
|
0.405 |
| Open surgery |
482 (97.4) |
278 (96.9) |
204 (98.1) |
|
| Laparoscopic surgery |
13 (2.6) |
9 (3.1) |
4 (1.9) |
|
| Liver resection range, n (%) |
|
|
|
0.570 |
| ≤3 liver segments |
333 (67.3) |
196 (68.3) |
137 (65.9) |
|
| >3 liver segments |
162 (32.7) |
91 (31.7) |
71 (34.1) |
|
| Antiviral therapy, n (%) |
155 (31.3) |
98 (34.1) |
57 (27.4) |
0.110 |
| Abnormal prothrombin time, n (%) |
188 (38.0) |
105 (36.6) |
83 (39.9) |
0.453 |
| Portal hypertension, n (%) |
82 (16.6) |
51 (17.8) |
31 (14.9) |
0.397 |
| Preoperative PLT/(L-1, ×109), x±s |
163.66±69.10 |
162.33±67.86 |
165.50±70.91 |
0.615 |
| Preoperative INR, x±s |
0.99±0.09 |
0.99±0.09 |
1.00±0.09 |
0.562 |
| Preoperative neutrophil ratio, x±s |
0.59±0.11 |
0.58±0.12 |
0.58±0.11 |
0.984 |
| Tumor maximum diameter/cm, M (QL, QU) |
5.00 (3.00, 7.00) |
5.00 (3.00, 7.00) |
5.00 (3.00, 8.25) |
0.079 |
| Porta hepatis occlusion time/min, M (QL, QU) |
17.0 (0.0, 31.0) |
21.0 (8.0, 38.0) |
4.5 (0.0, 21.5) |
<0.001 |
| Preoperative bilirubin/(U·L-1), M (QL, QU) |
12.60 (9.00, 17.20) |
12.80 (9.00, 17.20) |
12.20 (8.65, 19.10) |
0.415 |
| Preoperative ALT/(U·L-1), M (QL, QU) |
26.0 (18.0, 40.0) |
26.0 (17.0, 41.0) |
24.5 (19.0, 30.7) |
0.236 |
| Preoperative AST/(U·L-1), M (QL, QU) |
26.0 (19.0, 38.0) |
26.0 (19.0, 39.0) |
23.0 (19.5, 37.2) |
0.377 |
| Preoperative LDH/(U·L-1), M (QL, QU) |
173.0 (147.0, 200.0) |
175.0 (151.0, 200.0) |
167.0 (138.2, 208.5) |
0.931 |
| Preoperative CRP/(mg·L-1), M (QL, QU) |
5 (5, 5) |
5 (5, 5) |
5 (5, 5) |
0.639 |
| Preoperative WBC/(L-1, ×109), M (QL, QU) |
5.12 (3.94, 6.38) |
5.16 (3.97, 6.28) |
5.04 (3.86, 6.43) |
0.244 |
| PLC: Primary liver cancer; PLT: Platelet count; INR: International normalized ratio; ALT: Alanine transaminase; AST: Aspartate transaminase; LDH: Lactate dehydrogenase; CRP: C reactive protein; WBC: White blood cell count; M (QL, QU): Median (lower quartile, upper quartile). |
|
表 1 两组PLC患者一般临床资料的比较
Tab 1 Comparison of general clinical data of PLC patients between 2 groups
|
将预防性抗凝组患者以抗凝开始时间是否在术后12 h内分为2个亚组,其中术后12 h内抗凝的患者有112例、手术12 h后抗凝的患者有175例。2个亚组患者的年龄、性别、吸烟史、糖尿病史、高血压病史、既往肝脏手术史、肝硬化病史等临床特征差异均无统计学意义(均P>0.05),术后12 h内抗凝的患者肿瘤大小和术前CRP水平均低于手术12 h后抗凝的患者(均P<0.05)。见表 2。
表 2
(Tab 2)

表 2 不同抗凝时机PLC患者的一般临床资料比较
Tab 2 Comparison of general clinical data among PLC patients with different anticoagulation timings
| Index |
Anticoagulation within 12 h after surgery N=112 |
Anticoagulation after 12 h of surgery N=175 |
P value |
| Gender, n (%) |
|
|
0.327 |
| Female |
23 (20.5) |
28 (16.0) |
|
| Male |
89 (79.5) |
147 (84.0) |
|
| Age/year, x±s |
56.78±11.21 |
54.75±13.12 |
0.179 |
| History of hypertension, n (%) |
32 (28.6) |
34 (19.4) |
0.073 |
| History of diabetes mellitus, n (%) |
17 (15.2) |
14 (8.0) |
0.056 |
| Smoking history, n (%) |
47 (42.0) |
68 (38.9) |
0.600 |
| Drinking history, n (%) |
37 (33.0) |
48 (27.4) |
0.310 |
| History of hepatitis B, n (%) |
49 (43.8) |
81 (46.3) |
0.635 |
| Previous history of liver surgery, n (%) |
12 (10.7) |
22 (12.6) |
0.217 |
| Cirrhosis, n (%) |
58 (51.8) |
75 (42.9) |
0.139 |
| Ascites, n (%) |
10 (8.9) |
15 (8.6) |
0.917 |
| Intraoperative blood transfusion, n (%) |
13 (11.6) |
34 (19.4) |
0.411 |
| Surgical method, n (%) |
|
|
0.722 |
| Open surgery |
109 (97.3) |
169 (96.6) |
|
| Laparoscopic surgery |
3 (2.7) |
6 (3.4) |
|
| Liver resection range, n (%) |
|
|
0.514 |
| ≤3 liver segments |
79 (70.5) |
117 (66.9) |
|
| >3 liver segments |
33 (29.5) |
58 (33.1) |
|
| Antiviral therapy, n (%) |
44 (39.3) |
54 (30.9) |
0.142 |
| Abnormal prothrombin time, n (%) |
43 (38.4) |
62 (35.4) |
0.611 |
| Portal hypertension, n (%) |
16 (14.3) |
35 (20.0) |
0.217 |
| Preoperative PLT/(L-1, ×109), x±s |
163.96±66.29 |
161.30±69.01 |
0.549 |
| Preoperative INR, x±s |
0.99±0.11 |
0.99±0.08 |
0.143 |
| Preoperative neutrophil ratio, x±s |
0.57±0.11 |
0.58±0.12 |
0.833 |
| Tumor maximum diameter/cm, M (QL, QU) |
4.00 (3.00, 6.00) |
5.00 (3.00, 7.50) |
0.009 |
| Porta hepatis occlusion time/min, M (QL, QU) |
24.0 (15.0, 37.5) |
20.0 (7.0, 38.0) |
0.538 |
| Preoperative bilirubin/(U·L-1), M (QL, QU) |
13.2 (9.00, 17.30) |
12.60 (8.90, 17.10) |
0.845 |
| Preoperative ALT/(U·L-1), M (QL, QU) |
24.0 (15.0, 38.0) |
29.0 (18.0, 42.0) |
0.146 |
| Preoperative AST/(U·L-1), M (QL, QU) |
24.0 (18.0, 36.0) |
27.0 (19.0, 41.0) |
0.319 |
| Preoperative LDH/(U·L-1), M (QL, QU) |
172.0 (145.3, 199.8) |
177.0 (152.0, 201.0) |
0.208 |
| Preoperative CRP/(mg·L-1), M (QL, QU) |
5 (5, 5) |
5 (5, 5) |
0.047 |
| Preoperative WBC/(L-1, ×109), M (QL, QU) |
5.00 (3.70, 6.10) |
5.20 (4.10, 6.40) |
0.121 |
| The mean ranks of preoperative CRP were 138.67 and 147.41 in the subgroups of anticoagulation within 12 h and after 12 h of surgery, respectively. PLC: Primary liver cancer; PLT: Platelet count; INR: International normalized ratio; ALT: Alanine transaminase; AST: Aspartate transaminase; LDH: Lactate dehydrogenase; CRP: C reactive protein; WBC: White blood cell count; M (QL, QU): Median (lower quartile, upper quartile). |
|
表 2 不同抗凝时机PLC患者的一般临床资料比较
Tab 2 Comparison of general clinical data among PLC patients with different anticoagulation timings
|
2.2 两组患者术后并发症的比较
495例患者肝切除术后总体并发症发生率为30.7%(152/495),按照发生率由高到低依次为感染、ARDS、术后出血、PHLF、VTE。抗凝组术后VTE、ARDS、PHLF发生率均低于常规治疗组(均P<0.05),但与常规治疗比较预防性抗凝并未增加术后出血的发生率(P>0.05)。见表 3。
表 3
(Tab 3)

表 3 PLC患者术后并发症发生情况的比较
Tab 3 Comparison of postoperative complications in PLC patients
| n (%) |
| Complication |
Total N=495 |
Anticoagulant group N=287 |
Conventional treatment group N=208 |
P value |
| VTE |
14 (2.8) |
4 (1.4) |
10 (4.8) |
0.024 |
| Infection |
45 (9.1) |
28 (9.8) |
17 (8.2) |
0.545 |
| ARDS |
32 (6.5) |
11 (3.8) |
21 (10.1) |
0.005 |
| PHLF |
30 (6.1) |
11 (3.8) |
19 (9.1) |
0.015 |
| Bleeding |
31 (6.3) |
14 (4.9) |
17 (8.2) |
0.135 |
| PLC: Primary liver cancer; VTE: Venous thromboembolism; ARDS: Acute respiratory distress syndrome; PHLF: Post-hepatectomy liver failure. |
|
表 3 PLC患者术后并发症发生情况的比较
Tab 3 Comparison of postoperative complications in PLC patients
|
2.3 术后VTE影响因素的logistic回归分析
单因素logistic回归分析结果提示,年龄、肝硬化、门静脉高压、肿瘤数量、预防性抗凝、术前PLT与术后VTE有关(均P<0.05,表 4);多因素logistic回归分析结果提示,年龄、门静脉高压、肿瘤数量是术后VTE的独立危险因素,而预防性抗凝是术后VTE的独立保护因素(均P<0.05,表 5)。
表 4
(Tab 4)

表 4 PLC患者术后VTE影响因素的单因素logistic回归分析
Tab 4 Univariate logistic regression analysis of influencing factors of postoperative VTE in PLC patients
| Variable |
Regression coefficient |
Standard error |
Wald |
OR (95% CI) |
P value |
| Gender |
-17.881 |
4 167.817 |
0.000 |
0.000 |
0.997 |
| Age |
0.063 |
0.026 |
5.738 |
1.065 (1.012, 1.122) |
0.017 |
| History of hypertension |
-0.588 |
0.771 |
0.581 |
0.556 (0.122, 2.520) |
0.446 |
| History of diabetes mellitus |
-0.476 |
1.048 |
0.206 |
0.621 (0.080, 4.844) |
0.650 |
| Smoking history |
1.023 |
0.566 |
3.271 |
2.781 (0.918, 8.425) |
0.071 |
| Drinking history |
0.860 |
0.544 |
2.503 |
2.364 (0.814, 6.862) |
0.114 |
| Previous history of liver surgery |
0.877 |
0.669 |
1.722 |
2.404 (0.649, 8.914) |
0.189 |
| Cirrhosis |
1.529 |
0.658 |
5.403 |
4.613 (1.271, 16.747) |
0.020 |
| Portal hypertension |
2.309 |
0.572 |
16.286 |
10.060 (3.278, 30.871) |
<0.001 |
| Ascites |
-0.518 |
1.048 |
0.244 |
0.596 (0.076, 4.643) |
0.621 |
| Number of tumors |
0.480 |
0.200 |
5.757 |
1.616 (1.092, 2.392) |
0.016 |
| Tumor maximum diameter |
0.046 |
0.062 |
0.553 |
1.047 (0.928, 1.181) |
0.457 |
| Hepatic hilum obstruction |
-0.276 |
0.323 |
0.726 |
0.759 (0.403, 1.431) |
0.394 |
| Porta hepatis occlusion time |
0.005 |
0.008 |
0.460 |
1.005 (0.990, 1.021) |
0.498 |
| Intraoperative blood loss |
0.000 |
0.000 |
2.322 |
1.000 (1.000, 1.001) |
0.128 |
| Intraoperative blood transfusion |
0.288 |
0.601 |
0.229 |
1.333 (0.410, 4.334) |
0.632 |
| Laparoscopic surgery |
-17.693 |
11 147.524 |
0.000 |
0.000 |
0.999 |
| Liver resection range |
0.137 |
0.566 |
0.058 |
1.146 (0.378, 3.478) |
0.809 |
| Prophylactic anticoagulation |
-1.273 |
0.599 |
4.523 |
0.280 (0.087, 0.905) |
0.033 |
| Antiviral therapy |
-0.527 |
0.659 |
0.640 |
0.590 (0.162, 2.147) |
0.424 |
| HBV DNA |
0.154 |
0.542 |
0.081 |
1.167 (0.403, 3.377) |
0.776 |
| Preoperative ALP |
-0.209 |
0.548 |
0.145 |
0.812 (0.277, 2.377) |
0.703 |
| Preoperative bilirubin |
-0.033 |
0.042 |
0.623 |
0.968 (0.892, 1.050) |
0.430 |
| Preoperative ALT |
0.005 |
0.005 |
1.150 |
1.005 (0.996, 1.015) |
0.284 |
| Preoperative AST |
0.005 |
0.005 |
0.950 |
1.005 (0.995, 1.015) |
0.330 |
| Preoperative LDH |
-0.008 |
0.007 |
1.322 |
0.992 (0.978, 1.006) |
0.250 |
| Preoperative CRP |
-0.004 |
0.027 |
0.021 |
0.996 (0.945, 1.050) |
0.885 |
| Preoperative WBC |
-0.281 |
0.176 |
2.539 |
0.755 (0.535, 1.067) |
0.111 |
| Preoperative neutrophil ratio |
-0.001 |
0.023 |
0.002 |
0.999 (0.954, 1.046) |
0.965 |
| Preoperative PCT |
0.044 |
0.032 |
1.901 |
1.045 (0.982, 1.112) |
0.168 |
| Preoperative PLT |
-0.016 |
0.006 |
7.981 |
0.984 (0.974, 0.995) |
0.005 |
| Preoperative INR |
1.224 |
2.619 |
0.218 |
3.400 (0.020, 576.555) |
0.640 |
| PLC: Primary liver cancer; VTE: Venous thromboembolism; HBV: Hepatitis B virus; ALP: Alkaline phosphatase; ALT: Alanine transaminase; AST: Aspartate transaminase; LDH: Lactate dehydrogenase; CRP: C reactive protein; WBC: White blood cell count; PCT: Procalcitonin; PLT: Platelet count; INR: International normalized ratio; OR: Odds ratio; CI: Confidence interval. |
|
表 4 PLC患者术后VTE影响因素的单因素logistic回归分析
Tab 4 Univariate logistic regression analysis of influencing factors of postoperative VTE in PLC patients
|
表 5
(Tab 5)

表 5 PLC患者术后VTE影响因素的多因素logistic回归分析
Tab 5 Multivariate logistic regression analysis of influencing factors of postoperative VTE in PLC patients
| Variable |
Regression coefficient |
Standard error |
Wald |
OR (95% CI) |
P value |
| Age |
0.072 |
0.030 |
5.707 |
1.075 (1.013, 1.141) |
0.017 |
| Portal hypertension |
1.981 |
0.793 |
6.237 |
7.248 (1.532, 34.298) |
0.013 |
| Number of tumors |
0.678 |
0.266 |
6.478 |
1.970 (1.169, 3.319) |
0.011 |
| Prophylactic anticoagulation |
-1.360 |
0.635 |
4.585 |
0.257 (0.074, 0.891) |
0.032 |
| PLC: Primary liver cancer; VTE: Venous thromboembolism; OR: Odds ratio; CI: Confidence interval. |
|
表 5 PLC患者术后VTE影响因素的多因素logistic回归分析
Tab 5 Multivariate logistic regression analysis of influencing factors of postoperative VTE in PLC patients
|
2.4 PHLF影响因素的logistic回归分析
单因素logistic回归分析结果提示,门静脉高压、腹水、肿瘤数量、肿瘤大小、术中出血、术中输血、预防性抗凝、术前降钙素原、术前PLT、术前国际标准化比值均与PLC患者术后发生PHLF与否有关(均P<0.05,表 6);多因素logistic回归分析结果提示,门静脉高压、术中出血、术中输血、术前降钙素原均是PLC患者术后发生PHLF的独立危险因素(均P<0.05,表 7)。
表 6
(Tab 6)

表 6 PLC患者PHLF影响因素的单因素logistic回归分析
Tab 6 Univariate logistic regression analysis of influencing factors of PHLF in PLC patients
| Variable |
Regression coefficient |
Standard error |
Wald |
OR (95% CI) |
P value |
| Gender |
-0.431 |
0.550 |
0.614 |
0.650 (0.221, 1.910) |
0.433 |
| Age |
0.008 |
0.016 |
0.266 |
1.008 (0.977, 1.041) |
0.606 |
| History of hypertension |
0.564 |
0.403 |
1.956 |
1.757 (0.798, 3.872) |
0.162 |
| History of diabetes mellitus |
-0.103 |
0.626 |
0.027 |
0.902 (0.264, 3.079) |
0.869 |
| Smoking history |
0.290 |
0.378 |
0.588 |
1.336 (0.637, 2.803) |
0.443 |
| Drinking history |
0.605 |
0.382 |
2.503 |
1.831 (0.866, 3.873) |
0.114 |
| Previous history of liver surgery |
-0.523 |
0.747 |
0.490 |
0.593 (0.137, 2.564) |
0.484 |
| Cirrhosis |
0.203 |
0.377 |
0.290 |
1.225 (0.585, 2.564) |
0.590 |
| Portal hypertension |
1.167 |
0.400 |
8.505 |
3.213 (1.466, 7.039) |
0.004 |
| Ascites |
1.516 |
0.417 |
13.200 |
4.554 (2.010, 10.319) |
<0.001 |
| Number of tumors |
0.411 |
0.156 |
6.914 |
1.508 (1.110, 2.049) |
0.009 |
| Tumor maximum diameter |
0.096 |
0.039 |
5.976 |
1.101 (1.019, 1.189) |
0.014 |
| Hepatic hilum obstruction |
-0.001 |
0.170 |
0.000 |
0.999 (0.716, 1.395) |
0.996 |
| Porta hepatis occlusion time |
0.006 |
0.005 |
1.376 |
1.006 (0.996, 1.017) |
0.241 |
| Intraoperative blood loss |
0.002 |
0.000 |
24.294 |
1.002 (1.001, 1.002) |
<0.001 |
| Intraoperative blood transfusion |
1.893 |
0.396 |
22.873 |
6.639 (3.056, 14.422) |
<0.001 |
| Laparoscopic surgery |
-18.490 |
11 147.524 |
0.000 |
0.000 |
0.999 |
| Liver resection range |
0.186 |
0.392 |
0.225 |
1.204 (0.559, 2.594) |
0.636 |
| Prophylactic anticoagulation |
-0.925 |
0.390 |
5.615 |
0.396 (0.184, 0.852) |
0.018 |
| Antiviral therapy |
-0.634 |
0.467 |
1.845 |
0.530 (0.212, 1.325) |
0.174 |
| HBV DNA |
0.017 |
0.378 |
0.002 |
1.017 (0.485, 2.133) |
0.963 |
| APTT |
0.379 |
0.410 |
0.855 |
1.460 (0.650, 3.260) |
0.355 |
| Preoperative bilirubin |
0.008 |
0.004 |
3.287 |
1.008 (0.999, 1.017) |
0.070 |
| Preoperative ALT |
0.003 |
0.004 |
0.545 |
1.003 (0.995, 1.012) |
0.460 |
| Preoperative AST |
0.003 |
0.004 |
0.562 |
1.003 (0.995, 1.012) |
0.454 |
| Preoperative LDH |
0.003 |
0.002 |
2.879 |
1.003 (1.000, 1.006) |
0.090 |
| Preoperative CRP |
-0.011 |
0.022 |
0.239 |
0.989 (0.947, 1.033) |
0.625 |
| Preoperative WBC |
-0.018 |
0.058 |
0.095 |
0.982 (0.877, 1.100) |
0.758 |
| Preoperative neutrophil ratio |
0.031 |
0.018 |
3.076 |
1.032 (0.996, 1.069) |
0.079 |
| Preoperative PCT |
0.064 |
0.024 |
6.921 |
1.066 (1.016, 1.118) |
0.009 |
| Preoperative PLT |
-0.009 |
0.003 |
7.759 |
0.991 (0.984, 0.997) |
0.005 |
| Preoperative INR |
5.100 |
1.624 |
9.865 |
164.031 (6.804, 3 954.512) |
0.002 |
| PLC: Primary liver cancer; PHLF: Post-hepatectomy liver failure; HBV: Hepatitis B virus; APTT: Activated partial thromboplastin time; ALT: Alanine transaminase; AST: Aspartate transaminase; LDH: Lactate dehydrogenase; CRP: C reactive protein; WBC: White blood cell count; PCT: Procalcitonin; PLT: Platelet count; INR: International normalized ratio; OR: Odds ratio; CI: Confidence interval. |
|
表 6 PLC患者PHLF影响因素的单因素logistic回归分析
Tab 6 Univariate logistic regression analysis of influencing factors of PHLF in PLC patients
|
表 7
(Tab 7)

表 7 PLC患者PHLF影响因素的多因素logistic回归分析
Tab 7 Multivariate logistic regression analysis of influencing factors of PHLF in PLC patients
| Variable |
Regression coefficient |
Standard error |
Wald |
OR (95% CI) |
P value |
| Portal hypertension |
1.046 |
0.427 |
6.003 |
2.848 (1.233, 6.577) |
0.014 |
| Intraoperative blood loss |
0.001 |
0.000 |
5.368 |
1.001 (1.000, 1.002) |
0.021 |
| Intraoperative blood transfusion |
1.032 |
0.522 |
3.907 |
2.807 (1.009, 7.811) |
0.048 |
| Preoperative PCT |
0.054 |
0.025 |
4.633 |
1.055 (1.005, 1.108) |
0.031 |
| PLC: Primary liver cancer; PHLF: Post-hepatectomy liver failure; PCT: Procalcitonin; OR: Odds ratio; CI: Confidence interval. |
|
表 7 PLC患者PHLF影响因素的多因素logistic回归分析
Tab 7 Multivariate logistic regression analysis of influencing factors of PHLF in PLC patients
|
2.5 术后ARDS影响因素的logistic回归分析
单因素和多因素logistic回归结果均提示,腹水、术前胆红素是PLC患者术后发生ARDS的独立危险因素,而预防性抗凝是术后发生ARDS的独立保护因素(均P<0.05,表 8、9)。
表 8
(Tab 8)

表 8 PLC患者术后ARDS影响因素的单因素logistic回归分析
Tab 8 Univariate logistic regression analysis of influencing factors of postoperative ARDS in PLC patients
| Variable |
Regression coefficient |
Standard error |
Wald |
OR (95% CI) |
P value |
| Gender |
-0.237 |
0.501 |
0.223 |
0.789 (0.296, 2.107) |
0.636 |
| Age |
0.032 |
0.017 |
3.781 |
1.033 (1.000, 1.067) |
0.052 |
| History of hypertension |
0.463 |
0.397 |
1.357 |
1.589 (0.729, 3.462) |
0.244 |
| History of diabetes mellitus |
0.691 |
0.478 |
2.089 |
1.995 (0.782, 5.090) |
0.148 |
| Smoking history |
0.434 |
0.366 |
1.407 |
1.544 (0.753, 3.164) |
0.235 |
| Drinking history |
0.487 |
0.374 |
1.698 |
1.628 (0.782, 3.389) |
0.193 |
| Previous history of liver surgery |
-0.135 |
0.625 |
0.046 |
0.874 (0.257, 2.975) |
0.829 |
| Cirrhosis |
0.204 |
0.366 |
0.310 |
1.226 (0.599, 2.510) |
0.577 |
| Portal hypertension |
0.371 |
0.446 |
0.691 |
1.449 (0.605, 3.471) |
0.406 |
| Ascites |
1.058 |
0.436 |
5.899 |
2.882 (1.227, 6.771) |
0.015 |
| Number of tumors |
-0.288 |
0.302 |
0.908 |
0.750 (0.415, 1.356) |
0.341 |
| Tumor maximum diameter |
0.037 |
0.043 |
0.752 |
1.038 (0.954, 1.129) |
0.386 |
| Hepatic hilum obstruction |
-0.292 |
0.219 |
1.778 |
0.747 (0.487, 1.147) |
0.182 |
| Porta hepatis occlusion time |
-0.008 |
0.009 |
0.843 |
0.992 (0.975, 1.009) |
0.359 |
| Intraoperative blood loss |
0.000 |
0.000 |
1.428 |
1.000 (1.000, 1.001) |
0.232 |
| Intraoperative blood transfusion |
0.000 |
0.000 |
3.352 |
1.000 (1.000, 1.001) |
0.067 |
| Laparoscopic surgery |
0.193 |
1.057 |
0.033 |
1.212 (0.153, 9.629) |
0.855 |
| Liver resection range |
-0.586 |
0.439 |
1.785 |
0.556 (0.235, 1.315) |
0.182 |
| Prophylactic anticoagulation |
-1.036 |
0.384 |
7.276 |
0.355 (0.167, 0.753) |
0.007 |
| Antiviral therapy |
-0.163 |
0.406 |
0.161 |
0.850 (0.384, 1.882) |
0.688 |
| HBV DNA |
-0.385 |
0.377 |
1.046 |
0.680 (0.325, 1.424) |
0.306 |
| Preoperative ALP |
0.318 |
0.393 |
0.653 |
1.374 (0.636, 2.969) |
0.419 |
| Preoperative bilirubin |
0.011 |
0.004 |
8.140 |
1.012 (1.004, 1.020) |
0.004 |
| Preoperative ALT |
0.006 |
0.004 |
2.403 |
1.006 (0.999, 1.013) |
0.121 |
| Preoperative AST |
0.003 |
0.004 |
0.568 |
1.003 (0.995, 1.012) |
0.451 |
| Preoperative LDH |
0.001 |
0.002 |
0.101 |
1.001 (0.996, 1.005) |
0.750 |
| Preoperative CRP |
0.014 |
0.012 |
1.372 |
1.014 (0.991, 1.037) |
0.241 |
| Preoperative WBC |
-0.016 |
0.054 |
0.088 |
0.984 (0.885, 1.094) |
0.766 |
| Preoperative neutrophil ratio |
-0.003 |
0.016 |
0.041 |
0.997 (0.967, 1.028) |
0.840 |
| Preoperative PCT |
0.038 |
0.026 |
2.128 |
1.039 (0.987, 1.094) |
0.145 |
| Preoperative PLT |
-0.001 |
0.003 |
0.184 |
0.999 (0.994, 1.004) |
0.668 |
| Preoperative INR |
-0.645 |
2.085 |
0.096 |
0.525 (0.009, 31.267) |
0.757 |
| PLC: Primary liver cancer; ARDS: Acute respiratory distress syndrome; HBV: Hepatitis B virus; ALP: Alkaline phosphatase; ALT: Alanine transaminase; AST: Aspartate transaminase; LDH: Lactate dehydrogenase; CRP: C reactive protein; WBC: White blood cell count; PCT: Procalcitonin; PLT: Platelet count; INR: International normalized ratio; OR: Odds ratio; CI: Confidence interval. |
|
表 8 PLC患者术后ARDS影响因素的单因素logistic回归分析
Tab 8 Univariate logistic regression analysis of influencing factors of postoperative ARDS in PLC patients
|
表 9
(Tab 9)

表 9 PLC患者术后ARDS影响因素的多因素logistic回归分析
Tab 9 Multivariate logistic regression analysis of influencing factors of postoperative ARDS in PLC patients
| Variable |
Regression coefficient |
Standard error |
Wald |
OR (95% CI) |
P value |
| Prophylactic anticoagulation |
-0.934 |
0.391 |
5.697 |
0.393 (0.183, 0.846) |
0.017 |
| Ascites |
0.886 |
0.454 |
3.800 |
2.425 (0.995, 5.909) |
0.041 |
| Preoperative bilirubin |
0.011 |
0.004 |
5.899 |
1.011 (1.002, 1.019) |
0.015 |
| PLC: Primary liver cancer; ARDS: Acute respiratory distress syndrome; OR: Odds ratio; CI: Confidence interval. |
|
表 9 PLC患者术后ARDS影响因素的多因素logistic回归分析
Tab 9 Multivariate logistic regression analysis of influencing factors of postoperative ARDS in PLC patients
|
3 讨论
我国是世界上肝病发生率最高的国家之一,也是世界上肝切除病例最多的国家之一,目前肝切除术仍然是PLC患者的首选治疗方法。美国胸科医师学会抗栓治疗指南推荐腹部手术患者术后使用抗凝药物治疗[10],这一治疗方案同样对肝切除患者适用。在我国的实际临床工作中,因考虑到术后抗凝存在出血风险,即使在没有术后活动性出血的患者中,术后预防性抗凝仍未被普遍应用。本研究探究了PLC患者肝切除术后使用预防性抗凝治疗是否可降低术后并发症的发生率。
数据表明,普通外科未使用预防措施的患者术后深静脉血栓形成的发生率为6.1%[12-13]。由于手术时间长、术中出血多、肿瘤患者血液高凝等原因,接受PLC肝切除手术的患者被视为术后VTE发生的中-高风险人群,肝切除术后VTE发生率高达2.1%~4.7%[14-18]。本研究结果显示,VTE的总发生率为2.8%(14/495),其中抗凝组VTE的发生率为1.4%(4/287),低于常规治疗组的4.8%(10/208),进一步logistic回归分析结果显示术后发生VTE的独立危险因素包括年龄、门静脉高压和肿瘤数量,而术后预防性抗凝是VTE的独立保护因素。该结果与既往报道结果[16]一致,再次印证了PLC患者行肝切除术后VTE的发生率较高,术后早期预防性抗凝可降低VTE的发生率且不会增加出血风险。
HiSCO-05研究是一项中心随机对照试验,旨在分析肝切除术后使用抗凝血酶Ⅲ对PHLF发生率的影响[16]。HiSCO-05研究结果表明,使用抗凝血酶Ⅲ作为次要终点的方案是安全的,然而未观察到主要终点的疗效,即PHLF发生率降低。该研究同时指出,BMI≥25 kg/mm2和总胆红素≥15 mg/L为PHLF的独立危险因素。本研究结果显示,常规治疗组与抗凝组在PHLF发生率方面存在差异,进一步行单因素及多因素logistic回归分析发现,PLC患者发生PHLF的独立危险因素包括门静脉高压、术中出血、术中输血、术前降钙素原。本研究结果与HiSCO-05研究结果[16]存在差异,考虑本研究样本量较小,所得结果可能存在误差,且尚无大型临床研究明确肝切除术后抗凝与PHLF的发生相关或为PHLF发生的独立危险因素,故该结果仍需进一步验证。
有研究报道PLC术后肺部感染的发生率高达10%左右,原因可能为术后呼吸肌张力下降等导致肺活力降低及痰液淤积导致感染或术前原有疾病加重引起感染等[19]。但尚无临床研究证实肝切除术后肺部感染与ARDS有关,也未见有关肝切除术后ARDS危险因素的报道。本研究结果显示,常规治疗组与抗凝组在ARDS发生率方面存在差异,进一步分析发现PLC患者发生术后ARDS的独立危险因素包括腹水、术前胆红素,而预防性抗凝则是独立保护因素。但本研究样本量较小,后期仍需扩大样本量进一步验证该结果。
综上所述,PLC患者术后早期预防性抗凝可降低VTE、PHLF、ARDS的发生率,且不会增加术后出血的风险。但本研究为单中心研究,受到样本量、研究经费等因素限制,研究结果可能存在偏倚,后续将进一步扩大样本量进行多中心研究以验证该结果。
参考文献
| [1] |
CAI S W, YANG S Z, LV W P, et al. Sustained methylene blue staining to guide anatomic hepatectomy for hepatocellular carcinoma: initial experience and technical details[J]. Surgery, 2015, 158(1): 121-127. DOI:10.1016/j.surg.2015.01.018 |
| [2] | |
| [3] |
ISHIKAWA M, YOGITA S, MIYAKE H, et al. Clarification of risk factors for hepatectomy in patients with hepatocellular carcinoma[J]. Hepatogastroenterology, 2002, 49(48): 1625-1631. |
| [4] |
WEI A C, TUNG-PING POON R, FAN S T, et al. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma[J]. Br J Surg, 2003, 90(1): 33-41. DOI:10.1002/bjs.4018 |
| [5] | |
| [6] |
JOHNSTON M E 2 nd, SUSSMAN J J, PATEL S H. Surgical oncology and geriatric patients[J]. Clin Geriatr Med, 2019, 35(1): 53-63. DOI:10.1016/j.cger.2018.08.006 |
| [7] |
COSIC L, MA R, CHURILOV L, et al. Health economic implications of postoperative complications following liver resection surgery: a systematic review[J]. ANZ J Surg, 2019, 89(12): 1561-1566. DOI:10.1111/ans.15213 |
| [8] |
KIM B J, DAY R W, DAVIS C H, et al. Extended pharmacologic thromboprophylaxis in oncologic liver surgery is safe and effective[J]. J Thromb Haemost, 2017, 15(11): 2158-2164. DOI:10.1111/jth.13814 |
| [9] |
TZENG C W D, KATZ M H G, FLEMING J B, et al. Risk of venous thromboembolism outweighs post-hepatectomy bleeding complications: analysis of 5 651National Surgical Quality Improvement Program patients[J]. HPB (Oxford), 2012, 14(8): 506-513. DOI:10.1111/j.1477-2574.2012.00479.x |
| [10] |
GOULD M K, GARCIA D A, WREN S M, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9 th ed: American College of Chest Physicians evidence-based clinical practice guidelines[J]. Chest, 2012, 141(2 Suppl): e227S-e277S. DOI:10.1378/chest.11-2297 |
| [11] | |
| [12] | |
| [13] | |
| [14] |
REDDY S K, TURLEY R S, BARBAS A S, et al. Post-operative pharmacologic thromboprophylaxis after major hepatectomy: does peripheral venous thromboembolism prevention outweigh bleeding risks?[J]. J Gastrointest Surg, 2011, 15(9): 1602-1610. DOI:10.1007/s11605-011-1591-x |
| [15] |
NATHAN H, WEISS M J, SOFF G A, et al. Pharmacologic prophylaxis, postoperative INR, and risk of venous thromboembolism after hepatectomy[J]. J Gastrointest Surg, 2014, 18(2): 295-303. DOI:10.1007/s11605-013-2383-2 |
| [16] |
KURODA S, KOBAYASHI T, TASHIRO H, et al. A multicenter randomized controlled trial comparing administration of antithrombin Ⅲ after liver resection (HiSCO-05 trial)[J]. Surgery, 2021, 170(4): 1140-1150. DOI:10.1016/j.surg.2021.03.057 |
| [17] |
EJAZ A, SPOLVERATO G, KIM Y, et al. Defining incidence and risk factors of venous thromboemolism after hepatectomy[J]. J Gastrointest Surg, 2014, 18(6): 1116-1124. DOI:10.1007/s11605-013-2432-x |
| [18] |
BELLINI G, TENG A, KOTECHA N, et al. The identification of risk factors for venous thromboembolismin gastrointestinal oncologic surgery[J]. J Surg Res, 2016, 205(2): 279-285. DOI:10.1016/j.jss.2016.06.089 |
| [19] | |
 2024, Vol. 45
2024, Vol. 45


