急性阑尾炎是儿童最常见的外科急症之一。传统观念认为,儿童阑尾炎的治疗以急诊手术为原则。但随着抗生素的有效应用及研究和临床试验的不断深入,儿童单纯性阑尾炎早期非手术治疗越来越受到外科医师的认可[1-5],国外文献报道非手术治疗单纯性阑尾炎成功率为71%~94%[5]。而对于儿童急性化脓、坏疽或穿孔性阑尾炎,由于组织坏死的不可逆性,保守治疗可能导致弥漫性腹膜炎、腹腔脓肿,甚至危及生命,及时手术干预更为合理[6-8]。因此,早期识别化脓、坏疽、穿孔性阑尾炎等高危病例并实施手术具有实际的临床意义。本研究通过分析单中心行腹腔镜阑尾切除术患儿的临床资料,探讨急性非单纯性阑尾炎发病的危险因素并建立预测模型,以期为儿童急性阑尾炎的治疗策略提供参考。
1 资料和方法 1.1 研究对象收集2018年1月至2019年12月于我院普外科接受腹腔镜阑尾切除术且经术后病理证实为急性阑尾炎的582例患儿的临床资料,根据术中所见和病理类型,将患儿分为非单纯性阑尾炎组和单纯性阑尾炎组。所有患儿入院前均未使用抗生素或其他抗菌药物。纳入标准:(1)年龄>5岁;(2)腹腔镜阑尾切除术后且病理学检查确诊为阑尾炎;(3)临床资料完整。排除标准:(1)阑尾周围脓肿;(2)慢性阑尾炎及慢性阑尾炎急性发作;(3)合并其他炎症性疾病,如肺炎、胆囊炎等;(4)合并营养不良、血液系统疾病、自身免疫病、炎症性肠病或恶性肿瘤进展期。本研究获得我院伦理委员会审批(2021R023-E01)。
1.2 诊断标准单纯性阑尾炎诊断依据[9]:(1)术中发现阑尾轻度充血、肿胀,直径增粗,无坏疽、穿孔、脓液或腹腔脓性渗液等征象;(2)组织病理学检查证实阑尾仅有水肿及中性粒细胞浸润,无化脓、坏疽或穿孔。
非单纯性阑尾炎诊断依据[9]:(1)术中发现阑尾化脓,浆膜附有纤维素或脓苔,严重者阑尾发生节段性坏疽伴或不伴穿孔,腹腔有脓性渗液;(2)组织病理学检查证实病变累及阑尾全层,肌层见大量炎症细胞浸润,严重者阑尾管壁有广泛坏死组织或穿孔迹象。
临床与病理诊断不一致时,以病理诊断为准。我院对已经明确形成的脓肿采用抗生素和引流术等非手术治疗,故阑尾周围脓肿被排除在本研究之外。
1.3 研究方法收集所有患儿的临床资料,包括一般资料,如性别、年龄、BMI;发病时间(腹痛至入院);临床表现,如术前体温≥38.5 ℃及呕吐、腹泻、腹胀情况;反跳痛;实验室检查结果,如白细胞计数(white blood cell,WBC)、红细胞计数、血红蛋白、血细胞比容、血小板计数、中性粒细胞计数、中性粒细胞比例(neutrophil percentage,NP)、淋巴细胞计数、中性粒细胞与淋巴细胞比值、单核细胞计数、CRP、血清白蛋白(albumin,ALB)、CRP/ALB比值、血清钠、总胆红素、丙氨酸转氨酶、天冬氨酸转氨酶、纤维蛋白原。
1.4 统计学处理应用SPSS 24.0软件进行数据分析,应用MedCalc 15.0软件绘制ROC曲线。计量资料先行Shapiro-Wilk检验,符合正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验;呈偏态分布的计量资料以中位数(下四分位数,上四分位数)表示,两组间比较采用Mann-Whitney U检验。计数资料以例数和百分数表示,两组间比较采用χ2检验。将组间比较差异有统计学意义的变量纳入多因素logistic回归分析,筛选儿童急性非单纯性阑尾炎发病的独立危险因素。回归变量筛选采用逐步法,变量进入标准为P<0.05,排除标准为P>0.1。根据危险因素对应的回归系数构建回归方程,采用Hosmer-Lemeshow检验评价预测模型的拟合优度(P>0.05表明模型的拟合优度较好),采用ROC曲线评价预测模型的诊断价值。检验水准(α)为0.05。
2 结果 2.1 两组患儿的临床资料比较纳入研究的582例患儿中男349例、女233例,年龄5~15岁,平均年龄(8.7±2.6)岁,手术方式均为腹腔镜下阑尾切除术。单纯性阑尾炎组200例(34.4%);非单纯性阑尾炎组382例(65.6%),其中化脓性阑尾炎160例、坏疽性阑尾炎124例、穿孔性阑尾炎98例。两组患儿的年龄、发病时间、WBC、中性粒细胞计数、NP、淋巴细胞计数、中性粒细胞与淋巴细胞比值、单核细胞计数、CRP、ALB、CRP/ALB比值、血清钠、总胆红素、天冬氨酸转氨酶、纤维蛋白原、发热、呕吐、反跳痛等18个指标比较差异均有统计学意义(均P<0.05)。见表 1。
|
|
表 1 两组患儿临床资料比较 Tab 1 Comparison of clinical data between 2 groups |
2.2 儿童急性非单纯性阑尾炎发病影响因素的多因素logistic回归分析
对上述差异有统计学意义的指标进行共线性检验,对应的方差膨胀系数均<5,不存在多重共线性。将上述差异有统计学意义的变量作为自变量、阑尾炎类型为因变量(非单纯性=1,单纯性=0)进行多因素logistic回归分析,结果发现WBC、NP、CRP、ALB为儿童急性非单纯性阑尾炎发病的独立危险因素(均P<0.05)。见表 2。
|
|
表 2 儿童急性非单纯性阑尾炎影响因素的多因素logistic回归分析 Tab 2 Multivariate logistic regression analysis of influencing factors of acute non-simple appendicitis in children |
2.3 儿童急性非单纯性阑尾炎发病影响因素的logistic回归方程建立与模型评价
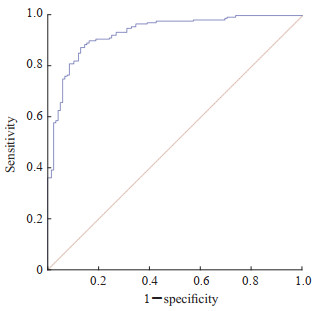
根据多因素logistic回归筛选出的4个危险因素及回归系数构建回归方程:logit(P)=-5.614+0.263×WBC+0.045×NP+0.045×CRP-0.027×ALB。概率模型为P=1/[1+Exp(-5.614+0.263×WBC+0.045×NP+0.045×CRP-0.027×ALB)]。Hosmer-Lemeshow检验结果显示建立的logistic回归方程拟合可行,预测值与观察值差异无统计学意义,预测模型的拟合度良好(χ2=6.288,P=0.622)。预测模型的AUC为0.931(95% CI 0.896~0.957,P<0.001),灵敏度为0.830,特异度为0.905,准确度为0.856,阳性预测值为0.943,阴性预测值为0.736,见图 1、表 3。
|
图 1 儿童急性非单纯性阑尾炎预测模型的ROC曲线 Fig 1 ROC curve of prediction model for acute non-simple appendicitis in children ROC: Receiver operating characteristic. |
|
|
表 3 回顾性评估预测模型对儿童急性非单纯性阑尾炎的诊断价值 Tab 3 Retrospective review of diagnostic value of prediction model for acute non-simple appendicitis in children |
收集2020年1月至12月于我院普外科行阑尾切除手术的160例临床资料完整的儿童急性阑尾炎病例,前瞻性验证预测模型对儿童非单纯性阑尾炎的诊断价值,结果显示预测模型的灵敏度为0.882,特异度为0.820,准确度为0.862,阳性预测值为0.915,阴性预测值为0.759。见表 4。
|
|
表 4 前瞻性评估预测模型对儿童非单纯性阑尾炎的诊断价值 Tab 4 Prospective review of diagnostic value of prediction model for non-simple appendicitis in children |
3 讨论
阑尾炎是儿童急腹症最常见的原因之一。尽管目前医疗水平取得了巨大进步,但世界各地仍有成千上万的患儿因阑尾炎而罹患严重的疾病。及时评估患儿阑尾炎的严重程度,根据病理阶段选择合适的治疗方式是良好预后的基础。既往临床试验通过腹部彩色超声及CT检查来判断急性阑尾炎的严重程度,然而其存在灵敏性低[10-12]、主观性强、费用高、操作不便及射线危害等不足。本研究建立的预测模型所纳入的临床资料为临床数据库中简单、易获取、客观的常规血液学指标,在临床上具有更好的普适性和简便性,特别是在不具备相应影像学检查条件的医疗环境下,可更加方便地指导外科医师及时做出合理治疗决策。
自1989年McBurney开创了阑尾切除术并建议阑尾炎应尽早手术以来,手术一直是急性阑尾炎治疗的经典和标准治疗方式。然而,近年来越来越多的证据提示儿童单纯性阑尾炎可以暂时不行阑尾切除术,抗生素等非手术治疗安全、有效[1-5, 13-14],这不仅避免了手术并发症,还可以显著降低治疗成本[13-14]。meta分析显示,非手术治疗单纯性阑尾炎的成功率达92%,其中只有16%的患儿需再次入院行阑尾切除术,且手术治疗与非手术治疗的并发症和住院时间没有差异[15]。免疫学家提出阑尾并不是废用器官,阑尾不仅是维持肠道微生物平衡的“储存池”,而且阑尾黏膜固有层的淋巴组织(主要是CD8+ T细胞)丰富,对机体的免疫功能起着重要作用[16]。儿童时期是免疫功能逐渐成熟和肠道菌群平衡建立的重要时期。单纯性阑尾炎的保守治疗可以保留阑尾,不仅有助于维持肠道菌群稳态和免疫系统发育,还可以避免手术并发症、增加患者满意度、降低医疗成本。因此,我们建议,如果预测模型显示患儿为非单纯性阑尾炎的可能性高时,则急诊行腹腔镜阑尾切除术;对于模型预测为单纯性阑尾炎的概率大且患儿家属有保守治疗倾向时,在充分尊重家属的知情权和选择权的基础上可采用抗感染保守治疗。
以坏疽和/或穿孔为代表的急性非单纯性阑尾炎的感染严重程度明显重于单纯性阑尾炎,本研究筛选出的高危因素中有3个均为炎症指标,根据其强弱依次为WBC、NP、CRP。WBC是人体血液中能够吞噬异物并产生抗体的细胞,WBC升高往往提示体内存在器官和组织细菌性感染,而且细菌性感染程度与WBC水平呈正相关。因此,WBC可以作为有效指标来预测非单纯性阑尾炎。Zani等[17]也发现非单纯性阑尾炎患儿整体WBC水平明显高于单纯性阑尾炎。中性粒细胞是机体的固有免疫细胞,是宿主防御病原体的效应阶段的主要参与者。本研究单因素分析显示,单纯性阑尾炎组和非单纯性阑尾炎组的中性粒细胞计数及NP差异有统计学意义(均P<0.001),而在进行多因素logistic回归分析时,只有NP是预测急性非单纯性阑尾炎发病的高危因素,提示机体严重感染时,NP较中性粒细胞计数对非单纯性阑尾炎具有更显著的预测效能。CRP是炎症反应时肝细胞合成的非特异性急性期蛋白质。当机体发生感染时,CRP在血液中的表达水平迅速上升,且机体炎症反应越强其表达水平越高[18-19]。CRP的合成在急性炎症或组织损伤后的4~6 h内开始增加,不受常用抗炎药物或免疫抑制剂的直接影响,且在人体内变异相对较低[20]。因此,血清CRP也可作为预测非单纯性阑尾炎客观、可靠的指标。Davis等[21]研究认为,没有任何一项实验室检查指标可以单独用来预测阑尾炎的发生,但CRP的灵敏性高于WBC和中性粒细胞计数。Gorter等[9]认为,虽然术前CRP水平不足以诊断急性阑尾炎,但CRP能够更准确判断急性阑尾炎的严重程度,较其他常见炎症指标(WBC、中性粒细胞计数等)诊断急性非单纯性阑尾炎的效能更高。由此可知,单项实验室指标很难判断阑尾炎的严重程度。本研究建立的预测模型纳入了上述3项感染指标,提高了急性非单纯性阑尾炎诊断的准确率。本研究还意外地发现非单纯性阑尾炎组血清ALB水平低于单纯性阑尾炎组(P=0.006),低ALB是急性非单纯性阑尾炎的危险因素(OR=0.973),推测其潜在机制可能为:首先,非单纯性阑尾炎患儿感染较重,当炎症反应持续存在时ALB合成受到抑制、分解亢进。其次,炎症因子可能导致血管通透性增加,使血清ALB由血管内向血管外渗漏到组织间隙及腹腔,引起ALB水平进一步降低;低蛋白血症又可引起血管内皮细胞的多糖包被层破坏,导致更多的白蛋白外渗到组织间隙[22]。本研究结果显示,通过血液学指标WBC、NP、CRP和ALB建立的预测模型对儿童急性非单纯性阑尾炎具有较好的诊断价值,ROC曲线的AUC为0.931,灵敏度为0.830,特异度为0.905,前瞻性验证的灵敏度为0.882,特异度为0.820,准确度为0.862。
鉴于既往研究结果及临床经验,急性阑尾炎合并阑尾粪石保守治疗的失败率高[4, 23];对于女性患儿可能会因延误手术或保守治疗失败而造成盆腔粘连,导致输卵管蠕动障碍甚至堵塞等严重并发症[24-25],因此对于预测模型提示非单纯性阑尾炎的女性患儿或合并阑尾粪石的患儿,强烈建议行腹腔镜阑尾切除术。临床实践过程中,阑尾切除术作为急性阑尾炎治疗的金标准,对假阳性者实施手术也是完全合理的,因此需要特别警惕假阴性,以避免延误手术而增加诊治风险。对于单纯性阑尾炎,有复发及治疗失败的风险,外科医师在制定保守治疗的临床决策前,一定要充分尊重患者的知情权和选择权。
本研究存在一些不足之处。(1)本研究是单中心、回顾性研究,在信息收集及患儿选择方面可能存在偏倚;(2)考虑影像学指标尽管诊断特异度高,但对坏疽或穿孔性阑尾炎的灵敏度低,故本研究未纳入影像学指标;(3)单纯性阑尾炎和非单纯性阑尾炎的定义是根据术中所见及术后病理结果,非手术切除病例被排除在外。在今后的研究中,需要开展多中心、前瞻性研究,并收集更多的数据,以进一步完善本研究所建立的模型,从而确保其准确性和可靠性。
| [1] |
BI L W, YAN B L, YANG Q Y, et al. Comparison of conservative treatment with appendectomy for acute uncomplicated pediatric appendicitis: a meta-analysis[J]. J Comp Eff Res, 2019, 8(10): 767-780. DOI:10.2217/cer-2019-0036 |
| [2] |
GEORGIOU R, EATON S, STANTON M P, et al. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis[J]. Pediatrics, 2017, 139(3): e20163003. DOI:10.1542/peds.2016-3003 |
| [3] |
PODDA M, GERARDI C, CILLARA N, et al. Antibiotic treatment and appendectomy for uncomplicated acute appendicitis in adults and children: a systematic review and meta-analysis[J]. Ann Surg, 2019, 270: 1028-1040. DOI:10.1097/SLA.0000000000003225 |
| [4] |
HUANG L, YIN Y, YANG L, et al. Comparison of antibiotic therapy and appendectomy for acute uncomplicated appendicitis in children: a meta-analysis[J]. JAMA Pediatr, 2017, 171(5): 426-434. DOI:10.1001/jamapediatrics.2017.0057 |
| [5] |
LÓPEZ J J, DEANS K, MINNECI P. Nonoperative management of appendicitis in children[J]. Curr Opin Pediatr, 2017, 29: 358-362. DOI:10.1097/MOP.0000000000000487 |
| [6] |
VAOS G, DIMOPOULOU A, GKIOKA E, et al. Immediate surgery or conservative treatment for complicated acute appendicitis in children? A meta-analysis[J]. J Pediatr Surg, 2019, 54(7): 1365-1371. DOI:10.1016/j.jpedsurg.2018.07.017 |
| [7] |
WANG V, KRIGER D, FANOUS E, et al. Should all complicated appendicitis be treated the same? The answer is no[J]. Am Surg, 2019, 85(10): 1179-1183. DOI:10.1177/000313481908501023 |
| [8] |
ZAVRAS N, VAOS G. Management of complicated acute appendicitis in children: still an existing controversy[J]. World J Gastrointest Surg, 2020, 12(4): 129-137. DOI:10.4240/wjgs.v12.i4.129 |
| [9] |
GORTER R R, WASSENAAR E C E, DE BOER O J, et al. Composition of the cellular infiltrate in patients with simple and complex appendicitis[J]. J Surg Res, 2017, 214: 190-196. DOI:10.1016/j.jss.2017.02.062 |
| [10] |
GONZALEZ D O, LAWRENCE A E, COOPER J N, et al. Can ultrasound reliably identify complicated appendicitis in children?[J]. J Surg Res, 2018, 229: 76-81. DOI:10.1016/j.jss.2018.03.012 |
| [11] |
KIM H Y, PARK J H, LEE S S, et al. CT in differentiating complicated from uncomplicated appendicitis: presence of any of 10 CT features versus radiologists' gestalt assessment[J]. Am J Roentgenol, 2019, 213(5): W218-W227. DOI:10.2214/ajr.19.21331 |
| [12] |
KIM H Y, PARK J H, LEE Y J, et al. Systematic review and meta-analysis of CT features for differentiating complicated and uncomplicated appendicitis[J]. Radiology, 2018, 287(1): 104-115. DOI:10.1148/radiol.2017171260 |
| [13] |
MINNECI P C, HADE E M, LAWRENCE A E, et al. Association of nonoperative management using antibiotic therapy vs laparoscopic appendectomy with treatment success and disability days in children with uncomplicated appendicitis[J]. JAMA, 2020, 324(6): 581-593. DOI:10.1001/jama.2020.10888 |
| [14] |
SIPPOLA S, HAIJANEN J, VⅡNIKAINEN L, et al. Quality of life and patient satisfaction at 7-year follow-up of antibiotic therapy vs appendectomy for uncomplicated acute appendicitis: a secondary analysis of a randomized clinical trial[J]. JAMA Surg, 2020, 155(4): 283-289. DOI:10.1001/jamasurg.2019.6028 |
| [15] |
MAITA S, ANDERSSON B, SVENSSON J F, et al. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis[J]. Pediatr Surg Int, 2020, 36(3): 261-269. DOI:10.1007/s00383-019-04610-1 |
| [16] |
GIRARD-MADOUX M J H, GOMEZ DE AGÜERO M, GANAL-VONARBURG S C, et al. The immunological functions of the appendix: an example of redundancy?[J]. Semin Immunol, 2018, 36: 31-44. DOI:10.1016/j.smim.2018.02.005 |
| [17] |
ZANI A, TEAGUE W J, CLARKE S A, et al. Can common serum biomarkers predict complicated appendicitis in children?[J]. Pediatr Surg Int, 2017, 33(7): 799-805. DOI:10.1007/s00383-017-4088-1 |
| [18] |
MCFADYEN J D, ZELLER J, POTEMPA L A, et al. C-reactive protein and its structural isoforms: an evolutionary conserved marker and central player in inflammatory diseases and beyond[J]. Subcell Biochem, 2020, 94: 499-520. DOI:10.1007/978-3-030-41769-7_20 |
| [19] |
BLOK G C G H, NIKKELS E D, VAN DER LEI J, et al. Added value of CRP to clinical features when assessing appendicitis in children[J]. Eur J Gen Pract, 2022, 28(1): 95-101. DOI:10.1080/13814788.2022.2067142 |
| [20] |
KANG H J, KANG H, KIM B, et al. Evaluation of the diagnostic performance of a decision tree model in suspected acute appendicitis with equivocal preoperative computed tomography findings compared with Alvarado, Eskelinen, and adult appendicitis scores: a STARD compliant article[J]. Medicine (Baltimore), 2019, 98(40): e17368. DOI:10.1097/MD.0000000000017368 |
| [21] |
DAVIS J, KASMIRE K. Utility of symptom duration and C-reactive protein, white blood cell count, and absolute neutrophil count in the evaluation of pediatric appendicitis[J]. J Emerg Med, 2021, 60(4): 428-435. DOI:10.1016/j.jemermed.2020.10.040 |
| [22] |
LOFTUS T J, BROWN M P, SLISH J H, et al. Serum levels of prealbumin and albumin for preoperative risk stratification[J]. Nutr Clin Pract, 2019, 34(3): 340-348. DOI:10.1002/ncp.10271 |
| [23] |
WANG N, LIN X, ZHANG S, et al. Appendicolith: an explicit factor leading to complicated appendicitis in childhood[J]. Arch Argent Pediatr, 2020, 118(2): 102-108. DOI:10.5546/aap.2020.eng.102 |
| [24] |
WEI L, MACDONALD T M, SHIMI S M. Appendicectomy is associated with increased pregnancy rate: a cohort study[J]. Ann Surg, 2012, 256(6): 1039-1044. DOI:10.1097/SLA.0b013e3182766250 |
| [25] |
PARENTE G, DI MITRI M, D'ANTONIO S, et al. Pelvic health assessment in adult females following pediatric appendicitis: a monocentric retrospective case-control study[J]. Children (Basel), 2022, 9(3): 346. DOI:10.3390/children9030346 |
 2024, Vol. 45
2024, Vol. 45


