引用本文

王丽珍, 李军, 李思源, 向清淋, 任艳霞, 王娅宁. 绝经后2型糖尿病患者护骨因子基因rs4355801、rs6993813位点多态性及突变与骨代谢的关系[J]. 海军军医大学学报, 2024, 45(2): 189-197

WANG Lizhen, LI Jun, LI Siyuan, XIANG Qinglin, REN Yanxia, WANG Yaning. Correlations of polymorphisms and mutations of osteoprotegerin gene rs4355801 and rs6993813 loci with bone metabolism in postmenopausal women with type 2 diabetes mellitus[J]. Academic Journal of Naval Medical University, 2024, 45(2): 189-197 (in Chinese with English abstract)

绝经后2型糖尿病患者护骨因子基因rs4355801、rs6993813位点多态性及突变与骨代谢的关系
王丽珍
1
, 李军
1

, 李思源
2, 向清淋
3, 任艳霞
1, 王娅宁
1
1. 石河子大学第一附属医院内分泌代谢科, 石河子 832000;
2. 石河子大学医学院组织学与胚胎学教研室, 石河子 832000;
3. 达州市中心医院重症医学科, 达州 635000
收稿日期: 2022-08-22 接受日期: 2023-03-06
基金项目: 2018年度兵团科技发展专项(2018BB040),石河子大学成果转化与技术推广项目(CGZH201911).
通信作者(Corresponding author):
李军, Tel: 0993-2850629, E-mail: xjlijun@163.com.
摘要: 目的 探讨绝经后2型糖尿病患者护骨因子(OPG)基因rs4355801、rs6993813位点多态性及突变与骨代谢的关系。方法 选取2020年10月至2021年10月就诊于石河子大学第一附属医院的绝经后女性200例,根据病情分为糖耐量及骨量正常组(A组,52例)、糖耐量正常但骨量异常组(B组,43例)、骨量正常的2型糖尿病组(C组,47例)、2型糖尿病合并骨量异常组(D组,58例)。收集患者年龄、身高、体重、绝经年限等基线资料,计算BMI、腰臀比。用罗氏全自动生化分析仪测定甘油三酯(TG)、高密度脂蛋白胆固醇(HDL-C)、低密度脂蛋白胆固醇(LDL-C)、血钙、血磷、碱性磷酸酶(ALP)、空腹血糖(FPG)等生物化学指标,HPLC测量糖化血红蛋白(HbA1c),双能X线测量L1~4椎体及股骨颈骨密度,飞行时间质谱测定OPG基因rs4355801、rs6993813位点多态性及基因型分型。采用多元线性回归分析筛选骨密度的影响因素。使用SHEsis软件进行单核苷酸多态性位点的连锁不平衡分析及单体型分析。结果 4组间年龄、BMI、腰臀比存在差异(P<0.05)。与A组、B组相比,C组、D组的FPG、HbA1c水平均升高(均P<0.05);与B组相比,C组HDL-C水平升高、ALP水平降低(均P<0.05);与C组相比,D组ALP水平升高(P<0.05);与A组、C组相比,B组、D组的L1~4椎体骨密度及股骨颈骨密度水平均降低(均P<0.05)。OPG基因rs4355801、rs6993813位点均符合Hardy-Weinberg平衡。rs4355801位点基因型及等位基因频率分布组间差异均无统计学意义(均P>0.05);与A组相比,C组、D组rs6993813位点基因型分布均存在差异(均P<0.05),而等位基因频率分布在各组间差异均无统计学意义(均P>0.05)。C组rs4355801位点突变型患者FPG、HbA1c水平均低于野生型患者(均P<0.05),D组rs4355801位点突变型患者L1~4椎体骨密度水平高于野生型患者(P<0.05),D组rs6993813位点突变型患者血磷水平低于野生型患者、股骨颈骨密度水平高于野生型患者(均P<0.05)。多元线性回归分析显示,绝经年限增加及BMI、TG、LDL-C、HDL-C降低是绝经后女性L1~4椎体骨密度降低的危险因素,绝经年限增加、HDL-C降低、血磷降低是股骨颈骨密度降低的危险因素;rs4355801位点AG基因型是绝经后女性L1~4椎体骨密度、股骨颈骨密度增加的保护因素(均P<0.05)。rs4355801、rs6993813位点野生型与突变型绝经后女性骨密度的差异均无统计学意义(均P>0.05)。OPG基因rs4355801、rs6993813位点之间存在明显连锁不平衡关系(D’>0.9,r2>0.3);携带GT单体型的绝经后女性骨量异常风险增高(P<0.05),携带AT单体型的绝经后女性骨量异常风险降低(P<0.05)。OPG基因rs4355801、rs6993813位点的交互作用未对绝经后女性骨密度产生影响(均P>0.05)。结论 rs4355801位点突变可能参与了绝经后女性的骨代谢、糖代谢,rs6993813位点突变及多态性参与了绝经后女性的骨代谢。OPG基因rs4355801、rs6993813位点的明显连锁关系可能影响绝经后女性的骨密度。
关键词:
绝经后女性 2型糖尿病 护骨因子 骨质疏松症 基因多态性 基因突变
Correlations of polymorphisms and mutations of osteoprotegerin gene rs4355801 and rs6993813 loci with bone metabolism in postmenopausal women with type 2 diabetes mellitus
WANG Lizhen
1
, LI Jun
1

, LI Siyuan
2, XIANG Qinglin
3, REN Yanxia
1, WANG Yaning
1
1. Department of Endocrinology and Metabolism, The First Affiliated Hospital of Shihezi University, Shihezi 832000, Xinjiang Uygur Autonomous Region, China;
2. Department of Histology and Embryology, Shihezi University School of Medicine, Shihezi 832000, Xinjiang Uygur Autonomous Region, China;
3. Department of Critical Care Medicine, Dazhou Central Hospital, Dazhou 635000, Sichuan, China
Supported by 2018 Corps Science and Technology Development Project (2018BB040) and Scientific Achievement Transformation and Technology Promotion Project of Shihezi University (CGZH201911).
Abstract: Objective To investigate the correlations of polymorphisms and mutations of osteoprotegerin (OPG) gene rs4355801 and rs6993813 loci with bone metabolism in postmenopausal women with type 2 diabetes mellitus (T2DM). Methods From Oct. 2020 to Oct. 2021, 200 postmenopausal women who visited The First Affiliated Hospital of Shihezi University were enrolled and divided into normal glucose tolerance and bone mass group (group A, n=52), normal glucose tolerance but abnormal bone mass group (group B, n=43), T2DM with normal bone mass group (group C, n=47), and T2DM with abnormal bone mass group (group D, n=58). The baseline data such as age, height, weight, and years since menopause (YSM) were collected, and body mass index (BMI) and waist-hip ratio (WHR) were calculated. Biochemical indicators such as triglyceride (TG), high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), blood calcium, blood phosphorus, alkaline phosphatase (ALP), and fasting blood glucose (FBG) were measured by Roche automatic biochemical analyzer. Glycosylated hemoglobin (HbA1c) was measured by high-performance liquid chromatography. Bone mineral densities (BMDs) of the lumbar spine (L1-4) and neck of femur (NOF) were measured by dual energy X-rays. Polymorphism and genotyping of OPG gene rs4355801 and rs6993813 loci were determined by time-of-flight mass spectrometry. Multiple linear regression analysis was used to analyze the influencing factors of BMD. Linkage disequilibrium analysis and haplotype analysis of single nucleotide polymorphism loci were performed using SHEsis software. Results There were significant differences in age, BMI, and WHR among the 4 groups (P < 0.05). Compared with groups A and B, the levels of FPG and HbA1c in groups C and D were significantly increased (all P < 0.05). Compared with group B, the level of HDL-C in group C was significantly increased, while the level of ALP was significantly decreased (P < 0.05). Compared with group C, the level of ALP in group D was significantly increased (P < 0.05). The BMD (L1-4) and BMD (NOF) in groups B and D were significantly decreased compared with groups A and C (all P < 0.05). The OPG gene rs4355801 and rs6993813 loci were in line with Hardy-Weinberg equilibrium. There were no significant differences between groups in rs4355801 genotype or allele frequency distribution (all P > 0.05). Compared with group A, there was a difference in rs6993813 genotype distribution in groups C and D (both P < 0.05), and there was no significant difference in allele frequency distribution among groups (all P > 0.05). The FPG and HbA1c levels of the patients with rs4355801 mutant type were both significantly lower than those with the wild type in group C (both P < 0.05). In group D, the BMD (L1-4) of rs4355801 mutant type was significantly higher than that with the wild type (P < 0.05). The patients with rs6993813 mutant type in group D had significantly lower blood phosphorus levels and higher BMD (NOF) compared to the wild type (both P < 0.05). Multiple linear regression analysis showed that the increase of YSM and the decreases of BMI, TG, LDL-C, and HDL-C were the risk factors for the decrease of BMD (L1-4); the increase of YSM and the decreases of HDL-C and blood phosphorus were the risk factors for the decrease of BMD (NOF); and rs4355801 AG genotype was a protective factor for the increases of BMD (L1-4) and BMD (NOF) in postmenopausal women (both P < 0.05). There was no significant difference in BMD between wild-type and mutant rs4355801 or rs6993813 loci in postmenopausal women (both P > 0.05). There was a significant linkage disequilibrium relationship between the OPG gene rs4355801 and rs6993813 loci (D' > 0.9, r2 > 0.3), and the risk of abnormal bone mass in postmenopausal women with GT haplotype was significantly higher (P < 0.05) and the risk of abnormal bone mass in postmenopausal women with AT haplotype was significantly lower (P < 0.05). The interactions of the OPG gene rs4355801 and rs6993813 loci had no effect on BMD in postmenopausal women (both P > 0.05). Conclusion OPG gene rs4355801 mutation may be involved in bone metabolism and glucose metabolism of postmenopausal women, and rs6993813 mutation and polymorphism are involved in bone metabolism of postmenopausal women. The significant linkage relationship of the OPG gene rs4355801 and rs6993813 loci may affect the BMD of postmenopausal women.
Key words:
postmenopausal women type 2 diabetes mellitus osteoprotegerin osteoporosis gene polymorphism gene mutation
随着我国社会老龄化加剧,骨质疏松导致的骨折发生率逐年递增[1]。绝经后骨质疏松是绝经后女性的一种常见多基因疾病[2],雌激素水平改变、氧化应激、肠道菌群失调、铁过载等在绝经后骨质疏松的发生和发展中起重要作用[3]。2型糖尿病是一种由多种病因所致胰岛素分泌不足或抵抗的慢性代谢性疾病,遗传因素在2型糖尿病的发生、发展中起关键作用[4]。护骨因子(osteoprotegerin,OPG)属于肿瘤坏死因子超家族,人类OPG基因位于染色体8q24[5]。目前研究已证实OPG可通过OPG-核因子κ B受体活化因子配体(receptor activator of nuclear factor κ B ligand,RANKL)-核因子κ B受体活化因子(receptor activator of nuclearfactor κ B,RANK)信号通路调控骨代谢[6-8],与糖代谢也有关[9]。目前关于绝经后2型糖尿病合并骨质疏松与OPG基因多态性及突变的研究鲜见报道。本研究探讨绝经后2型糖尿病患者OPG基因2个单核苷酸多态性(single nucleotide polymorphism,SNP)位点rs4355801、rs6993813及其突变与骨代谢的关系,研究SNP位点的连锁不平衡及交互作用对骨密度的影响。
1 材料和方法
1.1 研究对象
选择2020年10月-2021年10月在石河子大学第一附属医院就诊的200例自然绝经后汉族女性为研究对象。纳入标准:(1)具备完全民事行为能力;(2)自然绝经后女性;(3)于石河子大学第一附属医院行生物化学指标、口服葡萄糖耐量试验、双能X射线骨密度仪等检查。排除标准:(1)1型糖尿病、特殊类型糖尿病、妊娠期糖尿病;(2)合并影响钙和维生素D吸收与调节的疾病;(3)有使用影响骨调节的药物史;(4)合并严重肝肾功能不全、心功能不全、恶性肿瘤;(5)先天性骨质疏松。2型糖尿病的诊断参照1999年WHO推荐标准;骨密度的判断参照1994年WHO的推荐标准:T值≥-1.0标准差(standard deviation,SD)为骨量正常、>-2.5SD且<-1.0SD为骨量降低、≤-2.5SD为骨质疏松。本研究经石河子大学第一附属医院医学伦理委员会批准,所有研究对象均签署知情同意书。
1.2 研究分组
200例研究对象中52例纳入糖耐量及骨量正常组(A组)、43例纳入糖耐量正常但骨量异常组(B组)、47例纳入2型糖尿病骨量正常组(C组)、58例纳入2型糖尿病合并骨量异常组(D组)。
1.3 观察指标
收集研究对象的年龄、身高、体重、绝经年限等基线资料,计算BMI、腰臀比。空腹10~12 h,次日晨抽取肘部静脉血3~5 mL用于临床生物化学指标检验。使用罗氏全自动生化分析仪(型号Modular DPP-H7600)检测甘油三酯(triglyceride,TG)、高密度脂蛋白胆固醇(highdensity lipoprotein-cholesterol,HDL-C)、低密度脂蛋白胆固醇(low density lipoprotein-cholesterol,LDL-C)、血钙、血磷、碱性磷酸酶(alkaline phosphatase,ALP)、空腹血糖(fasting blood glucose,FBG)。采用HPLC测量糖化血红蛋白(glycosylated haemoglobin A1c,HbA1c)。使用双能X射线骨密度仪测量L1~4椎体及股骨颈骨密度。采用飞行时间质谱(time-of-flight mass spectrometry,TOF-MS)测定OPG基因rs4355801、rs6993813位点的基因多态性及基因型分型。
1.4 统计学处理
应用SPSS 25.0软件进行统计学分析。符合正态分布的计量资料以x±s表示,组间比较采用协方差分析或t检验;不符合正态分布的计量资料以中位数(下四分位数,上四分位数)表示,组间比较采用Kruskal-Wallis检验。计数资料以例数和百分数表示,组间比较采用χ2检验或Fisher确切概率法。采用多元线性回归分析筛选骨密度的影响因素。SNP位点的连锁不平衡分析及单体型分析使用SHEsis软件,Hardy-Weinberg平衡的检验采用χ2检验。应用PASS 2021软件行样本量估计。检验水准(α)为0.05。
2 结果
2.1 基线资料及临床生物化学指标、骨密度的比较
由表 1可见,4组绝经后女性的年龄、BMI、腰臀比存在差异(P<0.05)。
表 1
(Tab 1)
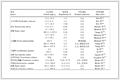
表 1 4组绝经后女性的基线资料比较
Tab 1 Comparison of baseline data among 4 groups of postmenopausal women
| M (QL, QU) |
| Variable |
Group A n=52 |
Group B n=43 |
Group C n=47 |
Group D n=58 |
| Age/year |
59.50 (48.25, 66.00) |
64.00 (56.00, 72.00) |
54.00 (49.00, 65.00) |
71.50 (65.75, 75.25)*△▲ |
| YSM/year |
12.00 (2.00, 17.00) |
17.00 (6.00, 22.00) |
5.00 (1.00, 16.00) |
22.50 (14.50, 26.25) |
| BMI/(kg·m-2) |
26.00 (23.44, 27.57) |
24.65 (22.67, 27.06) |
24.67 (22.41, 28.48)*△ |
24.31 (23.37, 27.79) |
| WHR |
0.90 (0.84, 0.97) |
0.88 (0.85, 0.93) |
0.91 (0.88, 0.95)△ |
0.90 (0.85, 0.94)*▲ |
| Group A: Normal glucose tolerance and bone mass group; Group B: Normal glucose tolerance but abnormal bone mass group; Group C: T2DM with normal bone mass group; Group D: T2DM with abnormal bone mass group. *P<0.05 vs group A; △P<0.05 vs group B; ▲P<0.05 vs group C. YSM: Years since menopause; BMI: Body mass index; WHR: Waist-hip ratio; T2DM: Type 2 diabetes mellitus; M (QL, QU): Median (lower quartile, upper quartile). |
|
表 1 4组绝经后女性的基线资料比较
Tab 1 Comparison of baseline data among 4 groups of postmenopausal women
|
与A、B组相比,C、D组FBG、HbA1c水平均升高(均P<0.05)。与B组相比,C组HDL-C水平升高、ALP水平降低(均P<0.05)。与C组相比,D组ALP水平升高(P<0.05)。与A组、C组相比,B组、D组的L1~4椎体骨密度、股骨颈骨密度水平均降低(均P<0.05)。见表 2。
表 2
(Tab 2)

表 2 4组绝经后女性生物化学指标及BMD比较
Tab 2 Comparison of biochemical indexes and BMD among 4 groups of postmenopausal women
| Variable |
Group A n=52 |
Group B n=43 |
Group C n=47 |
Group D n=58 |
| FBG/(mmol·L-1), M (QL, QU) |
5.11 (4.39, 5.52) |
4.91 (4.72, 5.66) |
8.83 (7.35, 10.40)*△ |
7.87 (6.91, 9.81)*△ |
| HbA1c/%, M (QL, QU) |
5.90 (5.60, 6.10) |
6.00 (5.60, 6.60) |
8.50 (7.20, 9.30)*△ |
7.55 (7.00, 8.80)*△ |
| TG/(mmol·L-1), M (QL, QU) |
1.29 (1.05, 1.93) |
1.14 (0.80, 1.47) |
1.39 (1.00, 2.75) |
1.45 (1.00, 1.92) |
| HDL-C/(mmol·L-1), M (QL, QU) |
1.37 (1.08, 1.59) |
1.27 (1.00, 1.56) |
1.45 (1.17, 2.38)△ |
1.31 (1.13, 1.87) |
| LDL-C/(mmol·L-1), M (QL, QU) |
2.76 (2.09, 3.13) |
2.26 (1.68, 2.93) |
1.90 (1.59, 3.18) |
2.41 (1.43, 3.53) |
| Calcium/(mmol·L-1), M (QL, QU) |
2.28 (2.23, 2.33) |
2.27 (2.21, 2.32) |
2.30 (2.20, 2.36) |
2.29 (2.18, 2.41) |
| Phosphorus/(mmol·L-1), M (QL, QU) |
1.11 (1.07, 1.25) |
1.19 (1.00, 1.29) |
1.18 (1.09, 1.25) |
1.10 (0.97, 1.20) |
| ALP/(U·L-1), M (QL, QU) |
72.50 (59.00, 88.50) |
79.00 (64.00, 91.00) |
58.0 (66.0, 78.0)△ |
76.00 (64.00, 91.25)▲ |
| BMD (L1-4)/(g·cm-2), x±s |
1.16±1.20 |
0.93±0.13* |
1.15±0.20△ |
0.95±0.10*▲ |
| BMD (NOF)/(g·cm-2), x±s |
1.02±0.25 |
0.78±0.13* |
1.02±0.15△ |
0.77±0.10*▲ |
| Group A: Normal glucose tolerance and bone mass group; Group B: Normal glucose tolerance but abnormal bone mass group; Group C: T2DM with normal bone mass group; Group D: T2DM with abnormal bone mass group. *P<0.05 vs group A; △P<0.05 vs group B; ▲P<0.05 vs group C. BMD: Bone mineral density; FBG: Fasting blood glucose; HbA1c: Glycated hemoglobin; TG: Triglyceride; HDL-C: High density lipoprotein-cholesterol; LDL-C: Low density lipoprotein-cholesterol; ALP: Alkaline phosphatase; BMD: Bone mineral density; NOF: Neck of femur; T2DM: Type 2 diabetes mellitus; M (QL, QU): Median (lower quartile, upper quartile). |
|
表 2 4组绝经后女性生物化学指标及BMD比较
Tab 2 Comparison of biochemical indexes and BMD among 4 groups of postmenopausal women
|
2.2 OPG基因SNP位点基因型及等位基因频率分析
经测定,OPG基因rs4355801、rs6993813位点均符合Hardy-Weinberg平衡。rs4355801位点的基因型及等位基因频率分布组间差异均无统计学意义(均P>0. 05)。与A组比较,C组、D组rs6993813位点基因型分布均存在差异(均P<0.05),等位基因频率分布在各组间差异均无统计学意义(均P>0.05)。见表 3。
表 3
(Tab 3)

表 3 OPG基因rs4355801、rs6993813位点基因型与等位基因在各组绝经后女性中的分布
Tab 3 Distribution of genotypes and alleles of OPG gene rs4355801 and rs6993813 loci in each group of postmenopausal women
| n (%) |
| Index |
Group A |
Group B |
Group C |
Group D |
| rs6993813 genotypea |
|
|
|
|
| CC |
15 (28.85) |
6 (13.95) |
15 (31.91) |
25 (43.10) |
| TT |
12 (23.08) |
12 (27.91) |
5 (10.64) |
8 (13.79) |
| TC |
25 (48.08) |
25 (58.14) |
27 (57.45) |
25 (43.10) |
| χ2 value |
|
2.869 |
118.960 |
150.877 |
| P value |
|
>0.05 |
<0.05 |
<0.05 |
| rs6993813 alleleb |
|
|
|
|
| T |
49 (47.12) |
49 (56.98) |
37 (39.36) |
41 (35.34) |
| C |
55 (53.88) |
37 (43.02) |
57 (60.64) |
75 (64.66) |
| χ2 value |
|
1.833 |
1.208 |
3.143 |
| P value |
|
>0.05 |
>0.05 |
>0.05 |
| rs4355801 genotypea |
|
|
|
|
| AA |
22 (42.31) |
16 (37.21) |
23 (48.94) |
34 (58.62) |
| GG |
5 (9.62) |
5 (11.63) |
2 (4.26) |
7 (12.07) |
| AG |
25 (48.08) |
22 (51.16) |
22 (46.81) |
17 (29.31) |
| χ2 value |
|
0.351 |
0.726 |
4.175 |
| P value |
|
>0.05 |
>0.05 |
>0.05 |
| rs4355801 alleleb |
|
|
|
|
| A |
69 (66.35) |
54 (62.79) |
68 (72.34) |
85 (73.28) |
| G |
35 (33.65) |
32 (37.21) |
26 (27.66) |
31 (26.72) |
| χ2 value |
|
0.261 |
0.832 |
1.254 |
| P value |
|
>0.05 |
>0.05 |
>0.05 |
| Group A: Normal glucose tolerance and bone mass group; Group B: Normal glucose tolerance but abnormal bone mass group; Group C: T2DM with normal bone mass group; Group D: T2DM with abnormal bone mass group. χ2 values and P values were calculated by comparison with group A. a: The total numbers of genotypes in groups A, B, C, and D were 52, 43, 47, and 58, respectively; b: The total numbers of alleles in groups A, B, C, and D were 104, 86, 94, and 116, respectively. OPG: Osteoprotegerin; T2DM: Type 2 diabetes mellitus. |
|
表 3 OPG基因rs4355801、rs6993813位点基因型与等位基因在各组绝经后女性中的分布
Tab 3 Distribution of genotypes and alleles of OPG gene rs4355801 and rs6993813 loci in each group of postmenopausal women
|
2.3 OPG基因SNP位点野生型和突变型基因型间生物化学指标比较
C组rs4355801位点突变型患者FBG、HbA1c水平均低于野生型患者(均P<0.05),D组rs4355801位点突变型患者L1~4椎体骨密度高于野生型患者(P<0.05),D组rs6993813位点突变型患者血磷水平低于野生型患者、股骨颈骨密度水平高于野生型患者(均P<0.05)。见表 4。
表 4
(Tab 4)

表 4 OPG基因SNP位点野生型和突变型绝经后T2DM患者生物化学指标比较
Tab 4 Comparison of biochemical indexes between wild-type and mutant postmenopausal women with T2DM at SNP of OPG gene
| Variable |
rs4355801 (group C) |
| Wide type (AA) n=23 |
Mutant (GG+AG) n=24 |
| FBG/(mmol·L-1), x±s |
9.78±2.68 |
8.33±1.90* |
| HbA1c/%, x±s |
9.05±1.86 |
7.70±1.83* |
| TG/(mmol·L-1), M (QL, QU) |
1.34 (0.77, 1.60) |
1.43 (1.03, 2.82) |
| HDL-C/(mmol·L-1), x±s |
1.97±1.19 |
1.88±0.91 |
| LDL-C/(mmol·L-1), x±s |
2.20±1.03 |
2.46±0.96 |
| Calcium/(mmol·L-1), x±s |
2.25±0.27 |
2.29±0.09 |
| Phosphorus/(mmol·L-1), x±s |
1.19±0.21 |
1.17±0.15 |
| ALP/(U·L-1), x±s |
69.56±14.97 |
64.55±17.60 |
| BMD (L1-4)/(g·cm-2), x±s |
1.16±0.23 |
1.14±0.17 |
| BMD (NOF)/(g·cm-2), x±s |
1.03±0.16 |
1.01±0.14 |
|
| Variable |
rs4355801 (group D) |
| Wide type (AA) n=34 |
Mutant (GG+AG) n=24 |
| FBG/(mmol·L-1), x±s |
8.49±2.70 |
8.52±2.45 |
| HbA1c/%, x±s |
8.15±2.04 |
8.15±1.64 |
| TG/(mmol·L-1), M (QL, QU) |
1.64 (1.03, 2.12) |
1.38 (0.86, 1.81) |
| HDL-C/(mmol·L-1), x±s |
1.48±0.50 |
1.60±0.74 |
| LDL-C/(mmol·L-1), x±s |
2.51±1.07 |
2.61±1.40 |
| Calcium/(mmol·L-1), x±s |
2.28±0.14 |
2.28±0.22 |
| Phosphorus/(mmol·L-1), x±s |
1.11±0.16 |
1.06±0.14 |
| ALP/(U·L-1), x±s |
69.56±14.97 |
73.70±19.33 |
| BMD (L1-4)/(g·cm-2), x±s |
0.98±0.12 |
1.16±0.23* |
| BMD (NOF)/(g·cm-2), x±s |
1.03±0.16 |
0.78±0.76 |
|
| Variable |
rs6993813 (group D) |
| Wide type (TT) n=8 |
Mutant (CC+TC) n=50 |
| FBG/(mmol·L-1), x±s |
9.01±3.06 |
8.23±2.08 |
| HbA1c/%, x±s |
8.53±2.01 |
7.93±0.73 |
| TG/(mmol·L-1), M (QL, QU) |
1.32 (0.83, 1.95) |
1.42 (1.03, 1.90) |
| HDL-C/(mmol·L-1), x±s |
1.45±0.57 |
1.60±0.65 |
| LDL-C/(mmol·L-1), x±s |
2.55±0.20 |
2.53±1.23 |
| Calcium/(mmol·L-1), x±s |
2.24±0.20 |
2.31±0.14 |
| Phosphorus/(mmol·L-1), x±s |
1.13±0.16 |
1.05±0.13* |
| ALP/(U·L-1), x±s |
80.64±17.07 |
73.64±18.36 |
| BMD (L1-4)/(g·cm-2), x±s |
0.94±0.10 |
0.93±0.12 |
| BMD (NOF)/(g·cm-2), x±s |
0.74±0.12 |
0.80±0.09* |
| Group C: T2DM with normal bone mass group; Group D: T2DM with abnormal bone mass group. *P<0. 05 vs wide type at the same locus. OPG: Osteoprotegerin; SNP: Single nucleotide polymorphism; T2DM: Type 2 diabetes mellitus; FBG: Fasting blood glucose; HbA1c: Glycated hemoglobin; TG: Triglyceride; HDL-C: High density lipoprotein-cholesterol; LDL-C: Low density lipoprotein-cholesterol; ALP: Alkaline phosphatase; BMD: Bone mineral density; NOF: Neck of femur; M (QL, QU): Median (lower quartile, upper quartile). |
|
表 4 OPG基因SNP位点野生型和突变型绝经后T2DM患者生物化学指标比较
Tab 4 Comparison of biochemical indexes between wild-type and mutant postmenopausal women with T2DM at SNP of OPG gene
|
2.4 绝经后女性骨密度的影响因素
多元线性回归分析结果显示,绝经年限增加及BMI、TG、HDL-C、LDL-C降低是绝经后女性L1~4椎体骨密度降低的危险因素;绝经年限增加、HDL-C降低、血磷降低是绝经后女性股骨颈骨密度降低的危险因素;rs4355801位点以AA基因型为参考类别,GG基因型与AA基因型绝经后女性的L1~4椎体骨密度、股骨颈骨密度均不相关(均P>0.05),AG基因型是绝经后女性L1~4椎体骨密度、股骨颈骨密度增加的保护因素(均P<0.05);rs6993813位点以TT基因型为参考类别,CC基因型、TC基因型与TT基因型绝经后女性的L1~4椎体骨密度、股骨颈骨密度均不相关(均P>0.05)。见表 5。
表 5
(Tab 5)

表 5 绝经后女性BMD影响因素的多元线性回归分析
Tab 5 Multiple linear regression analysis of influencing factors of BMD in postmenopausal women
| Variable |
BMD (L1-4) |
|
BMD (NOF) |
| b |
SE |
t value |
P value |
b |
SE |
t value |
P value |
| Age |
-0.092 |
0.013 |
-0.919 |
>0.05 |
|
-1.503 |
0.010 |
-1.503 |
>0.05 |
| YSM |
-0.221 |
0.016 |
-2.142 |
<0.05 |
-2.732 |
0.012 |
-2.732 |
<0.05 |
| BMI |
0.140 |
0.031 |
1.990 |
<0.05 |
0.823 |
0.023 |
0.823 |
>0.05 |
| WHR |
0.023 |
0.014 |
0.323 |
>0.05 |
1.157 |
0.011 |
1.157 |
>0.05 |
| FBG |
0.095 |
0.059 |
0.957 |
>0.05 |
0.180 |
0.044 |
0.180 |
>0.05 |
| HbA1c |
0.029 |
0.090 |
0.278 |
>0.05 |
0.657 |
0.068 |
0.657 |
>0.05 |
| TG |
0.143 |
0.066 |
1.997 |
<0.05 |
0.781 |
0.049 |
0.781 |
>0.05 |
| HDL-C |
0.170 |
0.179 |
2.103 |
<0.05 |
2.124 |
0.134 |
2.124 |
<0.05 |
| LDL-C |
0.167 |
0.124 |
2.159 |
<0.05 |
0.989 |
0.093 |
0.989 |
>0.05 |
| Calcium |
-0.030 |
0.754 |
-0.438 |
>0.05 |
-1.241 |
0.566 |
-1.241 |
>0.05 |
| Phosphorus |
0.002 |
0.616 |
0.023 |
>0.05 |
1.995 |
0.462 |
1.995 |
<0.05 |
| ALP |
0.013 |
0.002 |
0.194 |
>0.05 |
-0.956 |
0.002 |
-0.956 |
>0.05 |
| rs4355801 genotype (vs AA) |
|
|
|
|
|
|
|
|
| GG |
0.092 |
0.432 |
1.278 |
>0.05 |
0.020 |
0.338 |
0.273 |
>0.05 |
| AG |
0.207 |
0.238 |
2.860 |
<0.05 |
0.163 |
0.186 |
2.236 |
<0.05 |
| rs6993813 genotype (vs TT) |
|
|
|
|
|
|
|
|
| CC |
0.006 |
0.344 |
0.058 |
>0.05 |
0.004 |
0.270 |
0.045 |
>0.05 |
| TC |
0.124 |
0.318 |
1.269 |
>0.05 |
0.040 |
0.249 |
0.407 |
>0.05 |
| BMD: Bone mineral density; YSM: Years since menopause; BMI: Body mass index; WHR: Waist-hip ratio; FBG: Fasting blood glucose; HbA1c: Glycosylated hemoglobin; TG: Triglyceride; HDL-C: High density lipoprotein-cholesterol; LDL-C: Low density lipoprotein-cholesterol; ALP: Alkaline phosphatase; NOF: Neck of femur; b: Regression coefficient; SE: Standard error. |
|
表 5 绝经后女性BMD影响因素的多元线性回归分析
Tab 5 Multiple linear regression analysis of influencing factors of BMD in postmenopausal women
|
2.5 OPG基因SNP位点突变与绝经后女性骨量异常易感性的关系
根据骨量是否正常,将研究对象分为骨量正常组(99例)及骨量异常组(101例)。rs4355801位点和rs6993813位点野生型与突变型绝经后女性骨密度差异均无统计学意义(均P>0.05)。见表 6。
表 6
(Tab 6)

表 6 OPG基因rs4355801位点和rs6993813位点野生型、突变型与绝经后女性骨量异常的关系
Tab 6 Relationships between wild type and mutant OPG gene rs4355801 and rs6993813 loci and abnormal bone mass in postmenopausal women
| n (%) |
| Genotype |
Normal bone mass n=99 |
Abnormal bone mass n=101 |
χ2 value |
OR (95% CI) |
P value |
| rs4355801 |
|
|
0.329 |
0.850 (0.488, 1.481) |
>0.05 |
| Wide type (AA) |
45 (45.45) |
50 (49.50) |
|
|
|
| Mutant (GG+AG) |
54 (54.55) |
51 (50.50) |
|
|
|
| rs6993813 |
|
|
0.229 |
0.840 (0.410, 1.718) |
>0.05 |
| Wide type (TT) |
17 (17.17) |
20 (19.80) |
|
|
|
| Mutant (CC+TC) |
82 (82.83) |
81 (80.20) |
|
|
|
| OPG: Osteoprotegerin; OR: Odds ratio; CI: Confidence interval. |
|
表 6 OPG基因rs4355801位点和rs6993813位点野生型、突变型与绝经后女性骨量异常的关系
Tab 6 Relationships between wild type and mutant OPG gene rs4355801 and rs6993813 loci and abnormal bone mass in postmenopausal women
|
2.6 OPG基因SNP连锁不平衡分析及单体型分析
使用SHEsis软件行OPG基因SNP连锁不平衡分析及单体型分析,结果显示,OPG基因2个SNP位点rs4355801、rs6993813之间存在明显连锁不平衡关系(D’>0.9,r2>0.3)。GT单体型在骨量异常组中分布频率明显高于骨量正常组,提示携带GT单体型的绝经后女性骨量异常风险增高(P<0.05);而AT单体型在骨量异常组中分布频率明显低于骨量正常组,提示携带AT单体型绝经后女性骨量异常风险降低(P<0.05)。见表 7。
表 7
(Tab 7)

表 7 OPG基因rs4355801、rs6993813位点单体型分析
Tab 7 Haplotype analysis of loci in OPG gene rs4355801 and rs6993813
| Theoretical frequency (proportion/%) |
| Haploid |
Normal bone mass |
Abnormal bone mass |
χ2 value |
OR (95% CI) |
P value |
| AT |
61.81 (54.2) |
37.88 (40.3) |
4.005 |
1.755 (1.010, 3.051) |
<0.05 |
| AC |
24.19 (21.2) |
16.12 (17.2) |
0.545 |
1.301 (0.646, 2.618) |
>0.05 |
| GT |
1.19 (1.0) |
9.12 (9.7) |
8.209 |
0.098 (0.014, 0.676) |
<0.05 |
| GC |
26.81 (23.5) |
30.88 (32.8) |
2.236 |
0.629 (0.341, 1.158) |
>0.05 |
| OR: Odds ratio; CI: Confidence interval. |
|
表 7 OPG基因rs4355801、rs6993813位点单体型分析
Tab 7 Haplotype analysis of loci in OPG gene rs4355801 and rs6993813
|
2.7 OPG基因SNP的交互作用与各部位骨密度的关系
结果显示,OPG基因rs4355801、rs6993813位点的交互作用对L1~4椎体骨密度(F=0.984,P=0.417)及股骨颈骨密度(F=1.408,P=0.233)均未产生影响。
3 讨论
骨质疏松症是一种以骨密度降低为主要表现的多因素疾病,是非创伤性或低创伤性骨折(即骨质疏松性骨折)的重要原因[5],降低了世界各地老龄化人口的生活质量,给社会造成了巨大的经济负担[10]。研究显示,2型糖尿病患者是骨质疏松症的高发人群[11],高龄、女性、糖尿病病程长、高HbA1c、高FBG、低BMI、低胰岛素水平、低胰岛素样生长因子1可促进2型糖尿病合并骨质疏松症的发生,其发病受遗传和环境等因素的影响[12]。绝经前女性OPG通过OPG-RANKL-RANK信号通路拮抗RANKL与RANK的相互作用抑制破骨细胞形成来调节骨吸收[7],使骨吸收与骨形成呈平衡状态。而绝经后女性RANKL增加超过OPG导致骨吸收增加,最终导致绝经后骨质疏松[13],因此OPG基因被认为是可能与绝经后骨质疏松易感性相关的候选基因。
本研究结果显示,绝经后女性OPG基因rs4355801、rs6993813位点均符合Hardy-Weinberg平衡定律,表明研究对象具有群体代表性。虽rs4355801位点突变型与野生型绝经后女性的骨密度差异无统计学意义,但多元线性回归分析发现rs4355801位点AG基因型是绝经后女性L1~4椎体骨密度、股骨颈骨密度的保护因素。这与Richards等[14]的报道相似,说明OPG基因rs4355801位点基因突变可能参与了绝经后女性骨量异常的发生及发展。对于rs6993813位点,多元线性回归分析结果发现野生型与突变型绝经后女性的L1~4椎体骨密度、股骨颈骨密度的差异均无统计学意义(均P>0.05),但单因素分析发现D组野生型患者股骨颈骨密度低于突变型患者(P<0.05)、血磷水平高于突变型患者。说明OPG基因rs6993813位点基因突变可能参与了绝经后女性骨量异常的发生及发展,这与既往研究结果[15]一致。
本研究结果显示在OPG基因2个SNP位点中,rs4355801位点的基因型和等位基因分布频率在组间差异均无统计学意义(均P>0.05),与骨量异常的发病风险可能无关联,表明rs4355801可能不影响氨基酸编码,在OPG蛋白的功能改变上不起主要作用;rs6993813位点基因型分布在C组与A组及D组间存在差异(均P<0.05),但rs6993813位点野生型与突变型绝经后女性骨密度差异无统计学意义(P>0.05),提示该位点多态性可能与绝经后女性骨量异常无关,这与既往研究结果[16]不同,可能与地域因素有关。
OPG基因SNP位点对骨质疏松症的影响与研究对象的遗传背景、种族差异相关。在OPG基因rs4355801位点,沙特阿拉伯绝经后女性GG型者的骨密度较AA型、AG型者明显升高[17],在伊朗[18]、欧洲和香港人群[19]突变型(GG+AG)的骨密度明显高于野生型(AA)者。还有研究发现墨西哥Maya-Mestizo女性绝经后,rs4355801位点的多态性与骨密度明显相关[20],该位点基因多态性也影响欧洲人群骨密度(髋部)[21]。
本研究结果显示OPG基因rs4355801、rs6993813位点的交互作用并未对绝经后女性骨密度产生影响。目前鲜有此2个位点交互作用的研究,但既往研究发现OPG基因T950C位点与钙的摄入量对青春前期女童骨密度的交互作用具有统计学意义[22],雌激素受体基因、肌细胞增强因子2、转录因子FoxLl等3个基因的交互作用与骨质疏松性骨折有关[23]。位于染色体上某区域的一组相关联SNP等位位点称为单体型[24]。本研究结果显示,rs4355801位点基因多态性与绝经后女性骨量异常无相关,但OPG基因2个SNP位点rs4355801、rs6993813之间存在明显连锁不平衡关系,进一步行单体型分析发现,携带GT单体型能显著增加绝经后女性骨量异常的发病风险,而携带AT单体型则能明显降低绝经后女性骨量异常的发病风险。目前鲜有关于OPG基因rs4355801、rs6993813位点之间连锁不平衡及单体型的研究,其作用机制仍需进一步研究。
在C组rs4355801位点野生型患者与突变型患者的FBG、HbA1c水平差异具有统计学意义,但未发现与脂代谢相关,提示rs4355801位点基因突变可能参与了绝经后女性的糖代谢。本研究未发现rs6993813位点基因多态性及突变与糖、脂代谢明显相关。多元线性回归分析发现,绝经年限增加、HDL-C降低是L1~4椎体及股骨颈骨密度降低的危险因素,BMI、TG、LDL-C降低是L1~4椎体骨密度降低的危险因素,血磷降低是股骨颈骨密度降低的危险因素,与既往研究[25-29]结果一致。提示当患者绝经年限增加、HDL-C降低、BMI降低、TG降低、LDL-C降低时,要及时检测骨密度,以预防骨质疏松症的发生。
综上所述,OPG基因rs4355801、rs6993813位点存在明显连锁不平衡,且两位点基因多态性及突变在骨质疏松症的发生中起重要作用。基于疾病分子机制的精准医学在肿瘤诊疗中取得了变革性突破[30],精准医学已显示出巨大的临床应用价值,从基因遗传学进行诊疗,已成为未来疾病研究的重要方向。基于此,可通过对OPG基因测序,精准识别骨质疏松症高危人群,研发靶向药物为骨质疏松症的治疗提供更多的选择,实现骨质疏松症精准治疗。其次,应重点关注rs4355801位点野生型及GT单体型人群,此类人群应完善双能X射线骨密度等相关检查,做到早发现、早诊断、早预防骨质疏松症。由于骨质疏松症是一种遗传、环境因素共同作用的疾病[12],不同地域及不同民族之间存在明显差异[31],本研究为单中心研究,样本量较小,研究结果存在一定局限性。在今后需开展多民族、多地域、大规模的多中心、前瞻性临床研究,进一步评估OPG基因rs4355801、rs6993813位点多态性及突变是否可以成为骨质疏松症发生及风险预测的新型分子标志物,以促进骨质疏松症精准医学的应用。
参考文献
| [1] | |
| [2] | |
| [3] | |
| [4] |
BANERJEE M, VATS P, KUSHWAH A S, et al. Interaction of antioxidant gene variants and susceptibility to type 2 diabetes mellitus[J]. Br J Biomed Sci, 2019, 76(4): 166-171. DOI:10.1080/09674845.2019.1595869 |
| [5] |
LI X, CHENG J, DONG B, et al. Common variants of the OPG gene are associated with osteoporosis risk: a meta-analysis[J]. Genet Test Mol Biomark, 2021, 25(9): 600-610. DOI:10.1089/gtmb.2020.0282 |
| [6] |
ZHOU J, ZHAO Y. Osteoprotegerin gene ( OPG) polymorphisms associated with peri-implantitis susceptibility in a Chinese Han population[J]. Med Sci Monit, 2016, 22: 4271-4276. DOI:10.12659/msm.897592 |
| [7] |
CORREA-RODRÍGUEZ M, SCHMIDT-RIOVALLE J, RUEDA-MEDINA B. RANKL/ RANK/ OPG polymorphisms and heel quantitative ultrasound in young adults[J]. Nurs Res, 2017, 66(2): 145-151. DOI:10.1097/nnr.0000000000000202 |
| [8] |
GIACOBBO L C, PERIN M A A, PEREIRA T M, et al. RANK/ RANKL/ OPG gene polymorphisms and loss of orthodontic mini-implants[J]. Orthod Craniofac Res, 2020, 23(2): 210-222. DOI:10.1111/ocr.12360 |
| [9] |
BAUD'HUIN M, DUPLOMB L, TELETCHEA S, et al. Osteoprotegerin: multiple partners for multiple functions[J]. Cytokine Growth Factor Rev, 2013, 24(5): 401-409. DOI:10.1016/j.cytogfr.2013.06.001 |
| [10] |
WU F, ZHOU D, SHEN G, et al. Association of VDR and OPG gene polymorphism with osteoporosis risk in Chinese postmenopausal women[J]. Climacteric, 2019, 22(2): 208-212. DOI:10.1080/13697137.2018.1554643 |
| [11] | |
| [12] | |
| [13] | |
| [14] |
RICHARDS J B, RIVADENEIRA F, INOUYE M, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study[J]. Lancet, 2008, 371(9623): 1505-1512. DOI:10.1016/S0140-6736(08)60599-1 |
| [15] |
SHENG X, CAI G, GONG X, et al. Common variants in OPG confer risk to bone mineral density variation and osteoporosis fractures[J]. Sci Rep, 2017, 7(1): 1739. DOI:10.1038/s41598-017-01579-6 |
| [16] | |
| [17] |
ABDI S, BINBAZ R A, MOHAMMED A K, et al. Association of RANKL and OPG gene polymorphism in Arab women with and without osteoporosis[J]. Genes (Basel), 2021, 12(2): 200. DOI:10.3390/genes12020200 |
| [18] |
DASTGHEIB S A, GARTLAND A, TABEI S M, et al. A candidate gene association study of bone mineral density in an Iranian population[J]. Front Endocrinol, 2016, 7: 141. DOI:10.3389/fendo.2016.00141 |
| [19] |
STYRKARSDOTTIR U, HALLDORSSON B V, GUDBJARTSSON D F, et al. European bone mineral density loci are also associated with BMD in East-Asian populations[J]. PLoS One, 2010, 5(10): e13217. DOI:10.1371/journal.pone.0013217 |
| [20] |
CANTO-CETINA T, POLANCO REYES L, GONZÁLEZ HERRERA L, et al. Polymorphism of LRP5, but not of TNFRSF11B, is associated with a decrease in bone mineral density in postmenopausal Maya-Mestizo women[J]. Am J Hum Biol, 2013, 25(6): 713-718. DOI:10.1002/ajhb.22464 |
| [21] |
LIU J M, ZHANG M J, ZHAO L, et al. Analysis of recently identified osteoporosis susceptibility genes in Han Chinese women[J]. J Clin Endocrinol Metab, 2010, 95(9): E112-E120. DOI:10.1210/jc.2009-2768 |
| [22] |
何国鹏. OPG、COL1A2基因及钙摄入水平与青春前期女童骨密度的相关性研究[D]. 广州: 中山大学, 2006.
|
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] |
ADAMI S, BRAGA V, ZAMBONI M, et al. Relationship between lipids and bone mass in 2 cohorts of healthy women and men[J]. Calcif Tissue Int, 2004, 74(2): 136-142. DOI:10.1007/s00223-003-0050-4 |
| [29] | |
| [30] | |
| [31] | |
 2024, Vol. 45
2024, Vol. 45


