2. 海军军医大学(第二军医大学)第二附属医院神经内科, 上海 200003
2. Department of Neurology, The Second Affiliated Hospital of Naval Medical University (Second Military Medical University), Shanghai 200003, China
阿尔茨海默病(Alzheimer’s disease,AD)是一种以记忆缺陷和认知功能减退为特征的神经退行性疾病,其主要神经病理学特征为神经元外β-淀粉样蛋白(β-amyloid protein,Aβ)聚集成老年斑,以及细胞内高磷酸化Tau蛋白积累形成神经原纤维缠结。这些神经病理变化的潜在机制尚不清楚,目前主流的观点认为是由遗传和环境因素共同作用引起[1]。包括AD在内的痴呆症是全球第五大死因,可造成每年240万人死亡[2]。鉴于目前临床上对AD尚无有效的治疗药物以及人口老龄化问题的不断加剧,痴呆症病例数量在未来几十年里预计将显著增长[3]。如果没有新的AD治疗方法,越来越高的AD患病率将给整个医疗系统带来负担。
已上市的常规药物(如多奈哌齐等)只能改善AD的临床症状,并不能有效延缓病情进展。AD药物开发的失败揭示了这种疾病的复杂性和目前AD神经药理学研究正面临的挑战。随着对AD遗传学研究的深入,基因疗法或许可以从根本上治疗AD并能避免长期用药带来的不良反应,结合基因疗法在其他疾病领域的成果,目前成为了一种很有希望治愈的AD治疗方法。
根据有无阳性家族史及基因测序结果,AD可分为家族性AD和散发性AD。载脂蛋白E(apolipoprotein E,ApoE)基因、烟酰胺单核苷酸腺苷酰转移酶3基因、簇集蛋白基因等是散发性AD发病的主要基因[4]。其中,已有确切研究证据表明包含ApoEε4纯合型基因增加了该基因携带者散发性AD的患病风险。而目前公认的家族性AD涉及的致病基因有早老素1(presenilin 1, PS-1)基因、淀粉样前体蛋白(amyloid precursor protein,APP)基因和早老素2(presenilin 2, PS-2)基因[4]。因此基因疗法是现在研究AD治疗手段的新方向。
血脑屏障的存在使众多药物无法进入神经系统组织内发挥作用,严重限制了AD治疗性药物的研发和应用。近些年来,纳米技术在疾病诊断、治疗等方面的应用日益广泛,在纳米药物和纳米给药系统等方面取得了重大突破,尤其是在纳米技术与肿瘤医学相结合方面取得了显著的成果。因此,纳米医学的发展为医学技术的发展带来了新的动力和思路。而基因疗法与纳米医学的结合在其他领域的进展也为AD治疗带来新的方向。
1 AD病理假说与基因治疗中的相关基因靶点AD的多种病理假说为基因治疗提供了大量潜在靶点,这些靶点包括Aβ生成密切相关的蛋白[β淀粉样前体蛋白裂解酶1(beta-site amyloid precursor protein cleaving enzyme 1,BACE1)[5-7]和APP[8]]、支持和维持神经元健康/生长与影响胆碱能的神经营养因子[脑源性神经营养因子[9-10]和神经生长因子(nerve growth factor,NGF)[11-12]]、ApoE及与Aβ降解相关的酶(如组织蛋白酶B与脑啡肽酶)[13-17]。以这些基因为目标的AD相关基因治疗研究结果见表 1。
|
|
表 1 AD相关基因治疗中的潜在靶向基因 |
1.1 淀粉样蛋白级联假说
淀粉样蛋白级联假说是由McKhann等[18]提出,该假说认为可溶性Aβ聚集体是产生神经毒性、造成认知功能紊乱的主要形式[19],Aβ的聚集与沉积导致老年斑的形成,可直接作用于神经元产生毒性,也可激活脑内的小胶质细胞和星形胶质细胞产生炎症因子[20]。BACE1在产生Aβ方面至关重要,无论是生理量还是病理量。BACE1通过在β-分泌酶位点切割APP来启动Aβ单体的产生[21]。γ-分泌酶复合物的进一步切割导致Aβ单体可能聚集在一起,产生有毒的寡聚体和原纤维,最终形成淀粉样斑块[22]。据报道,在AD患者尸检的大脑组织中BACE1水平升高,这意味着BACE1升高会增加Aβ的形成,并推动疾病的初始进展[23]。
1.2 胆碱能与NGF现已能确定AD患者中枢神经系统的乙酰胆碱酯酶和乙酰胆碱的合成与释放均有缺陷[24],所以,增加神经中枢内的胆碱能递质浓度就可以改善AD患者认知功能。现在胆碱酯酶抑制剂已被广泛应用于AD患者以解决AD造成的认知障碍及其他临床症状[25]。第一个被鉴定的生长因子NGF作用于基底前脑胆碱能神经细胞(其中最重要的是Meynert基底核),并支持其正常生理功能,在海马和皮质区域提供大量的乙酰胆碱释放[26],在AD中这些神经细胞急剧恶化,导致认知能力下降。NGF缺乏促进胆碱能异常[27]。由于NGF不能有效地通过血脑屏障,目前已经使用了在AD患者大脑中注射转基因细胞或病毒载体或在Meynert基底核中植入包封细胞的方法,这些尝试取得了部分成功,但也有局限性[28]。
2 纳米医学在AD基因疗法中的应用基因疗法可以增加相关酶的活性,也可以调控低水平的生物活性物质的表达。相对于其他药物治疗方法,基因治疗具有潜在的好处和优势,例如基因表达选择性、安全性和高效性[29]。基因疗法已经在许多AD动物模型进行探索,并取得了令人鼓舞的结果。2005年公布的第一个采用离体基因疗法(与NGF相关)的临床试验结果表明:离体基因治疗组AD患者的认知能力、大脑代谢活动和胆碱能神经元的形态状态都有所改善[30-31]。研究表明,NGF基因治疗可以减轻AD的临床和行为表现[32]。siRNA在基因治疗中的应用最近引起了相当大的关注,因为它能够特异性下调目标基因,并且其治疗靶点的数量巨大,甚至包括某些曾被认为不可成药的靶点。涉及疾病病理、单个基因和病毒病原体的基因是RNA干扰的候选基因[29]。RNA干扰通过使用转录后基因沉默机制调节蛋白质生产来调节基因表达,双链RNA的较长部分可分裂成siRNA片段并激活RNA干扰。siRNA是最适合于针对编码蛋白质的基因进行短期沉默的手段[33]。然而,选择不恰当的给药方式或者递送载体都可能会改变siRNA的药代动力学特性,降低基因沉默活性,并增加脱靶效应[34]。
尽管基因疗法在治疗神经系统疾病方面具有明显的优势,然而在血液中的稳定性差、血脑屏障穿透能力差和细胞摄取乏力等因素限制了其治疗效果,因此需要开发一种递送基因治疗药物的载体,该载体可以保护药物免受血液中的早期破坏,同时能有效地将药物穿透血脑屏障后递送到大脑的病变区域[29]。在神经系统疾病的治疗,特别是在AD的治疗中,传统的药物因很难顺利通过血脑屏障并对神经组织进行作用,且存在药物毒性和不良反应的问题而逐渐使人们将研究的重点转向其他治疗手段,将纳米技术与基因疗法相结合已成为一种可能的解决方案。与常规药物相比,纳米药物在许多方面具有明显的优势:(1)为治疗药物在血液中的转运提供了良好的载体平台,并通过血脑屏障渗透到中枢神经系统;(2)提高治疗药物的生物相容性,纳米载体本身具有生物相容性和生物降解性;(3)提高治疗药物在血液和组织中的稳定性,避免酶过早降解;(4)通过药物的连续释放,提高药代动力学和药代动力学指标,延长半衰期和作用时间;(5)有效降低药物用量、用药频率和不良反应发生率。纳米药物载体不仅可以有效搭载实验药物,且其靶向性也相对传统药物更为有效,人们将普通的基因疗法的重点逐渐转向了可搭载高效药物且更具有靶向特征的纳米药物研发。纳米药物在动物实验中所显示出的毒性相对传统药物更少,不良反应也相对低于传统药物,其靶向性有望提高AD基因药物的疗效。
纳米药物载体的核心成分包括各种各样的材料,如脂类、聚合物和金属,它们可以封装具有不同化学性质的分子。此外,这些纳米颗粒可以促进生物活性分子的保护和传递,从而降低其潜在毒性,进而提高其溶解性、稳定性、生物分布和药代动力学。封装在纳米颗粒中的内容物广泛,分子范围从小分子、多肽、蛋白质到遗传物质[35]。对于慢性中枢神经系统疾病至关重要的是,纳米颗粒能将生物活性分子传递到难以到达的组织中,下面简要介绍几种纳米药物载体。
2.1 脂质纳米颗粒脂质体是一种直径为50~1 000 nm的球状脂质双分子层,可作为生物活性化合物有应用前景的药物递送载体,主要通过减少药物单独使用时的毒性作用[36],或增加药物的循环时间和有效性来发挥作用。根据脂质体的组成和细胞内传递机制可分为以下几种类型:常规脂质体、长循环脂质体、装饰脂质体(表面修饰的囊泡和免疫脂质体)和生物反应性脂质体(阳离子型脂质体、温度敏感型、pH敏感型)[37]。有研究表明,脂质体的亲水性和疏水性部分可以包封水溶性和脂溶性药物,以此来增加生物利用度,有助于药物穿过血脑屏障,这也使得脂质体作为纳米载体被广泛应用于治疗脑部疾病[38]。Canovi等[39]研究了用抗Aβ单克隆抗体包被的纳米脂质体的吸附亲和力,结果显示Aβ单克隆抗体包被的脂质体附着在AD脑尸检样本中的Aβ斑块上,为使用这种脂质体作为潜在的疾病诊断和治疗提供了证据。为了防止阳离子纳米颗粒的细胞毒性,使用聚乙二醇(PEG)-脂质体(阴离子)和靶向肽(阳离子)构建了阴离子siRNA纳米复合物,通过使用这些纳米复合物实现了针对BACE1的抑制,从而延缓AD的发生与发展[40]。此外脂质纳米颗粒也可以高效搭载其他药物靶向进入脑内以缓解病情,Mourtas等[41]通过合成含有姜黄素衍生物和血脑屏障转运介质的多功能脂质体,对AD患者死后大脑样本的解剖分析表明,姜黄素衍生物脂质体和姜黄素衍生物抗TrF脂质体对AD患者死后大脑样本中的淀粉样沉淀物具有高度亲和力。
2.2 聚合物纳米颗粒聚合物纳米颗粒是将药物包载于载体材料骨架内或者将药物通过共价链接/吸附作用修饰在载体材料的表面形成的纳米药物递送系统,其设计目的是防止药物在体内被降解和失活,并提高药物的生物利用度[42]。由于其稳定性较好、易于制备、可与小分子药物结合,还易于修饰配体,能达到靶向递送药物的作用,被广泛用于实验研究[43]。反义寡核苷酸可以封装在葡萄糖安装的聚合物纳米颗粒中,该聚合物纳米颗粒可以与葡萄糖转运蛋白-1偶联[44],这些反义寡核苷酸靶向APP或其加工酶的信使RNA,从而达到了降低毒性Aβ水平的目的。Yang等[45]构建了一种用于治疗AD的多功能纳米颗粒(Rapa@DAK/siRNA),该纳米颗粒以橙黄网孢盘菌凝集素和Aβ结合肽修饰的聚乙二醇化树突聚赖氨酸为基础,通过鼻内给药以实现β-前体蛋白切割酶-1的siRNA与雷帕霉素共同递送到脑内,这种聚合物纳米颗粒经鼻内给药后被证实可改善转基因AD小鼠的认知能力。Gao等[46]的研究发现,可以将聚乙二醇-神经酰胺纳米胶束通过自噬诱导小鼠N2a细胞中的Tau蛋白降解,这种聚合物纳米胶束实验证实了聚合物纳米药物在神经退行性疾病中的靶向治疗可行性。
2.3 金属及金属氧化物纳米颗粒纳米金属制剂也被用于中枢神经系统药物的靶向给药研究。氧化铈(CeO2)纳米颗粒具有自由基清除、抗干扰和抗凋亡的活性,铈的各种价态(氧化还原状态)均具有自由基清除活性,可用于创造抗氧化微环境和促进脑细胞修复的作用[47]。有研究表明,直径小于5 nm的CeO2纳米颗粒通过其超氧化物歧化酶模拟和过氧化氢酶模拟其活性,通过氧原子的可逆结合和在其表面上的Ce3+(还原)和Ce4+(氧化)状态之间的转换,CeO2纳米颗粒展现出了强大的氧自由基清除能力[48]。而金纳米颗粒已经被探索作为一种潜在的抗Aβ治疗药物,Hou等[49]设计和制备了直径为3.3 nm的L-和D-谷胱甘肽稳定的金纳米颗粒,这2种手性纳米颗粒都能抑制APP与Aβ42的聚集,并在静脉注射后穿过血脑屏障,且无明显毒性。与L3.3对映体相比,D3.3对APP与Aβ42具有更大的结合亲和力和更高的脑生物分布,对AD模型小鼠的Aβ42纤颤有更强的抑制作用,并更好地挽救行为损伤。这种小纳米颗粒与手性识别部分的结合为AD提供了一种潜在的治疗方法。
2.4 与siRNA纳米颗粒耦合的多级纳米载体缺氧聚集的Aβ积累是AD的一个关键致病事件,它由APP通过BACE1和γ-分泌酶的连续裂解产生。鉴于BACE1在Aβ生成过程中的关键作用,抑制其酶活性可以减少Aβ的产生,因此BACE1被视为是一个很好的治疗靶点。使用表达siRNA的慢病毒载体降低BACE1水平可减少AD小鼠中Aβ的产生以及改善神经变性和行为缺陷[50]。siRNA通过特异性沉默BACE1对AD治疗有很大的前景。然而,由于血脑屏障的存在和缺乏有效的siRNA脑传递方法限制了这一治疗策略。Zhou等[50]通过开发了一种糖基化的“三相互作用”稳定的聚合siRNA纳米药物(Gal-NP@siRNA),在APP/PS-1转基因AD小鼠模型中靶向BACE1。Gal-NP@siRNA表现出优越的血液稳定性,可以通过血糖控制的葡萄糖转运体-1介导的转运有效地穿透血脑屏障,从而确保siRNA降低BACE1的表达并改变相对通路。值得注意的是,给予Gal-NP@siBACE1恢复了AD小鼠认知能力,且没有明显的不良反应。实验还发现Gal-NP@siRNA纳米药物具有较高的大脑积累作用。Gal-NP@siBACE1降低了BACE1的表达,导致Aβ斑块水平降低,以及抑制磷酸化tau蛋白水平和受损髓鞘再生的额外好处[51]。这些积极的病理生理效应可能有助于恢复Gal-NP@siBACE1处理的转基因小鼠的认知能力。目前,许多BACE1的小分子抑制剂还处于临床前或临床研究的不同阶段。然而,有研究结果显示,抑制BACE1对突触功能和认知产生了不良影响[52]。
3 小结与展望目前尚无有效根治或者延缓AD病情进展的药物[53]。对于AD的致病机制有许多的观点及假说,对应的存在很多不同的治疗靶点。考虑到AD是多因素作用下而发生的一种难治性神经系统退行性疾病,基因治疗可以同时选择多个靶点,以抑制或增强的方式靶向不同AD假说所支持的关键蛋白质。因此,基因治疗AD是最具创新性和应用前景的新方法之一。关于AD新的基因靶点和药物靶点相结合的研究在逐年增加,结合最新纳米载体的研究,基于纳米技术的AD基因疗法在未来很可能实现突破性的研究进展,将会为AD患者带来更为有效的治疗效果,甚至改变AD患者的未来。
然而,在纳米技术被实际用作基因递送方法之前,仍有几个问题需要解决,例如转染效率低和潜在的毒性/耐受性问题、基因治疗是否会产生不良影响等[29]。关于毒性的问题,构建纳米载体的各种成分有可能会改变中枢神经系统的内环境和各种脑细胞的生理功能。此外,纳米载体在脑组织的代谢方式和转归目前尚不明确,由于AD的基因治疗可能需要多次重复给药,纳米载体在脑组织中的蓄积很可能会通过一些潜在机制引起神经元细胞的凋亡或坏死。为了解决这个问题,有必要更好地了解不同的细胞结构成分、细胞代谢途径和微环境,并构建具有靶向中枢神经系统递送药物特性的新型纳米结构[29]。当前生物相容性较好的仿生纳米材料成为了纳米药物研究的热点,且已取得了较为满意的研究结果,有希望在未来应用到临床治疗中。另外,可以通过优化纳米药物的给药剂量和持续治疗时间,以尽量减少纳米药物介导的不良影响。AD的基因疗法需要将治疗基因或者治疗药物递送到脑病变部位的特定靶点,因此需要成熟稳定的药物递送平台,以尽量减少出现脱靶的可能。
AD是一种多因素参与的疾病,目前APP、PS-1、PS-2和ApoE基因是已发现的与AD密切相关的4个主要基因[54],单基因治疗可能无法获得满意的治疗效果,同时针对多靶点的治疗可能是治疗AD的关键。随着AD病理不断的完善以及基因学说和纳米药物的不断发展,正确的基因治疗靶点与合适的纳米药物递送平台相结合可能是治疗AD和其他神经退行性疾病的一个重大突破(图 1)。
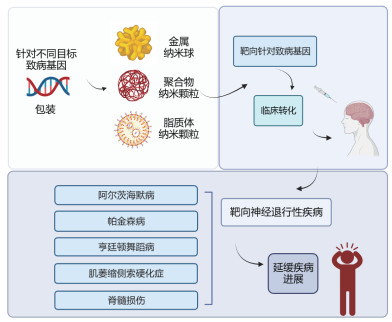
|
图 1 纳米医学结合基因疗法治疗阿尔茨海默病等神经退行性疾病的未来展望 |
| [1] |
REITZ C. Genetic diagnosis and prognosis of Alzheimer's disease: challenges and opportunities[J]. Expert Rev Mol Diagn, 2015, 15(3): 339-348. DOI:10.1586/14737159.2015.1002469 |
| [2] |
Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and metaanalysis[J]. Alzheimers Dement, 2013, 9(1): 63-75.e2. DOI:10.1016/j.jalz.2012.11.007 |
| [3] |
SCHELTENS P, DE STROOPER B, KIVIPELTO M, et al. Alzheimer's disease[J]. Lancet, 2021, 397(10284): 1577-1590. DOI:10.1016/s0140-6736(20)32205-4 |
| [4] |
FARKAS S, SZABÓ A, TÖRÖK B, et al. Ovariectomy-induced hormone deprivation aggravates Aβ1-42 deposition in the basolateral amygdala and cholinergic fiber loss in the cortex but not cognitive behavioral symptoms in a triple transgenic mouse model of Alzheimer's disease[J]. Front Endocrinol (Lausanne), 2022, 13: 985424. DOI:10.3389/fendo.2022.985424 |
| [5] |
SINGER O, MARR R A, ROCKENSTEIN E, et al. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model[J]. Nat Neurosci, 2005, 8(10): 1343-1349. DOI:10.1038/nn1531 |
| [6] |
MARINO M, ZHOU L, RINCON M Y, et al. AAV-mediated delivery of an anti-BACE1 VHH alleviates pathology in an Alzheimer's disease model[J]. EMBO Mol Med, 2022, 14(4): e09824. DOI:10.15252/emmm.201809824 |
| [7] |
GRICIUC A, FEDERICO A N, NATASAN J, et al. Gene therapy for Alzheimer's disease targeting CD33 reduces amyloid beta accumulation and neuroinflammation[J]. Hum Mol Genet, 2020, 29(17): 2920-2935. DOI:10.1093/hmg/ddaa179 |
| [8] |
DODART J C, MARR R A, KOISTINAHO M, et al. Gene delivery of human apolipoprotein E alters brain Abeta burden in a mouse model of Alzheimer's disease[J]. Proc Natl Acad Sci U S A, 2005, 102(4): 1211-1216. DOI:10.1073/pnas.0409072102 |
| [9] |
NARISAWA-SAITO M, WAKABAYASHI K, TSUJI S, et al. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer's disease[J]. Neuroreport, 1996, 7(18): 2925-2928. DOI:10.1097/00001756-199611250-00024 |
| [10] |
MUELLER-STEINER S, ZHOU Y, ARAI H, et al. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease[J]. Neuron, 2006, 51(6): 703-714. DOI:10.1016/j.neuron.2006.07.027 |
| [11] |
MALKKI H. Alzheimer disease: NGF gene therapy activates neurons in the AD patient brain[J]. Nat Rev Neurol, 2015, 11(10): 548. DOI:10.1038/nrneurol.2015.170 |
| [12] |
FALCICCHIA C, PAOLONE G, EMERICH D F, et al. Seizure-suppressant and neuroprotective effects of encapsulated BDNF-producing cells in a rat model of temporal lobe epilepsy[J]. Mol Ther Meth Clin Dev, 2018, 9: 211-224. DOI:10.1016/j.omtm.2018.03.001 |
| [13] |
ROSENBERG J B, KAPLITT M G, DE B P, et al. AAVrh.10-mediated APOE2 central nervous system gene therapy for APOE4-associated Alzheimer's disease[J]. Hum Gene Ther Clin Dev, 2018, 29(1): 24-47. DOI:10.1089/humc.2017.231 |
| [14] |
HONG C S, GOINS W F, GOSS J R, et al. Herpes simplex virus RNAi and neprilysin gene transfer vectors reduce accumulation of Alzheimer's disease-related amyloid-beta peptide in vivo[J]. Gene Ther, 2006, 13(14): 1068-1079. DOI:10.1038/sj.gt.3302719 |
| [15] |
HOOK V, YOON M, MOSIER C, et al. Cathepsin B in neurodegeneration of Alzheimer's disease, traumatic brain injury, and related brain disorders[J]. Biochim Biophys Acta Proteins Proteom, 2020, 1868(8): 140428. DOI:10.1016/j.bbapap.2020.140428 |
| [16] |
EL-AMOURI S S, ZHU H, YU J, et al. Neprilysin: an enzyme candidate to slow the progression of Alzheimer's disease[J]. Am J Pathol, 2008, 172(5): 1342-1354. DOI:10.2353/ajpath.2008.070620 |
| [17] |
MARR R A, ROCKENSTEIN E, MUKHERJEE A, et al. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice[J]. J Neurosci, 2003, 23(6): 1992-1996. DOI:10.1523/JNEUROSCI.23-06-01992.2003 |
| [18] |
MCKHANN G M, KNOPMAN D S, CHERTKOW H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease[J]. Alzheimers Dement, 2011, 7: 263-269. DOI:10.1016/j.jalz.2011.03.005 |
| [19] |
SHARMA S, VERMA S, KAPOOR M, et al. Alzheimer's disease like pathology induced six weeks after aggregated amyloid-beta injection in rats: increased oxidative stress and impaired long-term memory with anxiety-like behavior[J]. Neurol Res, 2016, 38(9): 838-850. DOI:10.1080/01616412.2016.1209337 |
| [20] |
GOEDERT M. Alzheimer's and Parkinson's diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein[J]. Science, 2015, 349(6248): e1255555. DOI:10.1126/science.1255555 |
| [21] |
LIANG Z, LI X, LUO X, et al. The aptamer Ob2, a novel AChE inhibitor, restores cognitive deficits and alleviates amyloidogenesis in 5×FAD transgenic mice[J]. Mol Ther Nucleic Acids, 2022, 28: 114-123. DOI:10.1016/j.omtn.2022.02.018 |
| [22] |
HAMPEL H, VASSAR R, DE STROOPER B, et al. The β-secretase BACE1 in Alzheimer's disease[J]. Biol Psychiatry, 2021, 89(8): 745-756. DOI:10.1016/j.biopsych.2020.02.001 |
| [23] |
DAMIAN HOLSINGER R M, MCLEAN C A, BEYREUTHER K, et al. Increased expression of the amyloid precursor beta-secretase in Alzheimer's disease[J]. Ann Neurol, 2002, 51(6): 783-786. DOI:10.1002/ana.10208 |
| [24] |
HAMPEL H, MESULAM M M, CUELLO A C, et al. The cholinergic system in the pathophysiology and treatment of Alzheimer's disease[J]. Brain, 2018, 141(7): 1917-1933. DOI:10.1093/brain/awy132 |
| [25] |
KARAMI A, ERIKSDOTTER M, KADIR A, et al. CSF cholinergic index, a new biomeasure of treatment effect in patients with Alzheimer's disease[J]. Front Mol Neurosci, 2019, 12: 239. DOI:10.3389/fnmol.2019.00239 |
| [26] |
ZHOU L T, ZHANG J, TAN L, et al. Elevated levels of miR-144-3p induce cholinergic degeneration by impairing the maturation of NGF in Alzheimer's disease[J]. Front Cell Dev Biol, 2021, 9: 667412. DOI:10.3389/fcell.2021.667412 |
| [27] |
SUN L, JIN Y, DONG L, et al. Coccomyxa gloeobotrydiformis improves learning and memory in intrinsic aging rats[J]. Int J Biol Sci, 2015, 11(7): 825-832. DOI:10.7150/ijbs.10861 |
| [28] |
ERIKSDOTTER M, MITRA S. Gene and cell therapy for the nucleus basalis of Meynert with NGF in Alzheimer's disease[J]. Handb Clin Neurol, 2021, 179: 219-229. DOI:10.1016/B978-0-12-819975-6.00012-1 |
| [29] |
UNNISA A, GREIG N H, KAMAL M A. Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer's disease[J]. Neural Regen Res, 2023, 18(10): 2127-2133. DOI:10.4103/1673-5374.369096 |
| [30] |
TUSZYNSKI M H, THAL L, PAY M, et al. A phase 1clinical trial of nerve growth factor gene therapy for Alzheimer disease[J]. Nat Med, 2005, 11(5): 551-555. DOI:10.1038/nm1239 |
| [31] |
STEPANICHEV M. Gene editing and Alzheimer's disease: is there light at the end of the tunnel?[J]. Front Genome Ed, 2020, 2: 4. DOI:10.3389/fgeed.2020.00004 |
| [32] |
PENG L, BESTARD-LORIGADOS I, SONG W. The synapse as a treatment avenue for Alzheimer's disease[J]. Mol Psychiatry, 2022, 27(7): 2940-2949. DOI:10.1038/s41380-022-01565-z |
| [33] |
BELOOR J, MAES N, ULLAH I, et al. Small interfering RNA-mediated control of virus replication in the CNS is therapeutic and enables natural immunity to West Nile virus[J]. Cell Host Microbe, 2018, 23(4): 549-556.e3. DOI:10.1016/j.chom.2018.03.001 |
| [34] |
SIOUD M. RNA and CRISPR interferences: past, present, and future perspectives[J]. Methods Mol Biol, 2020, 2115: 1-22. DOI:10.1007/978-1-0716-0290-4_1 |
| [35] |
EDIS Z, WANG J, WAQAS M K, et al. Nanocarriers-mediated drug delivery systems for anticancer agents: an overview and perspectives[J]. Int J Nanomedicine, 2021, 16: 1313-1330. DOI:10.2147/ijn.s289443 |
| [36] |
DARAEE H, ETEMADI A, KOUHI M, et al. Application of liposomes in medicine and drug delivery[J]. Artif Cells Nanomed Biotechnol, 2016, 44(1): 381-391. DOI:10.3109/21691401.2014.953633 |
| [37] |
KULKARNI J A, WITZIGMANN D, CHEN S, et al. Lipid nanoparticle technology for clinical translation of siRNA therapeutics[J]. Acc Chem Res, 2019, 52(9): 2435-2444. DOI:10.1021/acs.accounts.9b00368 |
| [38] |
NIU X, CHEN J, GAO J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: focus on recent advances[J]. Asian J Pharm Sci, 2019, 14(5): 480-496. DOI:10.1016/j.ajps.2018.09.005 |
| [39] |
CANOVI M, MARKOUTSA E, LAZAR A N, et al. The binding affinity of anti-Aβ1-42 MAb-decorated nanoliposomes to Aβ1-42 peptides in vitro and to amyloid deposits in post-mortem tissue[J]. Biomaterials, 2011, 32(23): 5489-5497. DOI:10.1016/j.biomaterials.2011.04.020 |
| [40] |
TAGALAKIS A D, LEE D H D, BIENEMANN A S, et al. Multifunctional, self-assembling anionic peptide-lipid nano complexes for targeted siRNA delivery[J]. Biomaterials, 2014, 35(29): 8406-8415. DOI:10.1016/j.biomaterials.2014.06.003 |
| [41] |
MOURTAS S, LAZAR A N, MARKOUTSA E, et al. Multifunctional nanoliposomes with curcumin-lipid derivative and brain targeting functionality with potential applications for Alzheimer disease[J]. Eur J Med Chem, 2014, 80: 175-183. DOI:10.1016/j.ejmech.2014.04.050 |
| [42] |
VAN GIAU V, AN S S A, HULME J P. Mitochondrial therapeutic interventions in Alzheimer's disease[J]. J Neurol Sci, 2018, 395: 62-70. DOI:10.1016/j.jns.2018.09.033 |
| [43] |
WANG X, WU M, LI H, et al. Enhancing penetration ability of semiconducting polymer nanoparticles for sonodynamic therapy of large solid tumor[J]. Adv Sci (Weinh), 2022, 9(6): e2104125. DOI:10.1002/advs.202104125 |
| [44] |
MIN H S, KIM H J, NAITO M, et al. Systemic brain delivery of antisense oligonucleotides across the blood-brain barrier with a glucose-coated polymeric nanocarrier[J]. Angew Chem Int Ed, 2020, 59(21): 8173-8180. DOI:10.1002/anie.201914751 |
| [45] |
YANG X, YANG W, XIA X, et al. Intranasal delivery of BACE1 siRNA and rapamycin by dual targets modified nanoparticles for Alzheimer's disease therapy[J]. Small, 2022, 18(30): e2203182. DOI:10.1002/smll.202203182 |
| [46] |
GAO J, CHEN X, MA T, et al. PEG-ceramide nanomicelles induce autophagy and degrade tau proteins in N2a cells[J]. Int J Nanomedicine, 2020, 15: 6779-6789. DOI:10.2147/ijn.s258311 |
| [47] |
WANG S, YAO Z, ZHANG X, et al. Energy-supporting enzyme-mimic nanoscaffold facilitates tendon regeneration based on a mitochondrial protection and microenvironment remodeling strategy[J]. Adv Sci (Weinh), 2022, 9(31): e2202542. DOI:10.1002/advs.202202542 |
| [48] |
SIPOSOVA K, HUNTOSOVA V, GARCAROVA I, et al. Dual-functional antioxidant and antiamyloid cerium oxide nanoparticles fabricated by controlled synthesis in water-alcohol solutions[J]. Biomedicines, 2022, 10(5): 942. DOI:10.3390/biomedicines10050942 |
| [49] |
HOU K, ZHAO J, WANG H, et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer's disease[J]. Nat Commun, 2020, 11(1): 4790. DOI:10.1038/s41467-020-18525-2 |
| [50] |
ZHOU Y, ZHU F, LIU Y, et al. Blood-brain barrier-penetrating siRNA nanomedicine for Alzheimer's disease therapy[J]. Sci Adv, 2020, 6(41): eabc7031. DOI:10.1126/sciadv.abc7031 |
| [51] |
GU X, SONG Q, ZHANG Q, et al. Clearance of two organic nanoparticles from the brain via the paravascular pathway[J]. J Control Release, 2020, 322: 31-41. DOI:10.1016/j.jconrel.2020.03.009 |
| [52] |
FILSER S, OVSEPIAN S V, MASANA M, et al. Pharmacological inhibition of BACE1 impairs synaptic plasticity and cognitive functions[J]. Biol Psychiatry, 2015, 77(8): 729-739. DOI:10.1016/j.biopsych.2014.10.013 |
| [53] |
程文彬, 陈晓晗, 孙文静, 等. 阿尔茨海默病精准诊断和药物研发的焦点与挑战[J]. 海军军医大学学报, 2023, 44(9): 1013-1025. CHENG W, CHEN X, SUN W, et al. Precision diagnosis and drug development for Alzheimer's disease: focus and challenges[J]. Acad J Naval Med Univ, 2023, 44(9): 1013-1025. DOI:10.16781/j.CN31-2187/R.20230210 |
| [54] |
LALWANI A K, KRISHNAN K, BAGABIR S A, et al. Network theoretical approach to explore factors affecting signal propagation and stability in dementia's protein-protein interaction network[J]. Biomolecules, 2022, 12(3): 451. DOI:10.3390/biom12030451 |
 2024, Vol. 45
2024, Vol. 45


