2. 海军军医大学(第二军医大学)海军医学系航海特殊损伤防护教研室,上海 200433
2. Department of Nautical Injury Protection, Faculty of Naval Medicine, Naval Medical University (Second Military Medical University), Shanghai 200433, China
晕船是严重影响海上作业的重要问题,舰艇的异常加速度环境导致人体前庭觉-视觉-本体觉感觉信息与中枢感受“内模式”之间出现冲突,是引起晕船的主要原因[1-3]。目前,晕船防治主要采用适应性训练和使用抗晕药物2种方法。现有的一线抗晕药物如毒蕈碱型乙酰胆碱受体阻断剂(如东莨菪碱)、组胺H1受体阻断剂(如苯海拉明)具有中枢抑制作用,显著影响人体的学习记忆和作业能力,而交感神经兴奋药物右旋苯丙胺虽然有中枢兴奋作用,但具有成瘾性。而且这些抗晕药物大多需要提前服用,一旦发生晕船其止吐效果并不明显[4]。前庭核与孤束核、迷走神经背核、臂旁核等内脏调控核团间具有谷氨酸能直接和间接通路,外界加速度变化通过前庭感受器传入中枢,再通过前庭-内脏反射诱发恶心、呕吐、流涎等一系列自主神经反应[5]。抗晕药物主要通过抑制前庭核的兴奋性降低自主神经反应起到预防晕动症的作用。有研究发现异常加速度刺激可通过对胃肠道的牵拉作用及迷走神经传入通路兴奋孤束核等自主神经中枢,诱发恶心、呕吐反应[6]。众所周知,胃肠道本身还可以分泌一系列胃肠激素如胃肠厌食激素胰高血糖素样肽-1(glucagon-like peptide-1,GLP-1)[7]、胆囊收缩素(cholecystokinin,CCK)[8]及瘦素(leptin)等,这些激素进入中枢并逆向调控摄食和自主神经功能,而胃促生长素(ghrelin)也具有调节食欲、胃排空和呕吐反射的效应[9]。此外,中枢神经肽类激素如神经肽Y(neuropeptide Y,NPY)[10]、促食欲素A(orexin A,OXA)也可调节胃肠道运动功能[11]。然而,胃肠食欲调控激素是否与晕动症发生有关并不明确。
本研究通过垂荡运动刺激筛选晕动症敏感及不敏感个体,检测两组人群垂荡刺激前后血浆胃肠激素和神经肽变化,以初步探讨胃肠功能调节肽类激素与晕动症发生的关系。
1 资料和方法 1.1 受试对象选择60名志愿者,均为男性,年龄18~29(21.20±2.42)岁,BMI为(21.66±1.47)kg/m2,身体健康,无前庭系统疾病、神经系统疾病及心血管系统疾病病史,近1年内均无舰艇出海经历,试验前3 d内未服用过解热镇痛药、催眠药及中枢兴奋药物。
1.2 伦理学声明本试验通过海军军医大学(第二军医大学)医学研究伦理委员会审批。根据伦理委员会要求,若受试对象出现剧烈呕吐等严重不适反应或受试者无法耐受试验立即停止刺激。受试人员均自愿同意参加本试验并签署知情同意书。
1.3 模拟垂荡刺激利用自行研制的人用可调垂荡模拟器(频率与加速度可调)[12],依据前期预试验结果,给予受试者正弦垂荡规律运动(运动频率0.42 Hz,最大加速度0.22 g)连续刺激45 min。
1.4 晕动症敏感性评价试验开始前要求受试者填写晕动症易感性问卷(motion sickness susceptibility questionnaire,MSSQ),采用本项目组设计的修改版公式计算MSSQ易感指数[13]。垂荡刺激后即刻,采用Graybiel量表评价晕动症症状严重程度。根据MSSQ易感指数和Graybiel评分综合判断受试者易感性,筛选出晕动症不敏感(Graybiel评分≤2分且MSSQ易感指数<5分)和晕动症敏感(Graybiel评分≥8分且MSSQ易感指数>21分)的受试者各15人。其余人员不纳入后续试验。
1.5 血样采集与处理晕动症不敏感组和晕动症敏感组受试者在垂荡刺激前后,每人抽取5 mL外周血液样本。各样本取100 μL,加入400 μL预冷的甲醇乙腈溶液(体积比为1∶1),涡旋60 s,-20 ℃静置1 h沉淀蛋白质,4 ℃ 2 000×g离心20 min,取上清液于-80 ℃保存备用。
1.6 ELISA实验方法采用ELISA法检测血浆胃促生长素、CCK、GLP-1、NPY、瘦素、OXA水平。首先设置标准品孔、空白孔和待测样品孔,标准品孔加不同浓度的标准品50 μL。在酶标包被板待测样品孔中先加样品稀释液40 μL,再加待测样品10 μL(样品最终稀释度为5倍)。将样品加于酶标包被板孔底部,尽量不触及孔壁,轻轻晃动混匀。除空白孔外,每孔加入酶标试剂100 μL。用封板膜封板后置于37 ℃温育60 min。将20×浓缩洗涤液用蒸馏水稀释20倍后备用。小心揭掉封板膜,弃液体,甩干,每孔加满洗涤液,静置30 s,弃洗涤液,如此重复5次,拍干。每孔先加入显色剂A50 μL,再加入显色剂B 50 μL,轻轻震荡混匀,37 ℃避光显色15 min。每孔再加终止液50 μL,终止反应(此时蓝色立转黄色)。以空白孔调零,在450 nm波长处依序测量各孔的光密度值,计算各样品中胃肠激素浓度。
1.7 统计学处理采用SPSS 21.0软件进行统计学分析。计量资料以x±s表示,两组间基础数据比较采用独立样本t检验;计数资料以人数和百分数表示,两组间比较采用χ2检验;组间及刺激前后胃肠激素水平的比较采用两因素方差分析,两两比较采用Bonferroni法。采用logistic回归模型分析血浆胃肠激素水平对晕动症易感性的预测效果并建立联合预测模型,采用ROC曲线分析模型的预测价值并计算灵敏度和特异度。检验水准(α)为0.05。
2 结果 2.1 一般资料晕动症敏感组和不敏感组受试者的年龄和BMI差异均无统计学意义(均P>0.05),晕动症敏感组受试者的MSSQ易感指数高于晕动症不敏感组(P<0.001);垂荡刺激后,晕动症敏感组受试者的Graybiel评分及恶心发生率均高于晕动症不敏感组(均P<0.001)。见表 1。
|
|
表 1 晕动症敏感组和不敏感组受试者的一般资料比较 Tab 1 Comparison of general information between motion sickness sensitive and insensitive groups |
2.2 血浆胃肠激素水平
两因素方差分析结果显示,血浆中胃促生长素、CCK、GLP-1、NPY、瘦素、OXA这6种胃肠激素均具有显著的敏感性效应和时间效应,其中胃促生长素、CCK、NPY、瘦素还具有敏感性-时间交互效应。见表 2。
|
|
表 2 血浆胃肠激素水平主效应和交互效应2×2方差分析 Tab 2 2×2 analysis of variance of main and interactive effects of plasma gastrointestinal hormone levels |
两两比较结果显示,与垂荡刺激前相比,晕动症敏感组垂荡刺激后血浆胃促生长素和CCK水平升高(均P<0.01),而NPY和瘦素水平下降(均P<0.01);与晕动症不敏感组相比,垂荡刺激后晕动症敏感组血浆胃促生长素和CCK水平较高(均P<0.01),而NPY和瘦素水平较低(均P<0.01)。见图 1。
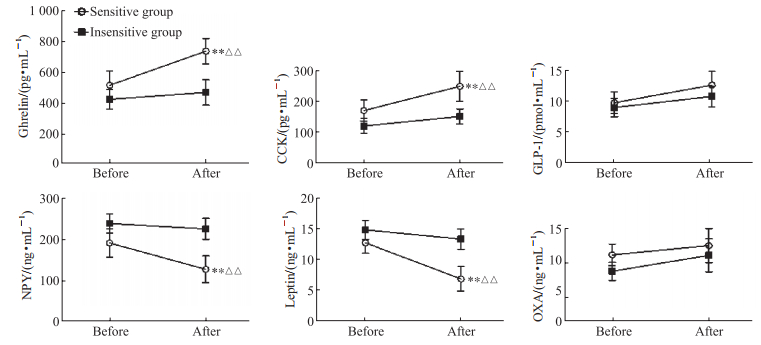
|
图 1 晕动症敏感组和不敏感组受试者在垂荡刺激前后的血浆胃肠激素水平 Fig 1 Plasma gastrointestinal hormone levels before and after vertical oscillation stimulation in motion sickness sensitive and insensitive groups **P<0.01 vs before vertical oscillation stimulation in the same group; △△P<0.01 vs insensitive group at the same stimulation period. n=15, x±s. CCK: Cholecystokinin; GLP-1: Glucagon-like peptide-1; NPY: Neuropeptide Y; OXA: Orexin A. |
2.3 血浆胃肠激素预测晕动症易感性的ROC曲线分析及多因素logistic回归分析
选取垂荡刺激前晕动症敏感组与不敏感组之间4种差异有统计学意义的激素(胃促生长素、CCK、NPY、瘦素)进行ROC曲线分析,结果显示这4种激素水平的AUC值均大于0.7,均对晕动症易感性具有预测价值(表 3)。
|
|
表 3 血浆胃肠激素预测晕动症易感性的ROC曲线分析结果 Tab 3 ROC curve analysis results of plasma gastrointestinal hormones in predicting motion sickness susceptibility |
多因素logistic回归分析结果显示,血浆胃促生长素、CCK、NPY是晕动症易感性的独立预测因素(均P<0.05,表 4),因此选择胃促生长素、CCK、NPY这3种血浆胃肠激素建立晕动症易感性预测模型,即logit(P)=-0.051×胃促生长素+0.060×NPY-0.169×CCK+33.397。对预测模型进行ROC曲线分析结果显示,其AUC值为0.988,灵敏度、特异度分别为100.0%、93.3%,预测效果优于胃促生长素(AUC=0.792)、CCK(AUC=0.880)、NPY(AUC=0.838)、瘦素(AUC=0.777)4种指标单独应用。
|
|
表 4 血浆胃肠激素预测晕动症易感性的多因素logistic回归分析结果 Tab 4 Multivariate logistic regression analysis of plasma gastrointestinal hormones in predicting motion sickness susceptibility |
3 讨论
本研究结果显示,晕动症敏感个体在垂荡刺激后血浆胃肠激素胃促生长素和CCK水平升高,NPY和瘦素水平下降。相对而言,晕动症不敏感的个体上述指标在刺激前后无显著变化。有研究发现,应激刺激后人体外周血胃促生长素及其mRNA显著升高,而给予外源性胃促生长素干预可使心率、血压下降[14-16]。动物实验亦发现,经侧脑室注射胃促生长素能够显著抑制交感神经活性,激活心脏迷走神经[17-19]。另外,外周血胃促生长素水平在午夜睡眠状态时升高,而此时人体心脏迷走神经活性亦占优势[20]。本研究中晕动症敏感个体在垂荡刺激后血浆胃促生长素水平升高。人体试验发现,毒蕈碱型乙酰胆碱受体激动剂可显著升高血浆胃促生长素水平,而给予毒蕈碱型乙酰胆碱受体阻断剂则使胃促生长素水平下降[21],而众所周知,毒蕈碱型乙酰胆碱受体阻断剂东莨菪碱具有显著的抗晕作用。上述证据提示血浆胃促生长素水平升高与晕动症发生具有密切关系。
动物摄食后,胃肠厌食激素通过刺激迷走传入神经将信号传入后脑干孤束核及最后区,产生饱腹感和厌食反应。呕吐毒素脱氧雪腐镰刀菌烯醇可导致小鼠厌食,并引起血液CCK、胃抑制肽、GLP-1和肽YY水平升高,给予CCK或GLP-1抑制剂则能显著缓解呕吐毒素厌食效应,但给予胃抑制肽或肽YY抑制剂则无此效果[22]。早期的研究发现,给予CCK静脉注射后,实验猴呕吐发生率显著升高,并伴有血浆精氨酸加压素的显著升高[23]。精氨酸加压素是呕吐反应的重要生物标志物,已有关于晕动症大鼠及人体出现精氨酸加压素升高的报道[24]。经外周给予CCK 1型受体激动剂可使大鼠脑干孤束核及下丘脑室旁核Fos蛋白表达增加,表明这些核团的神经元被显著激活[25],在晕动症模型大鼠中也观察到类似的结果[26],这一过程可能与CCK增加胃肠感觉迷走神经放电,导致孤束核GLP-1神经元激活有关[27]。还有文献报道,前庭核内CCK阳性神经元激活后,可通过前庭核-臂旁核通路诱导小鼠出现自发活动降低、摄食减少等晕动症样行为[28],提示CCK与晕动症的发生密切相关。
本研究还发现,垂荡刺激后晕动症敏感个体血浆NPY水平下降,这一结果与动物在体实验结果一致,即NPY表达下降可促进CCK引起的厌食反应[29]。同样地,作为厌食激素,瘦素在晕动症敏感组垂荡刺激后亦下降。研究发现,瘦素与CCK在抑制摄食方面具有协同作用,瘦素预处理可显著增强CCK诱导的饱腹感;然而,瘦素更倾向于长时程的摄食调控,而CCK主要参与短时摄食调控[30]。但本研究仅观察了垂荡刺激前后瘦素与CCK水平的变化,在晕动刺激过程中不同时间点的变化规律还需要进一步研究。
本研究通过多因素logistic回归构建了基于胃促生长素、CCK、NPY这3种血浆胃肠激素对晕动症易感性的预测模型,ROC曲线提示该预测模型对晕动症易感性的区分度及准确性高,可为预测晕动症易感个体提供指导。
| [1] |
QI R R, XIAO S F, SU Y, et al. Sea voyage training and motion sickness effects on working ability and life quality after landing[J]. Aerosp Med Hum Perform, 2021, 92(2): 92-98. DOI:10.3357/AMHP.5614.2021 |
| [2] |
LUCAS D, MEHANEZE M, LODDÉ B, et al. Seasickness and its impact on researchers' work on board French oceanographic vessels[J]. Int Marit Health, 2020, 71(3): 160-165. DOI:10.5603/IMH.2020.0029 |
| [3] |
IRMAK T, POOL D M, DE WINKEL K N, et al. Validating models of sensory conflict and perception for motion sickness prediction[J]. Biol Cybern, 2023, 117(3): 185-209. DOI:10.1007/s00422-023-00959-8 |
| [4] |
ZHANG L L, WANG J Q, QI R R, et al. Motion sickness: current knowledge and recent advance[J]. CNS Neurosci Ther, 2016, 22(1): 15-24. DOI:10.1111/cns.12468 |
| [5] |
CAI Y L, MA W L, LI M, et al. Glutamatergic vestibular neurons express Fos after vestibular stimulation and project to the NTS and the PBN in rats[J]. Neurosci Lett, 2007, 417(2): 132-137. DOI:10.1016/j.neulet.2007.01.079 |
| [6] |
YATES B J, CATANZARO M F, MILLER D J, et al. Integration of vestibular and emetic gastrointestinal signals that produce nausea and vomiting: potential contributions to motion sickness[J]. Exp Brain Res, 2014, 232(8): 2455-2469. DOI:10.1007/s00221-014-3937-6 |
| [7] |
FORTIN S M, CHEN J C, PETTICORD M C, et al. The locus coeruleus contributes to the anorectic, nausea, and autonomic physiological effects of glucagon-like peptide-1[J]. Sci Adv, 2023, 9(38): eadh0980. DOI:10.1126/sciadv.adh0980 |
| [8] |
DALY K, BURDYGA G, AL-RAMMAHI M, et al. Toll-like receptor 9 expressed in proximal intestinal enteroendocrine cells detects bacteria resulting in secretion of cholecystokinin[J]. Biochem Biophys Res Commun, 2020, 525(4): 936-940. DOI:10.1016/j.bbrc.2020.02.163 |
| [9] |
SANGER G J, FURNESS J B. Ghrelin and motilin receptors as drug targets for gastrointestinal disorders[J]. Nat Rev Gastroenterol Hepatol, 2016, 13(1): 38-48. DOI:10.1038/nrgastro.2015.163 |
| [10] |
WU Y, HE H, CHENG Z, et al. The role of neuropeptide Y and peptide YY in the development of obesity via gut-brain axis[J]. Curr Protein Pept Sci, 2019, 20(7): 750-758. DOI:10.2174/1389203720666190125105401 |
| [11] |
SINEN O, AKÇALI İ, AKKAN S S, et al. The role of hypothalamic orexin-A in stress-induced gastric dysmotility: an agonistic interplay with corticotropin releasing factor[J]. Neurogastroenterol Motil, 2024, 36(1): e14719. DOI:10.1111/nmo.14719 |
| [12] |
PAN L, QI R, XIAO S, et al. Predictive ability of motion sickness susceptibility questionnaire for motion sickness individual difference in Chinese young males[J]. Ocean Coast Manag, 2021, 203: 105505. DOI:10.1016/j.ocecoaman.2020.105505 |
| [13] |
潘磊磊, 王俊骎, 祁瑞瑞, 等. 修改版晕动症易感性问卷用于晕船易感性判断[J]. 第二军医大学学报, 2016, 37(2): 220-224. PAN L L, WANG J Q, QI R R, et al. Application of revised MSSQ for seasickness susceptibility evaluation[J]. Acad J Sec Mil Med Univ, 2016, 37(2): 220-224. DOI:10.16781/j.0258-879x.2016.02.0220 |
| [14] |
KISHIMOTO I, TOKUDOME T, HOSODA H, et al. Ghrelin and cardiovascular diseases[J]. J Cardiol, 2012, 59(1): 8-13. DOI:10.1016/j.jjcc.2011.11.002 |
| [15] |
HUDA M S B, MANI H, DOVEY T, et al. Ghrelin inhibits autonomic function in healthy controls, but has no effect on obese and vagotomized subjects[J]. Clin Endocrinol, 2010, 73(5): 678-685. DOI:10.1111/j.1365-2265.2010.03865.x |
| [16] |
ULRICH-LAI Y M, RYAN K K. Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications[J]. Cell Metab, 2014, 19(6): 910-925. DOI:10.1016/j.cmet.2014.01.020 |
| [17] |
SHIMIZU S, AKIYAMA T, KAWADA T, et al. Centrally administered ghrelin activates cardiac vagal nerve in anesthetized rabbits[J]. Auton Neurosci, 2011, 162(1/2): 60-65. DOI:10.1016/j.autneu.2011.04.001 |
| [18] |
YASUDA T, MASAKI T, KAKUMA T, et al. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats[J]. Neurosci Lett, 2003, 349(2): 75-78. DOI:10.1016/s0304-3940(03)00789-4 |
| [19] |
HU Z, ZHANG T, MEI Y, et al. Impact of ghrelin on ventricular arrhythmia and related mechanism after myocardial infarction[J]. Pharmacology, 2022, 107(1/2): 102-110. DOI:10.1159/000519330 |
| [20] |
CUMMINGS D E, FRAYO R S, MARMONIER C, et al. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues[J]. Am J Physiol Endocrinol Metab, 2004, 287(2): E297-E304. DOI:10.1152/ajpendo.00582.2003 |
| [21] |
BROGLIO F, GOTTERO C, VAN KOETSVELD P, et al. Acetylcholine regulates ghrelin secretion in humans[J]. J Clin Endocrinol Metab, 2004, 89(5): 2429-2433. DOI:10.1210/jc.2003-031517 |
| [22] |
YUE J, GUO D, GAO X, et al. Deoxynivalenol (vomitoxin)-induced anorexia is induced by the release of intestinal hormones in mice[J]. Toxins, 2021, 13(8): 512. DOI:10.3390/toxins13080512 |
| [23] |
VERBALIS J G, RICHARDSON D W, STRICKER E M. Vasopressin release in response to nausea-producing agents and cholecystokinin in monkeys[J]. Am J Physiol, 1987, 252(4 Pt 2): R749-R753. DOI:10.1152/ajpregu.1987.252.4.R749 |
| [24] |
LI X, JIANG Z L, WANG G H, et al. Plasma vasopressin, an etiologic factor of motion sickness in rat and human?[J]. Neuroendocrinology, 2005, 81(6): 351-359. DOI:10.1159/000088991 |
| [25] |
CANO V, CAICOYA E, RUIZ-GAYO M. Effect of peripheral cholecystokinin receptor agonists on c-Fos expression in brain sites mediating food consumption in rats[J]. Neurosci Lett, 2003, 343(1): 13-16. DOI:10.1016/s0304-3940(03)00277-5 |
| [26] |
PAN L, XIAO S, XU Z, et al. Orexin-A attenuated motion sickness through modulating neural activity in hypothalamus nuclei[J]. Br J Pharmacol, 2023. DOI:10.1111/bph.16307 |
| [27] |
MANISCALCO J W, EDWARDS C M, RINAMAN L. Ghrelin signaling contributes to fasting-induced attenuation of hindbrain neural activation and hypophagic responses to systemic cholecystokinin in rats[J]. Am J Physiol Regul Integr Comp Physiol, 2020, 318(5): R1014-R1023. DOI:10.1152/ajpregu.00346.2019 |
| [28] |
MACHUCA-MÁRQUEZ P, SÁNCHEZ-BENITO L, MENARDY F, et al. Vestibular CCK signaling drives motion sickness-like behavior in mice[J]. Proc Natl Acad Sci USA, 2023, 120(44): e2304933120. DOI:10.1073/pnas.2304933120 |
| [29] |
DE LA SERRE C B, KIM Y J, MORAN T H, et al. Dorsomedial hypothalamic NPY affects cholecystokinin-induced satiety via modulation of brain stem catecholamine neuronal signaling[J]. Am J Physiol Regul Integr Comp Physiol, 2016, 311(5): R930-R939. DOI:10.1152/ajpregu.00184.2015 |
| [30] |
AHN W, LATREMOUILLE J, HARRIS R B S. Leptin receptor-expressing cells in the ventromedial nucleus of the hypothalamus contribute to enhanced CCK-induced satiety following central leptin injection[J]. Am J Physiol Endocrinol Metab, 2022, 323(3): E267-E280. DOI:10.1152/ajpendo.00088.2022 |
 2024, Vol. 45
2024, Vol. 45


