在脑外伤、中风、缺血和阿尔茨海默病等众多中枢神经系统疾病中都有谷氨酸引起的神经兴奋性毒性损伤参与[1-4]。研究证实兴奋性毒性能使内质网功能失常,如钙离子释放和内质网应激,从而加重细胞损伤[5-6]。在对兴奋性毒性损伤的研究中,维持内质网功能一直是非常重要的研究方向。自噬是细胞维持内稳态的重要机制,异常自噬与外伤、肿瘤和神经退行性疾病等多种中枢神经系统疾病有关[7-9]。异常升高的谷氨酸能够诱导细胞凋亡和自噬[10-11],但自噬和谷氨酸兴奋性毒性损伤的关系仍需进一步厘清。
Homer1b/c是突触后结构蛋白Homer蛋白家族的一类,属于长链Homer蛋白[12-13]。长链Homer蛋白能够产生特殊的自缔合,并导致神经细胞内质网钙释放。虽然兴奋性毒性损伤的具体机制未完全明确,但研究表明细胞内钙释放是兴奋性毒性损伤的关键机制之一[14-15]。同时Homer1b/c与自噬的关系也未见研究报道。本研究探讨了Homer1b/c在谷氨酸兴奋性毒性损伤诱发细胞自噬中的作用和机制,旨在为相关神经系统疾病治疗提供新思路。
1 材料和方法 1.1 细胞与材料小鼠海马神经元HT22细胞购自武汉普诺赛生命科技有限公司。慢病毒载体制备所用的人胚肾细胞系293T细胞由上海吉凯生物基因技术有限公司提供。L-谷氨酸、钙离子螯合剂BAPTA-AM和内质网应激抑制剂4-PBA购自美国Sigma公司;FBS、DMEM购自美国Gibco公司;MTT、细胞蛋白质裂解液、PMSF购自上海碧云天生物技术股份有限公司;SDS-PAGE凝胶制备试剂盒购自上海鼎国生物技术有限公司;β-肌动蛋白、自噬效应蛋白微管相关蛋白1轻链3(microtubule-associated protein 1 light chain 3,LC3)一抗购自美国Sigma公司;Homer1b/c一抗购自美国Santa Cruz公司;自噬效应蛋白beclin-1、内质网应激标志蛋白C/EBP同源蛋白(C/EBP homologous protein,CHOP)和葡萄糖调节蛋白78(glucose regulated protein 78,GRP-78)一抗购自美国Cell Signaling Technology公司;HRP山羊抗兔IgG购自美国BioWorld公司;X-tremeGENE siRNA转染液购自德国Roche公司;乳酸脱氢酶(lactate dehydrogenase,LDH)检测试剂盒购自美国BioVision公司。
1.2 实验方法 1.2.1 细胞培养HT22细胞用含10% FBS及青霉素和链霉素双抗的DMEM于5% CO2、37 ℃恒温细胞培养箱中传代培养,隔天更换培养基,细胞密度达80%~90%时进行常规传代,细胞传代至第5~8代用于实验。实验中细胞密度为2×105/mL,MTT细胞活性检测实验和LDH细胞损伤检测实验以96孔板进行,每孔接种100 μL;蛋白质印迹法实验以6孔板进行,每孔接种2 mL。
1.2.2 细胞损伤模型建立取适量L-谷氨酸用PBS溶解,配制成浓度为10 mmol/L的溶液。HT22细胞以6孔板正常传代培养,每孔加入含L-谷氨酸的培养基20 mL,L-谷氨酸终浓度分别为10、100、500和1 000 μmol/L,处理12 h进行造模[16-17]。
1.2.3 siRNA慢病毒载体制备Homer1b/c siRNA序列为5'-GCATGCAGTTACTGTATCT-3',对照siRNA序列为5'-UUCUCCGAACGUGUCACGU-3',均由上海吉凯生物基因技术有限公司构建并扩增。(1)慢病毒载体制备:首先合成含干扰序列的单链DNA oligo,退火配对产生双链,然后通过其两端所含酶切位点直接连入酶切后的RNA慢病毒载体上;将连接产物转入制备好的细菌感受态细胞,经PCR鉴定阳性重组子后,通过基因测序进行验证,测序结果经比对确认正确的克隆即为构建成功的目的基因RNA干扰慢病毒载体。(2)滴度测定:对慢病毒载体慢病毒颗粒的重组病毒质粒及其2种辅助包装原件载体质粒分别进行高纯度无内毒素抽提。按照Lipofectamine 2000试剂(美国Invitrogen公司)说明书将质粒转染至293T细胞,转染后8 h更换为完全培养基,继续培养48 h后收集富含慢病毒颗粒的细胞上清液。对细胞上清液进行浓缩得到高滴度的慢病毒浓缩液,在293T细胞中测定并标定病毒滴度。
1.2.4 siRNA干扰慢病毒转染常规传代培养HT22细胞,转染前换液并对培养基进行定量(6孔板,1 mL/孔),然后按照1×105 TU/mL(TU为病毒的滴度单位)的滴度加入相应的慢病毒载体。病毒转染48 h后在荧光显微镜下观察转染情况,转染成功后进行细胞建模。
1.2.5 MTT法检测细胞存活率空白对照组只加入培养基,对照组与损伤组加入待建模细胞和正常培养基,损伤组细胞进行常规建模。用MTT粉末和DMSO配制MTT溶液(5 g/L),每孔各加入10 μL,在37 ℃恒温箱中孵育4 h,然后用移液枪吸去培养基,再加入DMSO后震荡溶解结晶。使用酶标仪测定光密度值(D),设置波长为570 nm。细胞存活率计算公式:细胞存活率(%)=(D损伤组-D空白对照组)/(D对照组-D空白对照组)×100%。
1.2.6 LDH法检测细胞损伤程度分别取0、2、4、6、8、10 μL NADH标准品(1.25 mmol/L)加入各组细胞96孔板中,使用检测缓冲液定量终体积至50 μL,根据实验需求在其余孔内加入50 μL细胞上清液。按照LDH检测试剂盒说明书制备反应混合液,每孔加入50 μL。完成后把96孔板置于酶标仪中,设置波长为450 nm,测定D1;然后将96孔板在37 ℃恒温箱中孵育30 min,测定D2,两者差值即D2-D1为最终D。根据标准曲线计算LDH水平以评估细胞损伤程度。
1.2.7 BAPTA-AM和4-PBA预处理细胞T22细胞用6孔板正常培养或经慢病毒转染后予BAPTA-AM和4-PBA预处理。BAPTA-AM和4-PBA用DMSO分别配成浓度为50 mg/mL和100 mg/mL的溶液,并以无血清培养基稀释,BAPTA-AM终浓度为10 μmol/L、4-PBA为10 mmol/L。吸取细胞原培养基,对照组加入2 mL新鲜无血清培养基,损伤组加入2 mL含BAPTA-AM或4-PBA的无血清培养基,空白对照组加入损伤组同比例的DMSO和培养基,预处理12 h后建立细胞损伤模型。
1.2.8 蛋白质印迹法检测蛋白表达常规6孔板培养细胞后用PBS冲洗处理,使用蛋白酶抑制剂PMSF和细胞蛋白质裂解液裂解细胞。提取细胞裂解液,加入5×上样缓冲液,煮沸并离心后取上清液。采用BCA法测定蛋白浓度。根据SDS-PAGE凝胶制备试剂盒说明书配置相应浓度的上、下层SDS-PAGE凝胶,采用上层胶电压90 V、下层胶电压120~180 V进行电泳,转膜1.5 h,再使用含5%脱脂奶粉的TBST室温封闭NC膜1 h。加入用TBST溶解的Homer1b/c、LC3、beclin-1、CHOP、GRP-78、β-肌动蛋白一抗(稀释比例分别为1∶1 500、1∶1 500、1∶1 000、1∶1 000、1∶1 000、1∶2 500)孵育后,用TBST冲洗;然后加入HRP山羊抗兔IgG孵育后,用TBST清洗去除未结合的抗体,显影曝光后采集图像信息。采用Image Pro Plus 6.0软件进行数据分析。
1.3 统计学处理应用SPSS 28.0软件进行统计学分析。所有实验至少重复3次。呈正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验,多组间比较采用单因素方差分析。检验水准(α)为0.05。
2 结果 2.1 谷氨酸兴奋性毒性损伤可诱发细胞自噬MTT法和LDH法检测结果显示,使用浓度为10、100、500和1 000 μmol/L L-谷氨酸处理HT22细胞12 h后,细胞存活率逐渐降低,细胞损伤程度逐渐升高(图 1A、1B)。蛋白质印迹法检测结果显示,与对照组比较,上述浓度的L-谷氨酸处理细胞后都能使beclin-1表达水平和LC3-Ⅱ/LC3-Ⅰ比值升高(均P<0.05,图 1C),表明谷氨酸兴奋性毒性损伤能诱发细胞自噬产生。由于500 μmol/L L-谷氨酸处理能在成功诱发自噬产生的同时,使细胞存活率保持在约80%,而使用1 000 μmol/L L-谷氨酸处理后细胞存活率下跌至约40%,因此后续实验采用500 μmol/L L-谷氨酸建模。
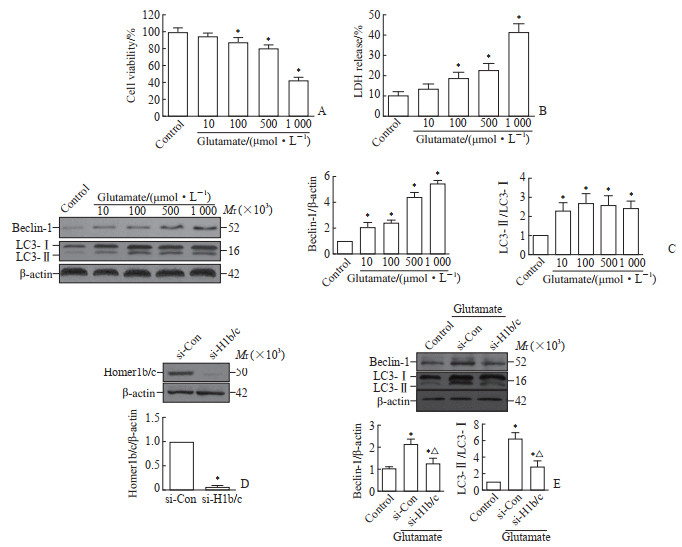
|
图 1 Homer1b/c调节谷氨酸兴奋性毒性损伤诱发的HT22细胞的自噬水平 Fig 1 Homer1b/c regulates autophagy of HT22 cells induced by glutamate excitotoxic injury A-C: After HT22 cells were treated with different concentrations of L-glutamate for 12 h, cell viability was decreased as detected by MTT assay (A), cell damage was increased as detected by LDH method (B), and beclin-1 level and LC3-Ⅱ/LC3-Ⅰ ratio were increased as detected by Western blotting (C). *P < 0.05 vs control group. n=3, x±s. D: After Homer1b/c siRNA lentivirus (si-H1b/c) and control siRNA lentivirus (si-Con) infected HT22 cells, the Homer1b/c expression was detected by Western blotting. *P < 0.05 vs si-Con group. n=3, x±s. E: After HT22 cells infected with si-H1b/c and si-Con were treated with 500 μmol/L L-glutamate, the expression of beclin-1 and LC3 was detected by Western blotting. *P < 0.05 vs control group; △P < 0.05 vs si-Con group. n=3, x±s. MTT: Methyl thiazolyl tetrazolium; LDH: Lactate dehydrogenase; LC3: Microtubule-associated protein 1 light chain 3; siRNA: Small interfering RNA. |
2.2 抑制Homer1b/c表达能够减少由谷氨酸兴奋性毒性损伤诱发的细胞自噬
蛋白质印迹法检测结果显示,与转染对照siRNA慢病毒的细胞相比,转染Homer1b/c siRNA慢病毒的HT22细胞中Homer1b/c蛋白表达水平下降(P<0.05,图 1D);在用500 μmol/L L-谷氨酸处理的细胞中,抑制Homer1b/c表达后HT22细胞中beclin-1表达水平和LC3-Ⅱ/LC3-Ⅰ比值均较转染对照组降低(均P<0.05,图 1E)。
2.3 细胞内钙离子和内质网应激在Homer1b/c调节谷氨酸兴奋性毒性损伤诱发的细胞自噬中发挥作用蛋白质印迹法检测结果显示,使用10 μmol/L钙离子螯合剂BAPTA-AM预处理降低了HT22细胞中L-谷氨酸引起的beclin-1表达和LC3-Ⅱ/LC3-Ⅰ比值变化(均P<0.05,图 2A)。与对照组相比,L-谷氨酸处理后HT22细胞中内质网应激标志蛋白CHOP和GRP-78表达增加,而经10 mmol/L的4-PBA预处理后CHOP、GRP-78、beclin-1表达和LC3-Ⅱ/LC3-Ⅰ比值均降低(均P<0.05,图 2B)。在抑制HT22细胞中Homer1b/c表达后,BAPTA-AM和4-PBA预处理未能进一步影响谷氨酸引起的beclin-1表达和LC3-Ⅱ/LC3-Ⅰ比值变化(图 2C)。结果表明下调Homer1b/c可抑制谷氨酸兴奋性毒性损伤诱发的HT22细胞自噬,在此基础上抑制钙离子释放和内质网应激并不能进一步削弱谷氨酸兴奋性毒性损伤诱发的细胞自噬。

|
图 2 细胞内钙离子和内质网应激参与Homer1b/c对谷氨酸兴奋性毒性损伤诱发的HT22细胞自噬的调控 Fig 2 Intracellular calcium ions and endoplasmic reticulum stress involved in Homer1b/c regulating autophagy of HT22 cells induced by glutamate excitotoxic injury The protein expression was detected by Western blotting. A: After HT22 cells were pretreated with calcium chelating agent BAPTA-AM (10 μmol/L) and then injured with 500 μmol/L L-glutamate for 12 h, the beclin-1 level and LC3-Ⅱ/LC3-Ⅰ ratio were decreased. *P < 0.05 vs control group; △P < 0.05 vs DMSO group. n=3, x±s. B: After HT22 cells were pretreated with endoplasmic reticulum stress inhibitor 4-PBA (10 mmol/L) and then injured with 500 μmol/L L-glutamate for 12 h, the levels of CHOP, GRP-78, beclin-1, and LC3-Ⅱ/LC3-Ⅰ ratio were decreased. *P < 0.05 vs control group; △P < 0.05 vs DMSO group. n=3, x±s. C: After HT22 cells infected with Homer1b/c siRNA lentivirus (si-H1b/c) and control siRNA lentivirus (si-Con) were pretreated with BAPTA-AM (10 μmol/L) or 4-PBA (10 mmol/L), the beclin-1 level or LC3-Ⅱ/LC3-Ⅰ ratio were not different from the si-H1b/c group. *P < 0.05 vs control group; △P < 0.05 vs si-Con group. n=3, x±s. DMSO: Dimethyl sulfoxide; BAPTA-AM: 1, 2-bis(2-aminophenoxy)ethane-N, N, N', N'-tetraacetic acid tetrakis (acetoxymethyl ester); LC3: Microtubule-associated protein 1 light chain 3; 4-PBA: 4-phenylbutyric acid; CHOP: C/EBP homologous protein; GRP-78: Glucose regulated protein 78; siRNA: Small interfering RNA. |
3 讨论
谷氨酸作为中枢神经系统一种主要的兴奋性递质,对兴奋性突触间的信号传递起到非常重要的作用[18-19]。谷氨酸过度释放造成的兴奋性毒性会引起谷氨酸受体信号传递过度活跃,还会影响神经细胞最终的死亡转归方式。研究显示,降低Homer1b/c蛋白表达水平能够减少谷氨酸引起的细胞凋亡[20-22],表明Homer1b/c是兴奋性毒性的一种重要调节因子,但是有关Homer1b/c在自噬中的作用并无相关研究。本研究使用小鼠海马神经元HT22细胞作为实验载体,用500 μmol/L L-谷氨酸培养液处理12 h进行造模,形成轻微细胞损伤模型的同时也成功诱发细胞自噬产生。进一步在该模型上观察到,抑制Homer1b/c表达能够减少由谷氨酸兴奋性毒性损伤诱发的细胞自噬,表明Homer1b/c对兴奋性毒性损伤造成的细胞自噬起重要调节作用。
自噬是指机体在受到外界因素如饥饿、感染等刺激后,细胞为了维持内稳态而进行的一个相对有益的生理过程[23]。阻断自噬产生会提高细胞对有害刺激的敏感程度,而自噬诱导剂能帮助细胞拮抗促细胞凋亡因子的刺激作用[24-25]。但有研究表明,不受控制的自噬产生也能导致细胞死亡[26-27],维持自噬在正常水平是多种神经系统疾病的研究热点。研究表明,细胞内钙稳态失衡在兴奋性毒性损伤中起关键作用,而内质网释放的钙离子对自噬有明显的调节作用[28]。本研究中,用钙离子螯合剂BAPTA-AM抑制钙离子释放能减少自噬产生,表明细胞内钙离子对于谷氨酸兴奋性毒性损伤诱发的自噬起到重要调节作用。内质网应激和钙离子释放密切相关,其能显著影响内质网功能,并使未折叠和/或异常折叠的蛋白过度积累,若蛋白酶体无法及时将之清除就会诱发自噬产生。本研究中,内质网应激抑制剂4-PBA能够减少自噬产生,表明内质网应激在谷氨酸兴奋性毒性损伤造成自噬的过程中也有着非常重要的作用。Homer蛋白相关研究表明,其为内质网释放钙离子的重要调节因子,而且降低Homer1b/c蛋白表达水平不仅能减少细胞内钙离子释放,也能抑制内质网应激的产生[29]。本研究结果表明,在抑制细胞内钙离子释放和内质网应激的前提下,下调Homer1b/c蛋白表达对于谷氨酸兴奋性毒性损伤诱发的自噬的阻断效果明显弱化,表明Homer1b/c对谷氨酸兴奋性毒性损伤诱发自噬的调节作用与其对细胞内钙离子释放和内质网应激的调节作用密切相关。
综上所述,轻度的谷氨酸兴奋性毒性损伤能够在HT22细胞中诱发自噬产生,而该效应在降低Homer1b/c表达后明显减弱,内质网功能失调在谷氨酸兴奋性毒性损伤诱导自噬产生中起到重要参与作用,且Homer1b/c对谷氨酸兴奋性毒性损伤诱发自噬产生的调节作用和内质网功能密切相关。因此,Homer1b/c极有可能是自噬产生的调节因子,且与内质网功能相关。未来或可在此基础上进一步研究Homer1b/c调节自噬的机制,以期寻找神经系统疾病的治疗新靶点。
| [1] |
CHEN T, YANG L K, AI P, et al. Edonerpic maleate regulates glutamate receptors through CRMP2- and Arc-mediated mechanisms in response to brain trauma[J]. Cell Death Discov, 2022, 8(1): 95. DOI:10.1038/s41420-022-00901-0 |
| [2] |
WANG X, PENG Y, ZHOU H, et al. The effects of enriched rehabilitation on cognitive function and serum glutamate levels post-stroke[J]. Front Neurol, 2022, 13: 829090. DOI:10.3389/fneur.2022.829090 |
| [3] |
NEVES D, SALAZAR I L, ALMEIDA R D, et al. Molecular mechanisms of ischemia and glutamate excitotoxicity[J]. Life Sci, 2023, 328: 121814. DOI:10.1016/j.lfs.2023.121814 |
| [4] |
ABD-ELRAHMAN K S, SARASIJA S, FERGUSON S S G. The role of neuroglial metabotropic glutamate receptors in Alzheimer's disease[J]. Curr Neuropharmacol, 2023, 21(2): 273-283. DOI:10.2174/1570159X19666210916102638 |
| [5] |
ZHANG W, YE F, PANG N, et al. Restoration of sarco/endoplasmic reticulum Ca2+-ATPase activity functions as a pivotal therapeutic target of anti-glutamate-induced excitotoxicity to attenuate endoplasmic reticulum Ca2+ depletion[J]. Front Pharmacol, 2022, 13: 877175. DOI:10.3389/fphar.2022.877175 |
| [6] |
NGUYEN D T, LE T M, HATTORI T, et al. The ATF6β-calreticulin axis promotes neuronal survival under endoplasmic reticulum stress and excitotoxicity[J]. Sci Rep, 2021, 11(1): 13086. DOI:10.1038/s41598-021-92529-w |
| [7] |
YAN C, LIU J, GAO J, et al. Correction: IRE1 promotes neurodegeneration through autophagy-dependent neuron death in the Drosophila model of Parkinson's disease[J]. Cell Death Dis, 2020, 11(2): 150. DOI:10.1038/s41419-020-2346-y |
| [8] |
HONG J M, MOON J H, PARK S Y. Human prion protein-mediated calcineurin activation induces neuron cell death via AMPK and autophagy pathway[J]. Int J Biochem Cell Biol, 2020, 119: 105680. DOI:10.1016/j.biocel.2019.105680 |
| [9] |
ZHANG Z, LI D, XU L, et al. Sirt1 improves functional recovery by regulating autophagy of astrocyte and neuron after brain injury[J]. Brain Res Bull, 2019, 150: 42-49. DOI:10.1016/j.brainresbull.2019.05.005 |
| [10] |
VUCICEVIC L, MISIRKIC M, CIRIC D, et al. Transcriptional block of AMPK-induced autophagy promotes glutamate excitotoxicity in nutrient-deprived SH-SY5Y neuroblastoma cells[J]. Cell Mol Life Sci, 2020, 77(17): 3383-3399. DOI:10.1007/s00018-019-03356-2 |
| [11] |
YANG T, XU Z, LIU W, et al. Oxidative stress accelerates synaptic glutamate dyshomeostasis and NMDARs disorder during methylmercury-induced neuronal apoptosis in rat cerebral cortex[J]. Environ Toxicol, 2020, 35(6): 683-696. DOI:10.1002/tox.22904 |
| [12] |
YAO Y X, ZHANG Y F, YANG Y, et al. Spinal synaptic scaffolding protein Homer 1b/c regulates CREB phosphorylation and c-fos activation induced by inflammatory pain in rats[J]. Neurosci Lett, 2014, 559: 88-93. DOI:10.1016/j.neulet.2013.11.049 |
| [13] |
BUONAGURO E F, MORLEY-FLETCHER S, AVAGLIANO C, et al. Glutamatergic postsynaptic density in early life stress programming: topographic gene expression of mGlu5 receptors and Homer proteins[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2020, 96: 109725. DOI:10.1016/j.pnpbp.2019.109725 |
| [14] |
SZYDLOWSKA K, TYMIANSKI M. Calcium, ischemia and excitotoxicity[J]. Cell Calcium, 2010, 47(2): 122-129. DOI:10.1016/j.ceca.2010.01.003 |
| [15] |
VERMA M, LIZAMA B N, CHU C T. Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration[J]. Transl Neurodegener, 2022, 11(1): 3. DOI:10.1186/s40035-021-00278-7 |
| [16] |
GAO L, WANG T, ZHUOMA D, et al. Farrerol attenuates glutamate-induced apoptosis in HT22 cells via the Nrf2/heme oxygenase-1 pathway[J]. Biosci Biotechnol Biochem, 2023, 87(9): 1009-1016. DOI:10.1093/bbb/zbad084 |
| [17] |
LI J, WANG G, ZHANG Y, et al. Protective effects of baicalin against L-glutamate-induced oxidative damage in HT-22 cells by inhibiting NLRP3 inflammasome activation via Nrf2/HO-1 signaling[J]. Iran J Basic Med Sci, 2023, 26(3): 351-358. DOI:10.22038/IJBMS.2023.64318.14149 |
| [18] |
KAYSER S, TEMPERINI P, POULIE C B M, et al. A diversity oriented synthesis approach to new 2, 3-trans-substituted l-proline analogs as potential ligands for the ionotropic glutamate receptors[J]. ACS Chem Neurosci, 2020, 11(5): 702-714. DOI:10.1021/acschemneuro.0c00005 |
| [19] |
FOSSATI M, ASSENDORP N, GEMIN O, et al. Trans-synaptic signaling through the glutamate receptor delta-1 mediates inhibitory synapse formation in cortical pyramidal neurons[J]. Neuron, 2019, 104(6): 1081-1094.e7. DOI:10.1016/j.neuron.2019.09.027 |
| [20] |
GIMSE K, GORZEK R C, OLIN A, et al. Hippocampal Homer1b/c is necessary for contextual fear conditioning and group Ⅰ metabotropic glutamate receptor mediated long-term depression[J]. Neurobiol Learn Mem, 2018, 156: 17-23. DOI:10.1016/j.nlm.2018.10.005 |
| [21] |
LV M M, CHENG Y C, XIAO Z B, et al. Down-regulation of Homer1b/c attenuates group Ⅰ metabotropic glutamate receptors dependent Ca2+ signaling through regulating endoplasmic reticulum Ca2+ release in PC12 cells[J]. Biochem Biophys Res Commun, 2014, 450(4): 1568-1574. DOI:10.1016/j.bbrc.2014.07.044 |
| [22] |
WU X Q, SU N, FEI Z, et al. Homer signaling pathways as effective therapeutic targets for ischemic and traumatic brain injuries and retinal lesions[J]. Neural Regen Res, 2022, 17(7): 1454-1461. DOI:10.4103/1673-5374.330588 |
| [23] |
LOEFFLER D A. Influence of normal aging on brain autophagy: a complex scenario[J]. Front Aging Neurosci, 2019, 11: 49. DOI:10.3389/fnagi.2019.00049 |
| [24] |
LUPITHA S S, CHANDRASEKHAR L, VARADARAJAN S N, et al. A reporter cell line for real-time imaging of autophagy and apoptosis[J]. Toxicol Lett, 2020, 326: 23-30. DOI:10.1016/j.toxlet.2020.02.011 |
| [25] |
GAO P, HAO F, DONG X, et al. The role of autophagy and beclin-1 in radiotherapy-induced apoptosis in thyroid carcinoma cells[J]. Int J Clin Exp Pathol, 2019, 12(3): 885-892. |
| [26] |
YAN X, ZHOU R, MA Z. Autophagy-cell survival and death[J]. Adv Exp Med Biol, 2019, 1206: 667-696. DOI:10.1007/978-981-15-0602-4_29 |
| [27] |
LINDER B, KÖGEL D. Autophagy in cancer cell death[J]. Biology, 2019, 8(4): 82. DOI:10.3390/biology8040082 |
| [28] |
SMAILI S S, PEREIRA G J S, COSTA M M, et al. The role of calcium stores in apoptosis and autophagy[J]. Curr Mol Med, 2013, 13(2): 252-265. DOI:10.2174/156652413804810772 |
| [29] |
BEQOLLARI D, KAMMERMEIER P J. The interaction between mGluR1 and the calcium channel CaV2.1 preserves coupling in the presence of long Homer proteins[J]. Neuropharmacology, 2013, 66: 302-310. DOI:10.1016/j.neuropharm.2012.05.038 |
 2024, Vol. 45
2024, Vol. 45


