2. 海军军医大学(第二军医大学)第三附属医院神经外科, 上海 200438;
3. 上海交通大学医学院附属瑞金医院功能神经外科, 上海 200025
2. Department of Neurosurgery, The Third Affiliated Hospital of Naval Medical University (Second Military Medical University), Shanghai 200438, China;
3. Department of Functional Neurosurgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China
帕金森病以静止性震颤、僵直和运动迟缓等运动障碍为主要特征[1],但在疾病进展过程中各种非运动特征并不少见,如认知功能下降和精神障碍等[2-3]。研究表明,帕金森病可能会影响到每个认知领域,包括注意、视觉空间功能、记忆和执行功能[3-4],其中执行功能障碍可能是帕金森病患者的主要认知障碍。
非侵入式脑刺激治疗帕金森病因不良反应少、安全性高、易于操作、治疗效果好而受到广泛关注,目前用于帕金森病治疗的非侵入式脑刺激方案主要包括经颅磁刺激(transcranial magnetic stimulation,TMS)、经颅直流电刺激(transcranial direct current stimulation,tDCS)、经颅交流电刺激(transcranial alternating current stimulation,tACS)。前额叶皮质是执行功能的主要神经基础,虽然TMS、tDCS、tACS调节皮质兴奋性的机制不同,但三者均被证明可以诱导长时程增强或抑制突触可塑性[5-10]。目前非侵入式脑刺激对帕金森病患者执行功能障碍的疗效尚不明确。本研究采用网状meta分析(network meta-analysis,NMA)方法评价上述3种非侵入式脑刺激方式对帕金森病患者执行功能的改善效果,以期为临床实践提供参考。
1 资料和方法 1.1 纳入和排除标准纳入标准:(1)研究类型为随机对照试验,其研究对象为根据诊断标准被判定为帕金森病患者,且无认知功能障碍;(2)研究的干预措施需至少包括TMS、tDCS、tACS其中一项,结局指标必须包括与执行功能相关的评分。排除标准:(1)无法获取全文及数据不全的研究;(2)相关结局指标仅用图片展示、无法获取确切数据的研究。
1.2 检索策略利用计算机检索5个数据库(Web of Science、PubMed、EMBASE、中国知网、万方数据)中关于非侵入式脑刺激治疗帕金森病患者且结局指标包括执行功能的随机对照试验,检索的时间范围为建库至2023年12月。在检索电子数据库的同时,手工检索所收录文章的参考文献。检索词采用主题词与自由词结合,中文检索词包括帕金森病、经颅电刺激、经颅直流电刺激、经颅交流电刺激、经颅磁刺激,英文检索词包括Parkinson disease、Parkinson’s disease、transcranial alternating current stimulation、transcranial direct current stimulation、transcranial electrical stimulation、transcranial magnetic stimulation。中文检索式(以万方数据为例)为主题: (“帕金森病”) AND题名或关键词: (经颅直流电刺激OR经颅磁刺激OR经颅交流电刺激OR经颅电刺激),英文检索式(以PubMed为例)为(Parkinson disease[MeSH] OR Parkinson’s disease) AND (transcranial alternating current stimulation [Title/Abstract] OR transcranial direct current stimulation [Title/Abstract] OR transcranial electrical stimulation [Title/Abstract] OR transcranial magnetic stimulation [Title/Abstract])。
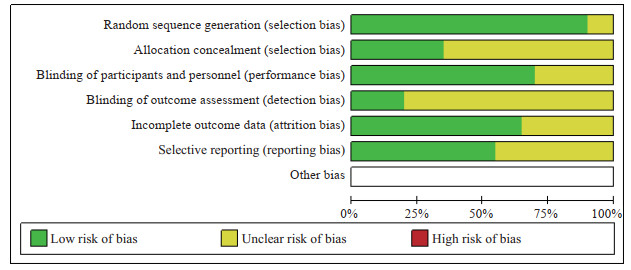
1.3 文献筛选、数据提取和质量评价由2名研究者根据预先确定的纳入和排除标准独立进行文献筛选和数据提取。提取每项研究的样本量、分组、干预情况、执行功能量表评分。使用Cochrane 5.4.0版推荐的偏倚风险评估工具对所有纳入研究的偏倚风险进行评估,每种偏倚分为低风险、不明确或高风险3个等级。2名研究者若遇到分歧,通过讨论解决;若讨论后分歧仍然存在,则咨询第3名研究者,然后做出最终决定。
1.4 统计学处理采用R 4.2.0软件进行统计分析。构建网状关系图对不同干预措施之间的关系进行可视化。NMA采用贝叶斯马尔科夫链-蒙特卡罗算法(Markov chain Monte Carlo,MCMC),建立4条MCMC链,进行20 000次自适应迭代和50 000次仿真迭代。使用Gelman-Rubin方法诊断模型的收敛程度。纳入研究的总体异质性采用χ2检验进行分析,若I2>50%则认为异质性较大。使用节点分割法对闭环研究进行不一致性检验。考虑到纳入研究中执行功能评分存在广泛差异性,使用标准化均数差(standardized mean difference,SMD)汇总结果,并提供95%贝叶斯可信区间(Bayesian credibility interval,CrI)。计算每种干预措施疗效的排序概率,通过累积排序曲线下面积(surface under the cumulative ranking curve,SUCRA)来衡量综合优选排序和不确定性。采用逐一剔除法进行敏感性分析。使用漏斗图评估发表偏倚。检验水准(α)为0.05。
2 结果 2.1 文献筛选结果及基本信息文献筛选流程和结果见图 1。共纳入20项随机对照试验[11-30],包括809例帕金森病患者。各研究的基本信息见表 1,偏倚风险评估见图 2。

|
图 1 文献筛选流程图 Fig 1 Flow diagram of study selection RCT: Randomized controlled trial. |
|
|
表 1 纳入研究的基本信息 Tab 1 Characteristics of included studies |
|
图 2 纳入研究的偏倚风险评估 Fig 2 Bias risk assessment of included studies |
2.2 纳入研究中不同干预措施的网状关系和一致性分析
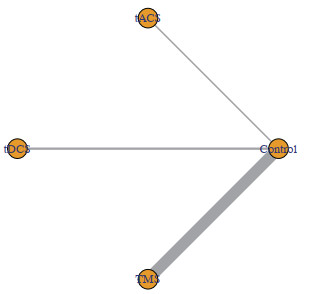
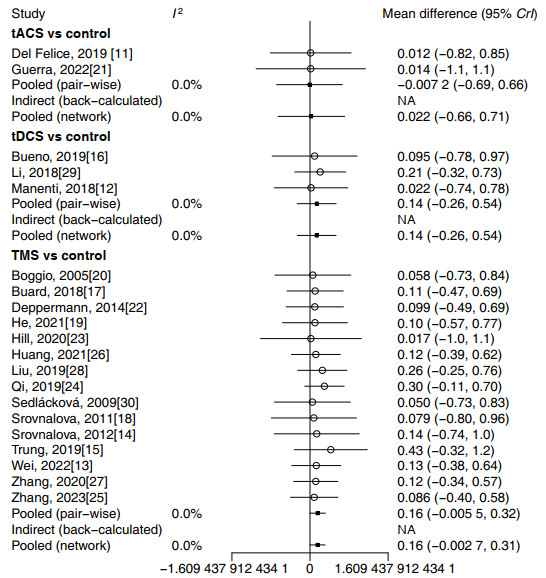
纳入的809例帕金森病患者中,412例接受积极非侵入式脑刺激治疗,不同干预措施的网状关系见图 3,直接比较和间接比较的异质性森林图见图 4。
|
图 3 纳入研究中不同干预措施的网状关系图 Fig 3 Network diagram of different interventions in included studies Lines represent treatments with direct comparisons. The thickness of edges represents the number of studies underlying each comparison. tACS: Transcranial alternating current stimulation; tDCS: Transcranial direct current stimulation; TMS: Transcranial magnetic stimulation. |
|
图 4 纳入研究中干预措施的直接和间接比较异质性森林图 Fig 4 Forest plots of heterogeneity of interventions compared directly and indirectly in included studies tACS: Transcranial alternating current stimulation; tDCS: Transcranial direct current stimulation; TMS: Transcranial magnetic stimulation; CrI: Bayesian credibility interval; NA: Not available. |
2.3 不同非侵入式脑刺激方式对帕金森病患者执行功能的疗效比较
NMA结果(表 2)显示,TMS与对照组相比显示出显著的益处(SMD=0.16,95% CrI 0.01~0.32),而tACS和tDCS与对照组相比并未显示疗效的改善(95% CrI均包含0,即无统计学意义)。在3种脑刺激方式的两两比较中,并未发现明显优于其他干预方法的干预措施。
|
|
表 2 不同干预措施对帕金森病执行功能影响的网状meta分析结果 Tab 2 Network meta-analysis of effects of different interventions on executive functions in patients with Parkinson's disease |
根据SUCRA结果,TMS最有可能是3种干预措施中治疗帕金森病患者执行功能障碍疗效最佳的干预措施(SUCRA=0.72,排序最佳的概率为37%),概率排序为TMS>tDCS>tACS>对照组(表 3)。
|
|
表 3 不同干预措施对帕金森病执行功能影响的概率排序结果 Tab 3 Probability ranking results of effectiveness of different interventions on executive functions in patients with Parkinson's disease |
2.4 发表偏倚检验和敏感性分析
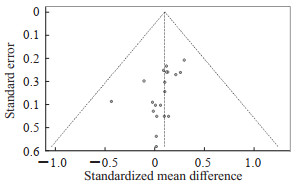
漏斗图呈现明显的不对称结构(图 5)。采用线性回归模型(Egger’s检验)检验漏斗图的对称性,结果显示有统计学意义(Egger’s=3.22,P<0.05),提示可能存在发表偏倚。敏感性分析结果显示,剔除个别研究后结论无明显方向性改变(表 4)。
|
图 5 纳入研究的发表偏倚漏斗图 Fig 5 Funnel plot of publication bias of included studies |
|
|
表 4 纳入研究的敏感性分析 Tab 4 Sensitivity analysis of included studies |
3 讨论
认知缺陷在帕金森病患者中很常见,多巴胺耗竭导致前额纹状体回路中断,进而导致认知能力下降[31-33]。帕金森病可能影响每个认知领域,包括记忆、语言、注意力、视觉空间和视觉建构能力及执行功能[31, 34]。既往文献中报道的一部分更广泛的认知缺陷实际上是潜在的执行功能障碍的一种表现[4, 34-35]。在帕金森病中观察到皮质-纹状体兴奋性和抑制性传递的改变[36-37],依赖前额叶、运动皮质和纹状体之间联系的执行功能受损可能是多巴胺缺乏的结果[38-39]。
非侵入式脑刺激技术可能通过调节潜在皮质组织的兴奋性促使与帕金森病相关的异常神经生理学正常化。目前临床上对非侵入式脑刺激的选择往往根据临床经验,缺乏循证医学证据支持。本研究采用NMA方法评价3种非侵入式脑刺激方式对帕金森病执行功能恢复效果的优劣。
本研究结果发现,TMS与对照组相比明显改善了帕金森病患者的执行功能,而tDCS、tACS对执行功能的改善无统计学意义。在最佳概率排序结果中,TMS可能疗效最优。但TMS对帕金森病患者执行功能的影响仍存在争议,如本研究纳入的2项研究中TMS对帕金森病患者的执行功能似乎没有改善[13-14],未来需要对这一治疗技术的相关参数和机制进行更详细的探索。本研究纳入的RCT中有2项关于tACS的研究检测了帕金森病患者的执行功能[11, 21],有3项关于tDCS的研究检测了帕金森病患者的执行功能[12, 16, 29],结果均显示帕金森病患者的执行功能有所改善,但受到纳入研究的数量限制,在NMA结果中tACS和tDCS对帕金森病执行功能的改善并无统计学意义。因此,未来还需要开展更多的对照试验,进一步探索tACS和tDCS是否有助于缓解帕金森病患者的执行功能障碍。
本研究的局限性在于可纳入分析的文献相对较少,并且部分纳入的研究显示出不明确的偏倚风险,各研究的刺激方案和参数也存在较大差异,难以对干预措施进行更具体或更详细的分析。我们考虑过将每个研究、每种干预策略进行分析,但这将大大降低NMA的统计能力。此外,本研究没有使用特定的量表来评估执行功能的有效性。
综上所述,根据当前有限的证据,TMS对帕金森病患者执行功能的干预效果可能优于tACS和tDCS。但受到纳入研究的局限性限制,该结论尚需进一步验证。
| [1] |
JANKOVIC J. Parkinson's disease: clinical features and diagnosis[J]. J Neurol Neurosurg Psychiatry, 2008, 79(4): 368-376. DOI:10.1136/jnnp.2007.131045 |
| [2] |
BIUNDO R, FIORENZATO E, ANTONINI A. Nonmotor symptoms and natural history of Parkinson's disease: evidence from cognitive dysfunction and role of noninvasive interventions[J]. Int Rev Neurobiol, 2017, 133: 389-415. DOI:10.1016/bs.irn.2017.05.031 |
| [3] |
GOLDMAN J G, SIEG E. Cognitive impairment and dementia in parkinson disease[J]. Clin Geriatr Med, 2020, 36(2): 365-377. DOI:10.1016/j.cger.2020.01.001 |
| [4] |
MUSLIMOVIĆ D, SCHMAND B, SPEELMAN J D, et al. Course of cognitive decline in Parkinson's disease: a meta-analysis[J]. J Int Neuropsychol Soc, 2007, 13(6): 920-932. DOI:10.1017/S1355617707071160 |
| [5] |
SAMMER G, REUTER I, HULLMANN K, et al. Training of executive functions in Parkinson's disease[J]. J Neurol Sci, 2006, 248(1/2): 115-119. DOI:10.1016/j.jns.2006.05.028 |
| [6] |
BIKSON M, INOUE M, AKIYAMA H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro[J]. J Physiol, 2004, 557(Pt 1): 175-190. DOI:10.1113/jphysiol.2003.055772 |
| [7] |
CREUTZFELDT O D, FROMM G H, KAPP H. Influence of transcortical d-c currents on cortical neuronal activity[J]. Exp Neurol, 1962, 5: 436-452. DOI:10.1016/0014-4886(62)90056-0 |
| [8] |
ESSER S K, HUBER R, MASSIMINI M, et al. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study[J]. Brain Res Bull, 2006, 69(1): 86-94. DOI:10.1016/j.brainresbull.2005.11.003 |
| [9] |
LIEBETANZ D, NITSCHE M A, TERGAU F, et al. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability[J]. Brain, 2002, 125(Pt 10): 2238-2247. DOI:10.1093/brain/awf238 |
| [10] |
MONTE-SILVA K, KUO M F, HESSENTHALER S, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation[J]. Brain Stimul, 2013, 6(3): 424-432. DOI:10.1016/j.brs.2012.04.011 |
| [11] |
DEL FELICE A, CASTIGLIA L, FORMAGGIO E, et al. Personalized transcranial alternating current stimulation (tACS) and physical therapy to treat motor and cognitive symptoms in Parkinson's disease: a randomized cross-over trial[J]. Neuroimage Clin, 2019, 22: 101768. DOI:10.1016/j.nicl.2019.101768 |
| [12] |
MANENTI R, COTELLI M S, COBELLI C, et al. transcranial direct current stimulation combined with cognitive training for the treatment of Parkinson disease: a randomized, placebo-controlled study[J]. Brain Stimul, 2018, 11(6): 1251-1262. DOI:10.1016/j.brs.2018.07.046 |
| [13] |
WEI W, YI X, WU Z, et al. Acute improvement in the attention network with repetitive transcranial magnetic stimulation in Parkinson's disease[J]. Disabil Rehabil, 2022, 44(25): 7958-7966. DOI:10.1080/09638288.2021.2004245 |
| [14] |
SROVNALOVA H, MARECEK R, KUBIKOVA R, et al. The role of the right dorsolateral prefrontal cortex in the Tower of London task performance: repetitive transcranial magnetic stimulation study in patients with Parkinson's disease[J]. Exp Brain Res, 2012, 223(2): 251-257. DOI:10.1007/s00221-012-3255-9 |
| [15] |
TRUNG J, HANGANU A, JOBERT S, et al. Transcranial magnetic stimulation improves cognition over time in Parkinson's disease[J]. Parkinsonism Relat Disord, 2019, 66: 3-8. DOI:10.1016/j.parkreldis.2019.07.006 |
| [16] |
BUENO M E B, DO NASCIMENTO NETO L I, TERRA M B, et al. Effectiveness of acute transcranial direct current stimulation on non-motor and motor symptoms in Parkinson's disease[J]. Neurosci Lett, 2019, 696: 46-51. DOI:10.1016/j.neulet.2018.12.017 |
| [17] |
BUARD I, SCIACCA D M, MARTIN C S, et al. Transcranial magnetic stimulation does not improve mild cognitive impairment in Parkinson's disease[J]. Mov Disord, 2018, 33(3): 489-491. DOI:10.1002/mds.27246 |
| [18] |
SROVNALOVA H, MARECEK R, REKTOROVA I. The role of the inferior frontal gyri in cognitive processing of patients with Parkinson's disease: a pilot rTMS study[J]. Mov Disord, 2011, 26(8): 1545-1548. DOI:10.1002/mds.23663 |
| [19] |
HE W, WANG J C, TSAI P Y. Theta burst magnetic stimulation improves parkinson's-related cognitive impairment: a randomised controlled study[J]. Neurorehabil Neural Repair, 2021, 35(11): 986-995. DOI:10.1177/1545968321104131 |
| [20] |
BOGGIO P S, FREGNI F, BERMPOHL F, et al. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson's disease and concurrent depression[J]. Mov Disord, 2005, 20(9): 1178-1184. DOI:10.1002/mds.20508 |
| [21] |
GUERRA A, COLELLA D, GIANGROSSO M, et al. Driving motor cortex oscillations modulates bradykinesia in Parkinson's disease[J]. Brain, 2022, 145(1): 224-236. DOI:10.1093/brain/awab257 |
| [22] |
DEPPERMANN S, VENNEWALD N, DIEMER J, et al. Does rTMS alter neurocognitive functioning in patients with panic disorder/agoraphobia? An fNIRS-based investigation of prefrontal activation during a cognitive task and its modulation via sham-controlled rTMS[J]. Biomed Res Int, 2014, 2014: 542526. DOI:10.1155/2014/542526 |
| [23] |
HILL A T, MCMODIE S, FUNG W, et al. Impact of prefrontal intermittent theta-burst stimulation on working memory and executive function in Parkinson's disease: a double-blind sham-controlled pilot study[J]. Brain Res, 2020, 1726: 146506. DOI:10.1016/j.brainres.2019.146506 |
| [24] |
齐美红. 低频重复经颅磁刺激对帕金森病患者运动及执行功能的影响[J]. 中国实用医刊, 2019, 46(18): 92-94. DOI:10.3760/cma.j.issn.1674-4756.2019.18.029 |
| [25] |
张黎明, 高磊, 薛翠萍, 等. 虚拟现实技术联合重复经颅磁刺激治疗帕金森病轻度认知障碍的临床疗效观察[J]. 中国康复, 2023, 38(3): 148-152. DOI:10.3870/zgkf.2023.03.005 |
| [26] |
黄丹霞, 吴志生, 庄一虹. 重复经颅磁刺激+康复训练在帕金森病患者中的应用效果及认知功能评分影响分析[J]. 中外医疗, 2021, 40(32): 39-42. DOI:10.16662/j.cnki.1674-0742.2021.32.039 |
| [27] |
张丽, 李富慧, 许鹏飞. 重复经颅磁刺激联合康复训练治疗帕金森病患者的效果[J]. 中国民康医学, 2020, 32(21): 57-59. DOI:10.3969/j.issn.1672-0369.2020.21.024 |
| [28] |
刘锦仪, 陈秀娟, 邱素丽, 等. 低频重复经颅磁刺激对早期帕金森病患者认知功能及生活质量的影响[J]. 内科, 2019, 14(4): 477-479. DOI:10.16121/j.cnki.cn45-1347/r.2019.04.28 |
| [29] |
李学, 张俊红, 祁亚伟, 等. 经颅直流电刺激对早期未治疗帕金森病患者认知功能及听觉事件相关电位的影响[J]. 中华物理医学与康复杂志, 2018, 40(3): 198-201. DOI:10.3760/cma.j.issn.0254-1424.2018.03.009 |
| [30] |
SEDLÁCKOVÁ S, REKTOROVÁ I, SROVNALOVÁ H, et al. Effect of high frequency repetitive transcranial magnetic stimulation on reaction time, clinical features and cognitive functions in patients with Parkinson's disease[J]. J Neural Transm, 2009, 116(9): 1093-1101. DOI:10.1007/s00702-009-0259-0 |
| [31] |
ZGALJARDIC D J, BOROD J C, FOLDI N S, et al. A review of the cognitive and behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry[J]. Cogn Behav Neurol, 2003, 16(4): 193-210. DOI:10.1097/00146965-200312000-00001 |
| [32] |
PERIÑÁN M T, MACÍAS-GARCÍA D, JESÚS S, et al. Homocysteine levels, genetic background, and cognitive impairment in Parkinson's disease[J]. J Neurol, 2023, 270(1): 477-485. DOI:10.1007/s00415-022-11361-y |
| [33] |
ROYALL D R, LAUTERBACH E C, CUMMINGS J L, et al. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association[J]. J Neuropsychiatry Clin Neurosci, 2002, 14(4): 377-405. DOI:10.1176/jnp.14.4.377 |
| [34] |
STUSS D T, ALEXANDER M P. Executive functions and the frontal lobes: a conceptual view[J]. Psychol Res, 2000, 63(3/4): 289-298. DOI:10.1007/s004269900007 |
| [35] |
BARNES J, BOUBERT L. Executive functions are impaired in patients with Parkinson's disease with visual hallucinations[J]. J Neurol Neurosurg Psychiatry, 2008, 79(2): 190-192. DOI:10.1136/jnnp.2007.116202 |
| [36] |
NIEOULLON A, KERKERIAN-LE GOFF L. Cellular interactions in the striatum involving neuronal systems using "classical" neurotransmitters: possible functional implications[J]. Mov Disord, 1992, 7(4): 311-325. DOI:10.1002/mds.870070404 |
| [37] |
ROBERTSON R G, CLARKE C A, BOYCE S, et al. The role of striatopallidal neurones utilizing gamma-aminobutyric acid in the pathophysiology of MPTP-induced Parkinsonism in the primate: evidence from[3H]flunitrazepam autoradiography[J]. Brain Res, 1990, 531(1/2): 95-104. DOI:10.1016/0006-8993(90)90762-z |
| [38] |
WILLIAMS-GRAY C H, EVANS J R, GORIS A, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort[J]. Brain, 2009, 132(Pt 11): 2958-2969. DOI:10.1093/brain/awp245 |
| [39] |
RAY N J, STRAFELLA A P. The neurobiology and neural circuitry of cognitive changes in Parkinson's disease revealed by functional neuroimaging[J]. Mov Disord, 2012, 27(12): 1484-1492. DOI:10.1002/mds.25173 |
 2024, Vol. 45
2024, Vol. 45


