2. 上海理工大学公利医疗技术学院精准医疗与再生医学系, 上海 200135
2. Department of Precision and Regenerative Medicine, School of Gongli Hospital Medical Technology, University of Shanghai for Science and Technology, Shanghai 200135, China
生长分化因子15(growth differentiation factor 15,GDF15)是TGF-β超家族的成员,最早发现产生于巨噬细胞,以自分泌方式通过负反馈调控机制抑制巨噬细胞的过度活化及TNF-α的表达,因此被称为巨噬细胞抑制因子1(macrophage inhibitory cytokine 1,MIC-1)[1-2]。在正常生理条件下,GDF15主要在胎盘和前列腺组织中高表达,故而也被称为非甾体抗炎药激活基因1(non-steroidal anti-inflammatory drug activated gene 1,NAG-1)、胎盘转化生长因子β(placental transforming growth factor β,PTGFB)、前列腺衍生因子(prostate-derived factor,PDF)和胎盘骨形态发生蛋白(placental bone morphogenetic protein,PLAB)等[3-4]。GDF15在乳腺、肝脏、肾脏等多种组织也有低表达,并且存在于血液循环中,其作用的靶细胞广泛,可通过不同信号转导作用引起细胞发生多种效应,从而使其在多种器官和组织中都参与调控作用。本文主要围绕GDF15在代谢、衰老和炎症相关疾病中的作用展开论述。
1 GDF15的转录调控及其受体人类Gdf15编码基因位于染色体19p12.1~13.1区域,包含长度分别为309 bp和891 bp的2个外显子片段,中间是长度为1 819 bp的内含子区,并且在起始密码子附近含有保守的TATA盒序列(TATAA)[5]。Gdf15基因的启动子可以结合多种顺式和反式启动子元件,包括特异性蛋白1(specificity protein 1,Sp1)、早期生长反应蛋白1(early growth response protein 1,EGR1)、p53、转录因子3A(activating transcription factor 3A,ATF3A)、NF-κB等[6]。细胞接收外界信号后,不同的启动子元件会结合在启动子上,通过促进或抑制GDF15的表达进而使细胞产生不同的应答反应。除了转录水平的调控,翻译和翻译后过程的调控也参与GDF15蛋白的表达和成熟。人GDF15前体蛋白(pro-GDF15)由308个氨基酸残基组成,包括信号肽、氨基端前肽和羧基端成熟区。信号肽切除后产生由167个氨基酸残基组成的分子量约为40 000的前肽。随后GDF15前肽进一步被水解,产生由112个氨基酸残基组成的分子量约为13 000的GDF15单体。GDF15前肽主要被3种蛋白酶水解,这3种蛋白酶都属于前蛋白转化酶枯草溶菌素(proprotein convertase subtilisin/kexin,PCSK)丝氨酸蛋白酶家族,分别是PCSK3、PCSK5和PCSK6。随后,2个GDF15蛋白单体通过半胱氨酸的二硫键连接形成同源二聚体蛋白,从而具有生物活性[7-8]。近期的研究发现β-抑制蛋白1(arrestin β1,ARRB1)具有促使GDF15蛋白成熟的作用,其主要作用是结合在GDF15前肽上帮助其转运到高尔基体,然后通过高尔基体中PCSK丝氨酸蛋白酶的水解促进GDF15进一步成熟;敲除Arrb1基因后,GDF15蛋白无法正常成熟和发挥生物学活性[8-9]。
尽管GDF15是TGF-β超家族的成员,但是它与任何已知的TGF-β受体都不能结合。胶质细胞源性神经营养因子家族受体α样(glial cell line derived neurotrophic factor family receptor α-like,GFRAL)蛋白是迄今为止发现的唯一GDF15受体。GFRAL是一种孤儿受体,主要表达分布于小鼠、大鼠和人类的脑干最后区(area postrema)和孤束核区[10-11]。GFRAL由394个氨基酸残基组成,分子量约为44 000,存在2种形式:一种是跨膜蛋白,含全部氨基酸序列;一种是胞内可溶性蛋白,包含238个氨基酸残基,含有GDF15蛋白结合结构域[12-13]。GFRAL与存在于脑干中的神经元表面受体酪氨酸激酶形成复合物,来自外周血中的GDF15与该复合物结合后,触发受体磷酸化,激活细胞内Akt、ERK1/2和磷脂酶C(phospholipase C,PLC)γ等信号通路,并进一步引发下游级联放大信号。近期研究显示,肝癌细胞分泌的GDF15可以与调节性T细胞(regulatory T cell,Treg)表面的CD48受体结合,诱导Treg的免疫抑制,因此CD48被认为是肿瘤免疫微环境中GDF15的潜在新受体[14]。
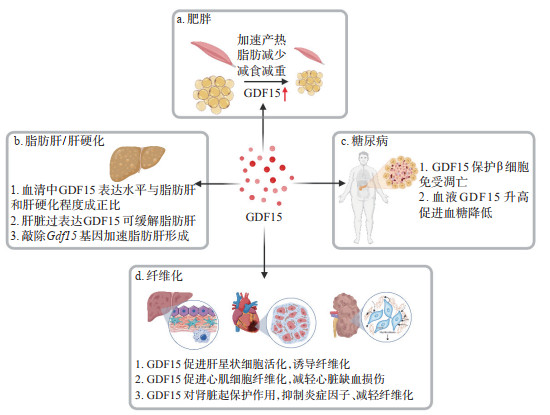
2 GDF15在机体稳态维持中的作用GDF15在健康个体和年轻受试者中表达量非常低,血浆中的浓度为0.1~1.2 ng/mL,但年龄的增加、运动及压力刺激等会促进血清中GDF15浓度升高[15]。肥胖、糖尿病、早期癌症等情况下GDF15的水平会达到0.8~3.0 ng/mL,晚期癌症和线粒体基因突变的条件下GDF15的水平会升高到2~10 ng/mL,而有些特殊的晚期癌症中GDF15的水平甚至会上升到10~100 ng/mL[2, 4]。研究表明,长时间的耐力运动会促进肝脏、骨骼肌和心肌细胞中GDF15蛋白表达的增加,使血清中GDF15浓度升高到只有在病理情况下才能观察到的水平[16]。用高脂饮食喂养小鼠1周后,血浆中GDF15的浓度并未明显改变,而继续喂养4周后,其浓度增加1倍;用缺乏赖氨酸的食物喂养小鼠1 h就可使血清中GDF15的水平升高约33%,喂养4 h后其水平则可升高1倍[17]。对小鼠给予外源性GDF15可以导致运动疲劳和条件性味觉厌恶,这表明GDF15可能会诱导对运动和营养应激的厌恶反应,使机体避免运动过度和营养过剩[16-17]。Patsalos等[18]近期发现了一个新的巨噬细胞亚群,该群细胞会通过表达和分泌GDF15促进成肌细胞和骨髓细胞启动再生,从而促进肌肉再生。Nakayasu等[19]使用促炎细胞因子IL-1β和干扰素γ联合处理离体人胰岛,结果显示促炎细胞因子处理后会引起β细胞内GDF15的表达降低,而给予GDF15能够阻断细胞内凋亡信号从而保护β细胞免受死亡;对临床组织标本进行免疫组织化学检测发现,1型糖尿病患者胰腺组织中GDF15的表达丰度显著降低,而2型糖尿病患者和正常人胰岛中GDF15的表达没有明显变化,这进一步说明低表达的GDF15失去了对炎症诱导β细胞凋亡的保护作用[19]。因此,GDF15在生理条件下是一种应激反应诱导因子或病理性代偿反应保护因子,对机体稳态的维持起着重要作用,当机体遇到应激、病理反应等条件刺激后,GDF15蛋白可代偿性表达来保护细胞。
3 GDF15在衰老中的作用随着年龄的增加,各器官组织中细胞会发生衰老并会表达衰老相关分泌表型(senescence associated secretory phenotype,SASP),导致组织微环境发生炎症并诱发衰老相关疾病。我们前期研究发现,在联体共生小鼠模型中实现年轻和老年血液的交换,可以有效逆转老年小鼠肝脏中衰老的肝细胞表型和功能,提高肝脏再生和代谢能力,从而逆转衰老引起的老年脂肪肝疾病[20]。对人和小鼠血液中蛋白表达的分析结果显示,老年人和小鼠血液中包括GDF15蛋白在内的衰老相关蛋白的浓度分别较年轻人和小鼠明显增加[21-22]。研究表明老年个体中GDF15蛋白表达水平增加可能与衰老过程中过度的氧化应激反应和线粒体功能障碍有关[23-24]。目前,GDF15已被认为是衰老相关分泌表型的一种,主要以自分泌或旁分泌途径在衰老微环境中发挥促炎或抑炎的作用[25]。如衰老心肌细胞分泌GDF15,GDF15与TGF-β相互作用促进心肌成纤维细胞活化并诱导纤维化发生[26]。诱导结肠成纤维细胞衰老后会促进GDF15的表达,从而激活MAPK和PI3K信号通路,加速结直肠癌细胞的增殖、迁移和侵袭,以促炎的方式加速了肿瘤的发展[27]。然而,GDF15在微环境中也可以作为一种抑炎因子。如GDF15可以维持Treg的免疫抑制功能并减缓炎症[28],是潜在的改善老年人免疫的新策略。敲除Gdf15基因后,小鼠局部及全身炎症反应显著加剧,并导致严重的肝脏损伤和脂肪沉积[29]。综上所述,随着年龄的增加,GDF15蛋白逐渐积累,并产生多种生物学效应,既能通过促炎作用诱导疾病发生,也能继续发挥抗炎作用以维持器官功能。
4 GDF15在代谢性疾病中的调控作用GDF15与肥胖和糖尿病有着密切的关系。研究显示,肥胖患者血浆中GDF15浓度显著高于正常人群,且GDF15与胰岛素抵抗指数等血糖控制相关指标呈正相关[30]。大量研究显示GDF15具有减重效应,主要机制可能是GDF15通过作用于中枢神经系统的GFRAL受体,一方面调控细胞内脂肪酸β氧化和脂质分解,抑制脂肪酸合成和摄取,减少炎症反应并降低脂肪变性,另一方面还通过调控食欲相关的神经元细胞引起食欲抑制、摄食减少、恶心呕吐,最终发挥减重作用[16-17, 31]。此外,GDF15-GFRAL轴还可促进脂肪细胞中脂肪分解相关酶和激素敏感性脂肪酶的表达升高,加速脂肪分解和产热[32]。二甲双胍是治疗2型糖尿病的一线药物,通过抑制肝脏葡萄糖的产生发挥降血糖作用。二甲双胍除了具有降血糖作用外还有减重的功效,促进血液中GDF15蛋白的水平升高是其重要的减重机制。二甲双胍通过上调肾脏中GDF15蛋白的合成增高血液中GDF15水平,并作用于脑干最后区的GFRAL受体,从而降低食物摄入量,达到减肥效果[33-34]。尽管GDF15在远端肠道和肾脏均有表达,但只有肾脏特异性的GDF15表达下调及脑干最后区特异性的GFRAL表达下调,才能阻断二甲双胍诱导的血液中GDF15水平的上升并消除其对食物摄入和体重的降低作用[35]。高脂膳食喂养的GDF15野生型(Gdf15+/+)小鼠口服二甲双胍11 d后,小鼠食物摄入量减少,体重降低;但高脂膳食喂养的GDF15缺陷(Gdf15-/-)小鼠口服二甲双胍后,与对照组小鼠相比体重并没有降低[34]。这表明二甲双胍的减重作用需要GDF15及其受体参与,通过升高GDF15的水平抑制摄食而减轻体重。二甲双胍还可通过促进肝脏和肌肉中GDF15的表达来激活5’-腺嘌呤核苷酸依赖的蛋白激酶(adenosine 5’-monophosphate-activated protein kinase,AMPK)信号通路,从而抑制高糖引起的葡萄糖耐受现象[36]。
然而,也有越来越多的研究表明GDF15需要与其他蛋白协同作用才能更加有效地参与肥胖和糖尿病的治疗。成纤维细胞生长因子21(fibroblast growth factor 21,FGF21)和GDF15都是重要的应激诱导因子,Patel等[37]在高脂喂养的小鼠中发现,血清中GDF15与FGF21一样主要来源于肝脏,与Gdf15敲除小鼠相比,Gdf15和Fgf21共敲除小鼠经高脂饮食诱导后会加速肝脏脂肪变性和胰岛素抵抗现象,说明GDF15和FGF21共同参与肝脏脂肪代谢及糖尿病胰岛素抵抗的调控。Breit等[38]研究发现,GDF15和瘦素联合输注所致的体重和脂肪减少明显大于任何一种单独治疗,在瘦素缺陷的小鼠和内源性瘦素作用被抑制的小鼠中,GDF15对体重和脂肪的影响明显降低,进一步研究发现瘦素受体(leptin receptor,LEPR)和GFRAL分别表达于不同的神经元,作者认为这两类神经元分别被各自的配体激活,通过直接或间接的协同神经元信号转导,最终共同促进脂肪分解。肝细胞表达的GDF15蛋白还受葡萄糖醛酸C5异构酶(glucuronyl C5-epimerase,Glce)的调控。肝脏特异性敲除Glce基因后抑制了GDF15单体向成熟形式的转化并促进GDF15蛋白的泛素化降解,从而通过肝脏-脂肪组织的信号转导降低棕色脂肪组织产热功能,促进白色脂肪组织分化,使小鼠血糖耐受能力和胰岛素敏感性显著下降,血清总胆固醇和甘油三酯含量升高,并加剧高脂饮食诱导的脂代谢紊乱[39]。过氧化物酶体增殖物激活受体β/δ(peroxisome proliferator-activated receptor β/δ,PPARβ/δ)也能够促进GDF15的表达,从而加速肝内脂肪酸氧化,缓解高脂饮食引起的脂肪肝[40]。更为有趣的是,GDF15不仅参与减重,还是维持能量燃烧和避免体重反弹的一个关键因子。其主要机制是GDF15/GFRAL信号激活了β-肾上腺素能依赖的信号轴,促进骨骼肌中脂肪酸氧化及钙的释放,肌肉细胞通过徒劳循环来消耗钙并在这个过程中释放能量,从而阻止节食期间新陈代谢的减慢[41]。由此可见,GDF15是治疗非酒精性脂肪性肝病(non-alcoholic fatty liver disease,NAFLD)和糖尿病的潜在靶点。
5 GDF15在炎症相关疾病中的调控作用在疾病发生过程中,GDF15可作为一种炎症标志物在多种细胞中表达。研究显示,IL-1β或衣霉素刺激的内质网应激可诱导肝细胞中GDF15的表达,而NAFLD中肝脏GDF15的表达与IL-1β含量和脂肪变性严重程度直接相关[42-43]。NAFLD伴肥胖患者GDF15的表达显著高于正常人群,NAFLD晚期纤维化患者GDF15的表达也明显高于NAFLD早期患者,并且GDF15的水平与肝脏硬度呈正相关[44]。一项转录组学分析显示,血清中GDF15浓度与NAFLD严重程度和纤维化分级呈正相关[45]。因此,GDF15可能是NAFLD晚期纤维化的有用生物标志物。在特发性肺纤维化疾病中,GDF15蛋白的表达水平随着肺纤维化的发生而升高,用GDF15抗体中和其表达后可显著降低肺纤维化程度,而给予重组GDF15蛋白则可促进成纤维细胞的增殖、加速肺纤维化[46]。该研究结果表明GDF15通过成纤维细胞的活化和分化介导肺纤维化,提示GDF15具有促进肺纤维化的潜在作用。
然而,GDF15蛋白也像一把“双刃剑”,在发挥促炎作用的同时也有抑炎作用。如在肝脏纤维化过程中,GDF15的表达降低了肝脏内促炎细胞因子的表达,抑制纤维化小鼠肝脏中T细胞的活化;而抑制GDF15表达后则会促进T细胞产生TNF-α,激活CD4+和CD8+ T细胞,从而加重肝脏损伤和纤维化[47]。另外,研究显示虽然内源性的GDF15过表达不足以预防肾脏损伤,但是给予外源性GDF15蛋白可在急性和慢性肾损伤时维持肾脏保护因子Klotho的表达,对肾脏起到保护作用[48]。而且,过表达GDF15可抑制晚期糖基化终末产物(advanced glycation end products,AGE)/晚期糖基化终末产物受体(receptor advanced glycation end products,RAGE)通路和Toll样受体4(Toll-like receptor 4,TLR4)/髓样分化因子88(myeloid differentiation factor 88,MyD88)/NF-κB信号通路,抑制炎症因子的表达,从而缓解肾纤维化[49]。正常生理状态下心脏组织中GDF15表达水平较低,而心肌梗死后心肌细胞中GDF15的表达增多,并且给予外源性GDF15能够抑制Gdf15缺陷小鼠心肌梗死后多形核白细胞的聚集,说明GDF15可抑制炎症细胞募集而起到心脏保护作用[50]。脂多糖刺激后,Gdf15敲除小鼠表现出更强的炎症反应并伴随严重的肾脏和心脏损伤,而Gdf15转基因小鼠则可免受脂多糖介导的器官损伤[51]。临床研究显示,非缺血性扩张型心肌病患者血清中GDF15浓度明显增高,且与肌纤维化和肾功能异常相关,而当心肌功能恢复后其表达水平迅速下降至正常水平[52]。还有研究者在针对新型冠状病毒感染患者的前瞻性研究中发现,高水平的GDF15与新型冠状病毒血症和低氧血症有关,强调了GDF15对预后的重要性[53]。
6 小结GDF15是一种内分泌激素,主要参与细胞生长、分化和组织修复,在生理状态下对机体稳态维持起着重要作用,是炎症反应时机体自分泌的保护因子,也是氧化应激和细胞衰老的标志物。目前认为GDF15最主要的作用是参与细胞的糖脂代谢,可谓是肥胖减重领域冉冉升起的“新星”。GDF15可作为炎症因子促进组织发生炎症,从而引起炎症相关纤维化等疾病。而且,在不同的疾病以及同一疾病的不同病理过程中,GDF15像一把“双刃剑”一样参与多种生物学效应(图 1)。尽管GDF15蛋白的作用非常广泛,但是GFRAL是目前发现的GDF15的唯一受体。GDF15-GFRAL的信号调控主要发生在细胞的代谢途径,而关于GDF15是如何促进细胞发生炎症,以及其作为炎症因子如何作用于靶细胞的分子机制,仍需进一步探索。深入研究GDF15的特定作用,建立个体化靶向GDF15的干预策略将对临床疾病治疗具有重要意义。
|
图 1 GDF15在代谢性疾病和炎症相关疾病中的作用 GDF15:生长分化因子15. |
| [1] |
CONTE M, GIULIANI C, CHIARIELLO A, et al. GDF15, an emerging key player in human aging[J]. Ageing Res Rev, 2022, 75: 101569. DOI:10.1016/j.arr.2022.101569 |
| [2] |
SIDDIQUI J A, POTHURAJU R, KHAN P, et al. Pathophysiological role of growth differentiation factor 15 (GDF15) in obesity, cancer, and cachexia[J]. Cytokine Growth Factor Rev, 2022, 64: 71-83. DOI:10.1016/j.cytogfr.2021.11.002 |
| [3] |
BAEK S J, HOROWITZ J M, ELING T E. Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. Basal transcription is mediated by Sp1 and Sp3[J]. J Biol Chem, 2001, 276(36): 33384-33392. DOI:10.1074/jbc.M101814200 |
| [4] |
ASSADI A, ZAHABI A, HART R A. GDF15, an update of the physiological and pathological roles it plays: a review[J]. Pflugers Arch, 2020, 472(11): 1535-1546. DOI:10.1007/s00424-020-02459-1 |
| [5] |
JIANG J, THALAMUTHU A, HO J E, et al. A meta-analysis of genome-wide association studies of growth differentiation factor-15 concentration in blood[J]. Front Genet, 2018, 9: 97. DOI:10.3389/fgene.2018.00097 |
| [6] |
TSUI K H, HSU S Y, CHUNG L C, et al. Growth differentiation factor-15: a p53- and demethylation-upregulating gene represses cell proliferation, invasion, and tumorigenesis in bladder carcinoma cells[J]. Sci Rep, 2015, 5: 12870. DOI:10.1038/srep12870 |
| [7] |
BAEK S J, ELING T. Growth differentiation factor 15 (GDF15): a survival protein with therapeutic potential in metabolic diseases[J]. Pharmacol Ther, 2019, 198: 46-58. DOI:10.1016/j.pharmthera.2019.02.008 |
| [8] |
WANG D, DAY E A, TOWNSEND L K, et al. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease[J]. Nat Rev Endocrinol, 2021, 17(10): 592-607. DOI:10.1038/s41574-021-00529-7 |
| [9] |
ZHANG Z, XU X, TIAN W, et al. ARRB1 inhibits non-alcoholic steatohepatitis progression by promoting GDF15 maturation[J]. J Hepatol, 2020, 72(5): 976-989. DOI:10.1016/j.jhep.2019.12.004 |
| [10] |
EMMERSON P J, WANG F, DU Y, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL[J]. Nat Med, 2017, 23(10): 1215-1219. DOI:10.1038/nm.4393 |
| [11] |
YANG L, CHANG C C, SUN Z, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand[J]. Nat Med, 2017, 23(10): 1158-1166. DOI:10.1038/nm.4394 |
| [12] |
BREIT S N, TSAI V W W, BROWN D A. Targeting obesity and cachexia: identification of the GFRAL receptor-MIC-1/GDF15 pathway[J]. Trends Mol Med, 2017, 23(12): 1065-1067. DOI:10.1016/j.molmed.2017.10.005 |
| [13] |
TSAI V W W, HUSAINI Y, SAINSBURY A, et al. The MIC-1/GDF15-GFRAL pathway in energy homeostasis: implications for obesity, cachexia, and other associated diseases[J]. Cell Metab, 2018, 28(3): 353-368. DOI:10.1016/j.cmet.2018.07.018 |
| [14] |
WANG Z, HE L, LI W, et al. GDF15 induces immunosuppression via CD48 on regulatory T cells in hepatocellular carcinoma[J]. J Immunother Cancer, 2021, 9(9): e002787. DOI:10.1136/jitc-2021-002787 |
| [15] |
MAZAGOVA M, BUIKEMA H, VAN BUITEN A, et al. Genetic deletion of growth differentiation factor 15 augments renal damage in both type 1 and type 2 models of diabetes[J]. Am J Physiol Renal Physiol, 2013, 305(9): F1249-F1264. DOI:10.1152/ajprenal.00387.2013 |
| [16] |
KLEIN A B, NICOLAISEN T S, ØRTENBLAD N, et al. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise[J]. Nat Commun, 2021, 12(1): 1041. DOI:10.1038/s41467-021-21309-x |
| [17] |
PATEL S, ALVAREZ-GUAITA A, MELVIN A, et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans[J]. Cell Metab, 2019, 29(3): 707-718. DOI:10.1016/j.cmet.2018.12.016 |
| [18] |
PATSALOS A, HALASZ L, MEDINA-SERPAS M A, et al. A growth factor-expressing macrophage subpopulation orchestrates regenerative inflammation via GDF-15[J]. J Exp Med, 2022, 219(1): e20210420. DOI:10.1084/jem.20210420 |
| [19] |
NAKAYASU E S, SYED F, TERSEY S A, et al. Comprehensive proteomics analysis of stressed human islets identifies GDF15 as a target for type 1 diabetes intervention[J]. Cell Metab, 2020, 31(2): 363-374. DOI:10.1016/j.cmet.2019.12.005 |
| [20] |
LIU Q, CHEN F, YANG T, et al. Aged-related function disorder of liver is reversed after exposing to young milieu via conversion of hepatocyte ploidy[J]. Aging Dis, 2021, 12(5): 1238-1251. DOI:10.14336/AD.2020.1227 |
| [21] |
LEHALLIER B, GATE D, SCHAUM N, et al. Undulating changes in human plasma proteome profiles across the lifespan[J]. Nat Med, 2019, 25(12): 1843-1850. DOI:10.1038/s41591-019-0673-2 |
| [22] |
TANAKA T, BIANCOTTO A, MOADDEL R, et al. Plasma proteomic signature of age in healthy humans[J]. Aging Cell, 2018, 17(5): e12799. DOI:10.1111/acel.12799 |
| [23] |
DAVIS R L, LIANG C, SUE C M. A comparison of current serum biomarkers as diagnostic indicators of mitochondrial diseases[J]. Neurology, 2016, 86(21): 2010-2015. DOI:10.1212/WNL.0000000000002705 |
| [24] |
FUJITA Y, TANIGUCHI Y, SHINKAI S, et al. Secreted growth differentiation factor 15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders[J]. Geriatr Gerontol Int, 2016, 16(Suppl 1): 17-29. DOI:10.1111/ggi.12724 |
| [25] |
BASISTY N, KALE A, JEON O H, et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development[J]. PLoS Biol, 2020, 18(1): e3000599. DOI:10.1371/journal.pbio.3000599 |
| [26] |
CHEN M S, LEE R T, GARBERN J C. Senescence mechanisms and targets in the heart[J]. Cardiovasc Res, 2022, 118(5): 1173-1187. DOI:10.1093/cvr/cvab161 |
| [27] |
GUO Y, AYERS J L, CARTER K T, et al. Senescence-associated tissue microenvironment promotes colon cancer formation through the secretory factor GDF15[J]. Aging Cell, 2019, 18(6): e13013. DOI:10.1111/acel.13013 |
| [28] |
MITTELBRUNN M, KROEMER G. Hallmarks of T cell aging[J]. Nat Immunol, 2021, 22(6): 687-698. DOI:10.1038/s41590-021-00927-z |
| [29] |
MOON J S, GOEMINNE L J E, KIM J T, et al. Growth differentiation factor 15 protects against the aging-mediated systemic inflammatory response in humans and mice[J]. Aging Cell, 2020, 19(8): e13195. DOI:10.1111/acel.13195 |
| [30] |
XIONG Y, WALKER K, MIN X, et al. Long-acting MIC-1/GDF15 molecules to treat obesity: evidence from mice to monkeys[J]. Sci Transl Med, 2017, 9(412): eaan8732. DOI:10.1126/scitranslmed.aan8732 |
| [31] |
BORNER T, SHAULSON E D, GHIDEWON M Y, et al. GDF15 induces anorexia through nausea and Emesis[J]. Cell Metab, 2020, 31(2): 351-362. DOI:10.1016/j.cmet.2019.12.004 |
| [32] |
SURIBEN R, CHEN M, HIGBEE J, et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice[J]. Nat Med, 2020, 26(8): 1264-1270. DOI:10.1038/s41591-020-0945-x |
| [33] |
DAY E A, FORD R J, SMITH B K, et al. Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss[J]. Nat Metab, 2019, 1(12): 1202-1208. DOI:10.1038/s42255-019-0146-4 |
| [34] |
COLL A P, CHEN M, TASKAR P, et al. GDF15 mediates the effects of metformin on body weight and energy balance[J]. Nature, 2020, 578(7795): 444-448. DOI:10.1038/s41586-019-1911-y |
| [35] |
ZHANG S Y, BRUCE K, DANAEI Z, et al. Metformin triggers a kidney GDF15-dependent area postrema axis to regulate food intake and body weight[J]. Cell Metab, 2023, 35(5): 875-886. DOI:10.1016/j.cmet.2023.03.014 |
| [36] |
AGUILAR-RECARTE D, BARROSO E, ZHANG M, et al. A positive feedback loop between AMPK and GDF15 promotes metformin antidiabetic effects[J]. Pharmacol Res, 2023, 187: 106578. DOI:10.1016/j.phrs.2022.106578 |
| [37] |
PATEL S, HAIDER A, ALVAREZ-GUAITA A, et al. Combined genetic deletion of GDF15 and FGF21 has modest effects on body weight, hepatic steatosis and insulin resistance in high fat fed mice[J]. Mol Metab, 2022, 65: 101589. DOI:10.1016/j.molmet.2022.101589 |
| [38] |
BREIT S N, MANANDHAR R, ZHANG H P, et al. GDF15 enhances body weight and adiposity reduction in obese mice by leveraging the leptin pathway[J]. Cell Metab, 2023, 35(8): 1341-1355. DOI:10.1016/j.cmet.2023.06.009 |
| [39] |
HE F, JIANG H, PENG C, et al. Hepatic glucuronyl C5-epimerase combats obesity by stabilising GDF15[J]. J Hepatol, 2023, 79(3): 605-617. DOI:10.1016/j.jhep.2023.05.011 |
| [40] |
AGUILAR-RECARTE D, BARROSO E, GUMÀ A, et al. GDF15 mediates the metabolic effects of PPARβ/δ by activating AMPK[J]. Cell Rep, 2021, 36(6): 109501. DOI:10.1016/j.celrep.2021.109501 |
| [41] |
WANG D, TOWNSEND L K, DESORMEAUX G J, et al. GDF15 promotes weight loss by enhancing energy expenditure in muscle[J]. Nature, 2023, 619(7968): 143-150. DOI:10.1038/s41586-023-06249-4 |
| [42] |
KIM K H, KIM S H, HAN D H, et al. Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice[J]. Sci Rep, 2018, 8(1): 6789. DOI:10.1038/s41598-018-25098-0 |
| [43] |
ADOLPH T E, GRABHERR F, MAYR L, et al. Weight loss induced by bariatric surgery restricts hepatic GDF15 expression[J]. J Obes, 2018, 2018: 7108075. DOI:10.1155/2018/7108075 |
| [44] |
KOO B K, UM S H, SEO D S, et al. Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease[J]. Liver Int, 2018, 38(4): 695-705. DOI:10.1111/liv.13587 |
| [45] |
GOVAERE O, COCKELL S, TINIAKOS D, et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis[J]. Sci Transl Med, 2020, 12(572): eaba4448. DOI:10.1126/scitranslmed.aba4448 |
| [46] |
RADWANSKA A, COTTAGE C T, PIRAS A, et al. Increased expression and accumulation of GDF15 in IPF extracellular matrix contribute to fibrosis[J]. JCI Insight, 2022, 7(16): e153058. DOI:10.1172/jci.insight.153058 |
| [47] |
CHUNG H K, KIM J T, KIM H W, et al. GDF15 deficiency exacerbates chronic alcohol- and carbon tetrachloride-induced liver injury[J]. Sci Rep, 2017, 7(1): 17238. DOI:10.1038/s41598-017-17574-w |
| [48] |
VALIÑO-RIVAS L, CUARENTAL L, CEBALLOS M I, et al. Growth differentiation factor-15 preserves Klotho expression in acute kidney injury and kidney fibrosis[J]. Kidney Int, 2022, 101(6): 1200-1215. DOI:10.1016/j.kint.2022.02.028 |
| [49] |
CHEN J, PENG H, CHEN C, et al. NAG-1/GDF15 inhibits diabetic nephropathy via inhibiting AGE/RAGE-mediated inflammation signaling pathways in C57BL/6 mice and HK-2 cells[J]. Life Sci, 2022, 311(Pt A): 121142. DOI:10.1016/j.lfs.2022.121142 |
| [50] |
KEMPF T, ZARBOCK A, WIDERA C, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice[J]. Nat Med, 2011, 17(5): 581-588. DOI:10.1038/nm.2354 |
| [51] |
ABULIZI P, LOGANATHAN N, ZHAO D, et al. Growth differentiation factor-15 deficiency augments inflammatory response and exacerbates septic heart and renal injury induced by lipopolysaccharide[J]. Sci Rep, 2017, 7(1): 1037. DOI:10.1038/s41598-017-00902-5 |
| [52] |
LOK S I, WINKENS B, GOLDSCHMEDING R, et al. Circulating growth differentiation factor-15 correlates with myocardial fibrosis in patients with non-ischaemic dilated cardiomyopathy and decreases rapidly after left ventricular assist device support[J]. Eur J Heart Fail, 2012, 14(11): 1249-1256. DOI:10.1093/eurjhf/hfs120 |
| [53] |
MYHRE P L, PREBENSEN C, STRAND H, et al. Growth differentiation factor 15 provides prognostic information superior to established cardiovascular and inflammatory biomarkers in unselected patients hospitalized with COVID-19[J]. Circulation, 2020, 142(22): 2128-2137. DOI:10.1161/CIRCULATIONAHA.120.050360 |
 2024, Vol. 45
2024, Vol. 45


