铁死亡(ferroptosis)是于2012年首次提出的概念,用来描述可以被erastin和RAS选择性致死化合物3(RAS-selective lethal compound 3,RSL3)所诱发的、铁依赖的、非凋亡的调节性细胞死亡形式[1-3]。铁死亡的特征是细胞内铁依赖的、过量的脂质过氧化物累积,并且在形态学、生物学和遗传学等方面明显不同于凋亡、坏死、自噬及其他形式的细胞死亡。铁死亡与体内异常代谢和生化过程密切相关,参与癌症、炎症、神经变性、肝肾损伤和心脏缺血再灌注损伤等病理过程[4]。
急性呼吸窘迫综合征(acute respiratory distress syndrome,ARDS)是一种严重危及生命的疾病,临床特征是双肺弥散性炎症性损伤,ARDS的柏林定义根据氧合指数将其分为轻度、中度和重度,其中轻度ARDS即急性肺损伤(acute lung injury,ALI)[5]。肺炎、胃内容物误吸、败血症和淹溺等诸多诱发因素可导致ARDS[6]。尽管引起ARDS的基础病因不同,但却有相似病理生理特征:肺泡上皮-毛细血管内皮细胞屏障损伤,随之而来的富含蛋白质的液体积聚在肺泡腔和组织间隙,细胞病变、气道炎症引起气流阻力增加和肺活量下降[7]。柏林定义是临床上诊断ARDS的金标准,由于目前动物模型研究中并无类似的明确定义,模拟ARDS的动物模型仍称为ALI模型[5]。ICU患者的ARDS发病率为10.4%,死亡率高达35%~46%[7]。ARDS的主要治疗措施为机械通气、体外膜肺氧合(extracorporeal membrane oxygenation,ECMO)等支持治疗,缺乏有效的药物治疗方法[7]。2019年12月开始,新型冠状病毒感染在全球范围内大流行,出现ARDS是新型冠状病毒感染患者死亡的主要原因,使ARDS的防治形势更加严峻。
近年来,阐明铁死亡在ARDS中的确切机制、考察其调节剂以开发ARDS新疗法已成为研究的热点。本文主要就铁死亡的定义、机制及其在ARDS中的基础和临床应用进展进行综述。
1 铁死亡的概念2003年在筛选选择性杀死RAS突变致癌性人成纤维瘤BJ细胞的药物时,Dolma团队意外发现小分子化合物erastin能够诱导细胞出现非凋亡性细胞死亡[2]。2008年,Yang和Stockwell[3]发现RSL3同样可以诱发铁依赖的、细胞内活性氧过量累积的非凋亡性细胞死亡,这一过程能够通过铁螯合剂来预防。2012年“ferroptosis”术语首次被用于描述这一调节性细胞死亡形式[1]。2017年,铁死亡正式被定义为铁依赖的、细胞内脂质过氧化物过量累积所导致的细胞死亡方式[4]。在20世纪50年代已有研究报道了具有铁死亡特征的细胞死亡,如在培养人癌细胞时胱氨酸剥夺导致细胞死亡并具有独特的微观形态[8],培养液中恢复谷胱甘肽(glutathione,GSH)时能够促进细胞生长[9];通过给大鼠肌肉注射过量右旋糖酐铁能诱发脂质组织中过氧化物的形成,这一现象可以被维生素E减弱并可被不饱和脂肪而加剧,铁和脂质过氧化之间存在联系[10]。尽管在当时这些发现显得毫无关联,但今天来看其已经概述了铁死亡的基本原则。随后研究发现了胱氨酸/谷氨酸逆向转运体和体内GSH合成机制,而且谷胱甘肽过氧化物酶4(glutathione peroxidase 4,GPX4)基因敲除的小鼠可出现脂质过氧化致特殊类型的细胞死亡[11]。
铁死亡在细胞形态、生物化学特征、遗传学等方面与凋亡、程序性坏死、自噬等明显不同,电镜下主要表现为线粒体外膜破坏、线粒体皱缩,而没有染色体的浓缩(凋亡)、细胞器的肿胀(坏死)及双层膜囊泡的形成(自噬)[1](表 1)。但是越来越多的证据表明铁死亡与自噬、凋亡和氧化应激等存在一定程度上的交叉重叠[12],不能用单一的铁死亡标志物来判断是否有铁死亡的发生。
|
|
表 1 铁死亡与其他细胞死亡方式的差异 |
2 影响铁死亡的代谢通路 2.1 铁稳态失衡
铁死亡的执行需要铁的参与,铁螯合剂可以阻止铁死亡,而铁超载可诱导铁死亡。细胞内铁稳态与铁的吸收、储存、循环和利用密切相关[13],并由一系列铁调节蛋白调控。人体肠道每天从饮食中吸收约1~2 mg铁[14]。膳食内的三价铁(Fe3+)在肠道刷状缘细胞色素b的作用下还原为具有催化活性的亚铁(Fe2+),然后在二价金属离子转运蛋白1的调节下进入肠小上皮细胞,进入细胞质的亚铁可以储存在铁蛋白重链中[15]。入血的Fe2+离子在肝素的作用下被氧化成Fe3+,与转铁蛋白结合,其他细胞对铁的吸收主要由质膜蛋白转铁蛋白受体1介导的内吞作用将与转铁蛋白结合的铁带入细胞。在生理条件下细胞内大多数Fe2+与铁蛋白结合,少量游离Fe2+用于维持正常新陈代谢[16]。铁蛋白尤其是其重链的减少会使细胞内不稳定铁库的Fe2+增多,使细胞对铁死亡敏感[17]。细胞内铁释放由膜铁转运蛋白1与多铜亚铁氧化酶(如铜蓝蛋白)介导,膜铁转运蛋白1或铜蓝蛋白减少会增加细胞对铁死亡的敏感性[16]。铁可以通过含铁蛋白的多泡体和外泌体以铁蛋白的形式从细胞排出,这一过程的主要驱动因素prominin2可以防止铁死亡[18]。核因子E2相关因子2及其靶基因血红素加氧酶1可释放血红素中的亚铁,发挥促铁死亡作用[19]。细胞内铁摄取增多或排出障碍时,胞内铁增加甚至会超过转铁蛋白的结合能力,形成非转铁蛋白结合铁,导致铁超载,过量Fe2+或非转铁蛋白结合铁通过芬顿反应或含铁脂氧合酶(lipoxygenase,LOX)产生脂质过氧化物的过量累积,引起铁死亡[20]。正常情况下细胞内有清除活性氧的酶系统,如超氧化物歧化酶、过氧化氢酶和谷胱甘肽过氧化物酶,以防止细胞损伤[16]。活性氧生成和清除失衡时发生氧化应激,大量的超氧化物和过氧化物从铁硫簇、血红素和其他含铁物质中诱导亚铁的释放,结果Fe2+浓度进一步升高,形成恶性循环,最终导致铁死亡(图 1)。
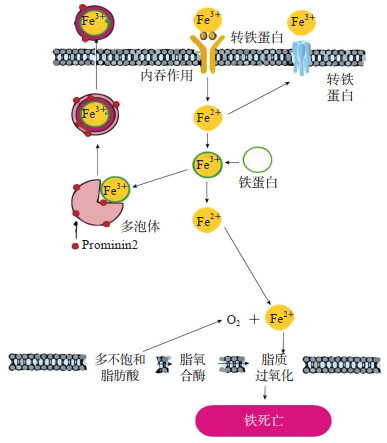
|
图 1 铁离子代谢与铁死亡 |
近年来,GSH在调节细胞内铁稳态中的作用引起了广泛关注。由Fe2+和GSH形成的复合物被认为是不稳定铁池中铁的主要形式[21]。实际上,铁与多聚胞嘧啶结合蛋白1的结合也是GSH依赖性的[22]。
2.2 脂质代谢异常研究发现,含有花生四烯酸基团或肾上腺素基团的磷脂酰乙醇胺是脂质过氧化的主要底物[23]。铁死亡的脂质过氧化涉及更广泛的多不饱和脂肪酸(poly-unsaturated fatty acid,PUFA),与饱和脂肪酸和单不饱和脂肪酸相比,PUFA更易被过氧化形成脂质过氧化物,哺乳动物细胞中的PUFA主要由膳食必需脂肪酸(亚油酸和α-亚麻酸)转化而来[24]。
铁死亡时脂质过氧化主要影响酯化PUFA,PUFA先在长链脂酰辅酶A合成酶4的催化下转化为酰基辅酶A酯,再在溶血卵磷脂酰基转移酶3催化下酰化为溶血磷脂[23]。含有足够PUFA的磷脂是铁死亡导致细胞死亡的先决条件[25],因此,涉及PUFA的吸收或降解过程均影响细胞对铁死亡的敏感性。
铁可能不仅通过芬顿反应促进脂质自氧化,还参与某些酶反应[26]。LOX,特别是12/15-LOX(即ALOX15),被认为在脂质过氧化和铁死亡中起中心作用[23]。p53通过其转录靶亚精胺/精胺N1-乙酰转移酶1间接促进ALOX15表达来调节铁死亡。但是ALOX15基因敲除不能挽救GPX4缺陷诱导的成纤维细胞铁死亡[27],因此铁死亡发生时脂质过氧化的具体机制仍需进一步探索。
2.3 氨基酸代谢障碍及GPX4耗竭胱氨酸/谷氨酸逆向转运体由调节亚基溶质载体家族3成员2和催化亚基溶质载体家族7成员11偶联组成,都位于细胞膜上,以1∶1的比例将细胞内谷氨酸和细胞外的胱氨酸互换,胱氨酸被还原成半胱氨酸,通过一系列的酶促反应生成GSH,而GSH是GPX4降解磷脂过氧化氢的必要底物[28]。此外,蛋氨酸可以通过转硫途径将硫原子转移到丝氨酸上生成半胱氨酸,而半胱氨酸-转移核糖核酸合成酶敲除可以上调该过程,使细胞对铁死亡具有抵抗力[29]。当细胞外谷氨酸含量增加时,或存在erastin等抑制剂,会降低胱氨酸/谷氨酸逆向转运体的活性,阻止胱氨酸的摄取,细胞内GSH减少,进一步使大量活性氧累积,促进铁死亡[30]。组织和血浆中含有大量的谷氨酰胺,经谷氨酰胺酶(包括谷氨酰胺酶1和2)催化后可转化为谷氨酸,而谷氨酰胺分解是三羧酸循环和脂质生物合成所必需的环节,并且其分解产物α-酮戊二酸酯与铁死亡有关[31]。p53基因可下调胱氨酸/谷氨酸逆向转运体的活性,使胱氨酸不能进入胞内,抑制了GSH的合成,导致细胞内的脂质活性氧不断增多,最终引起细胞铁死亡[32]。而且,谷氨酰胺酶2作为抑癌基因p53的靶基因与铁死亡密切相关[33]。
GPX4是一种硒蛋白,其生物合成依赖于硒代半胱氨酸的共翻译整合机制[34]。GPX4处于GSH代谢和脂质过氧化的交叉点,改变GPX4水平和/或活性将对细胞存活产生直接影响,基因敲除和药物抑制GPX4也会导致活性氧的产生和铁死亡[4]。
2.4 铁死亡抑制蛋白1(ferroptosis suppressor protein 1,FSP1)-NADH/还原型烟酰胺腺嘌呤二核苷酸磷酸(reduced nicotinamide adenine dinucleotide phosphate,NADPH)-辅酶Q10(coenzyme Q10,CoQ10)系统FSP1-NADH/NADPH-CoQ10系统与GSH-GPX4轴并行,两者具有很强的协同效应。FSP1属于Ⅱ型NADH: 泛醌氧化还原酶2家族,即呼吸链的复合物Ⅰ。FSP1能够利用NADH降低CoQ10水平[35]。FSP1主要定位于脂滴和质膜,可通过自由基捕获抗氧化剂(radical-trapping antioxidant,RTA)直接减少脂质自由基数量,或可能间接地通过α-生育酚的循环来减少脂质自由基数量[36]。FSP1抑制剂能诱导过表达FSP1的细胞发生铁死亡[24]。
2.5 鸟苷三磷酸环水解酶1(guanosine triphosphatecyclohydrolase 1,GCH1)-四氢生物蝶呤(tetrahy-drobiopterin,BH4)-二氢叶酸还原酶(dihydrofolate reductase,DHFR)系统基于全基因组CRISPR筛选技术发现GCH1是GPX4之外独立对抗铁死亡的分子,是BH4合成的限速酶[37]。BH4是一种有效的RTA,可以通过DHFR再生。阻断DHFR与抑制GPX4具有协同作用,可诱导铁死亡[38]。除了作为RTA的作用外,BH4还能通过将苯丙氨酸转化为酪氨酸促进CoQ10的合成,酪氨酸还能进一步转化为CoQ10的前体4-羟基-苯甲酸酯[37]。
3 铁死亡在ARDS中的作用在ALI动物模型中检测到与铁死亡相关的指标Fe2+、活性氧、丙二醛(malondialdehyde,MDA)水平升高和GSH水平降低,抑制铁死亡有可能减轻肺损伤。铁死亡与ARDS有关,但是其具体机制尚不清楚。
ARDS是由大量活性氧和炎症因子引发的氧化和抗氧化系统失衡所导致的炎症性疾病[39]。铁死亡是细胞死亡的一种免疫原性形式,与损伤相关分子模式和炎症细胞因子的持续释放有关,后者促进了一系列炎症反应,炎症细胞因子进一步促进铁死亡和其他形式的细胞死亡,从而形成一个自我放大环,加重了器官损伤[40](图 2)。
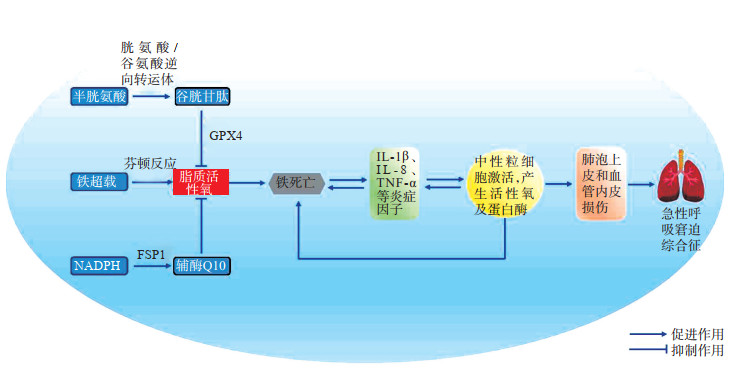
|
图 2 铁死亡在急性呼吸窘迫综合征中的作用 GPX4:谷胱甘肽过氧化物酶4;IL:白细胞介素;TNF-α:肿瘤坏死因子α;NADPH:还原型烟酰胺腺嘌呤二核苷酸磷酸;FSP1:铁死亡抑制蛋白1. |
在小鼠ALI模型中,铁死亡诱导剂可加重肺水肿和肺泡炎症,并伴有IL-1、IL-6和TNF-α等细胞因子水平的升高;铁死亡抑制剂起到保护作用[41]。小鼠尾静脉注射油酸制备的ALT模型出现了肺细胞线粒体收缩、线粒体膜破裂、肺组织铁超载、GSH缺乏和铁蛋白表达下调等铁死亡特点,在肺缺血再灌注损伤模型中也观察到类似的结果[42]。
3.1 铁超载和铁螯合剂铁代谢异常与ARDS患者肺组织损伤密切相关[43]。肺上皮细胞和巨噬细胞可以产生铁调节蛋白,维持铁稳态并保护肺组织免受氧化应激损伤[44]。在ARDS患者的支气管肺泡灌洗液中可以检测到Fe2+和铁调节剂的水平升高[43]。在油酸诱导的ALI模型小鼠的肺组织中可检测到铁超载[42]。预先补充铁会加剧对肺的损害,但这种作用是暂时的,不会影响ALI的炎症程度或恢复速度[41]。
3.2 炎症和抗炎治疗ARDS是肺内和/或全身危险因素引起的肺部炎症性疾病,适当的炎症反应有利于清除病原体,但是过度炎症反应会导致肺泡上皮-血管内皮屏障功能破坏,随后富含蛋白质的液体积聚在肺泡腔和组织间隙[45]。
ARDS患者的血浆和肺泡液中促炎细胞因子(如IL-1β、IL-8、TNF-α等)可以激活固有免疫。中性粒细胞通过趋化作用被激活,产生活性氧和多种蛋白酶,导致肺泡上皮和血管内皮的损伤甚至死亡,并形成恶性循环[45]。其中铁死亡时活性氧也会增多,可能与炎症存在一定交叉[12]。
抗炎治疗是ARDS药物治疗的一个方向。抗炎治疗同时抑制铁死亡可以治疗中风、心肌梗死和胰腺炎[40]。在ALI模型小鼠中,铁死亡抑制剂可以减少炎症细胞因子,改善肺水肿,从而减轻肺损伤[41]。铁死亡和炎症的相互作用关系还需进一步研究证实。
3.3 氧化应激和抗氧化治疗氧化应激在ARDS的发生、发展中起着重要作用。氧化应激也会对肺上皮和内皮细胞屏障造成损伤,大量中性粒细胞通过趋化作用聚集在肺组织,同时产生促炎细胞因子和大量活性氧。活性氧可进一步增加细胞因子水平,加重组织损伤和水肿,是ARDS的重要介质[41]。脂质过氧化产物MDA在ALI小鼠模型中升高,通常也被认为是铁死亡的标志物之一。
GSH是气道上皮中最重要的抗氧化剂,通过清除活性氧和修复细胞损伤发挥抗氧化作用,有利于减轻炎症、降低ARDS的严重程度[46]。在ALI患者和动物模型中均观察到GSH水平降低和氧化型GSH水平升高[41],肺泡液GSH缺乏使ARDS患者更易发生肺损伤[47];在脂多糖诱导的ALI模型中,补充GSH可减轻线粒体功能障碍和氧化损伤[48]。动物实验和临床研究均表明,N-乙酰半胱氨酸可促进GSH的产生,减轻ALI[49]。鉴于GSH在铁死亡中的重要性,它是否通过抑制铁死亡而对ARDS有治疗作用也值得探讨。
4 结语铁死亡是代谢失衡和氧化还原稳态紊乱导致的一种特殊的细胞死亡方式,与铁死亡相关的各代谢过程并不是独立的,而是机体复杂代谢网络的一部分,并与其他方式死亡和病变有一定程度的交叉。动物实验和临床试验初步结果均表明,铁死亡与很多疾病和病理过程密切相关,抑制铁死亡能够延缓疾病进展、改善临床症状,也为很多难治性疾病提供了新的治疗靶点。ARDS是由各种因素引起的严重危及生命的肺部炎症性疾病,目前采取以机械通气为主的支持治疗,缺乏特效药物。基础实验和临床研究均表明铁死亡在ARDS中起着重要作用,进一步深入了解其确切的作用机制,进而对铁死亡关键代谢途径进行干预并联合其他治疗策略为ARDS的治疗提供了新思路。
| [1] |
DIXON S J, LEMBERG K M, LAMPRECHT M R, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death[J]. Cell, 2012, 149(5): 1060-1072. DOI:10.1016/j.cell.2012.03.042 |
| [2] |
DOLMA S, LESSNICK S L, HAHN W C, et al. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells[J]. Cancer Cell, 2003, 3(3): 285-296. DOI:10.1016/s1535-6108(03)00050-3 |
| [3] |
YANG W S, STOCKWELL B R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells[J]. Chem Biol, 2008, 15(3): 234-245. DOI:10.1016/j.chembiol.2008.02.010 |
| [4] |
STOCKWELL B R, FRIEDMANN ANGELI J P, BAYIR H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease[J]. Cell, 2017, 171(2): 273-285. DOI:10.1016/j.cell.2017.09.021 |
| [5] |
ARDS Definition Task Force, RANIERI V M, RUBENFELD G D, THOMPSON B T, et al. Acute respiratory distress syndrome: the Berlin definition[J]. JAMA, 2012, 307(23): 2526-2533. DOI:10.1001/jama.2012.5669 |
| [6] |
SLUTSKY A S, VILLAR J, PESENTI A. Happy 50th birthday ARDS![J]. Intensive Care Med, 2016, 42(5): 637-639. DOI:10.1007/s00134-016-4284-9 |
| [7] |
MATTHAY M A, ZEMANS R L. The acute respiratory distress syndrome: pathogenesis and treatment[J]. Annu Rev Pathol, 2011, 6: 147-163. DOI:10.1146/annurev-pathol-011110-130158 |
| [8] |
EAGLE H. The specific amino acid requirements of a human carcinoma cell (Stain HeLa) in tissue culture[J]. J Exp Med, 1955, 102(1): 37-48. DOI:10.1084/jem.102.1.37 |
| [9] |
EAGLE H. Amino acid metabolism in mammalian cell cultures[J]. Science, 1959, 130(3373): 432-437. DOI:10.1126/science.130.3373.432 |
| [10] |
GOLBERG L, SMITH J P. Changes associated with the accumulation of excessive amounts of iron in certain organs of the rat[J]. Br J Exp Pathol, 1958, 39(1): 59-73. |
| [11] |
SEILER A, SCHNEIDER M, FÖRSTER H, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death[J]. Cell Metab, 2008, 8(3): 237-248. DOI:10.1016/j.cmet.2008.07.005 |
| [12] |
LIU J, KUANG F, KROEMER G, et al. Autophagy-dependent ferroptosis: machinery and regulation[J]. Cell Chem Biol, 2020, 27(4): 420-435. DOI:10.1016/j.chembiol.2020.02.005 |
| [13] |
GALARIS D, BARBOUTI A, PANTOPOULOS K. Iron homeostasis and oxidative stress: an intimate relationship[J]. Biochim Biophys Acta Mol Cell Res, 2019, 1866(12): 118535. DOI:10.1016/j.bbamcr.2019.118535 |
| [14] |
CAMASCHELLA C, NAI A, SILVESTRI L. Iron metabolism and iron disorders revisited in the hepcidin era[J]. Haematologica, 2020, 105(2): 260-272. DOI:10.3324/haematol.2019.232124 |
| [15] |
FUQUA B K, VULPE C D, ANDERSON G J. Intestinal iron absorption[J]. J Trace Elem Med Biol, 2012, 26(2/3): 115-119. DOI:10.1016/j.jtemb.2012.03.015 |
| [16] |
LATUNDE-DADA G O. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy[J]. Biochim Biophys Acta Gen Subj, 2017, 1861(8): 1893-1900. DOI:10.1016/j.bbagen.2017.05.019 |
| [17] |
CHEN P H, WU J, DING C C, et al. Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism[J]. Cell Death Differ, 2020, 27(3): 1008-1022. DOI:10.1038/s41418-019-0393-7 |
| [18] |
BROWN C W, AMANTE J J, CHHOY P, et al. Prominin2 drives ferroptosis resistance by stimulating iron export[J]. Dev Cell, 2019, 51(5): 575-586.e4. DOI:10.1016/j.devcel.2019.10.007 |
| [19] |
HASSANNIA B, WIERNICKI B, INGOLD I, et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma[J]. J Clin Invest, 2018, 128(8): 3341-3355. DOI:10.1172/JCI99032 |
| [20] |
YE Z, LIU W, ZHUO Q, et al. Ferroptosis: final destination for cancer?[J]. Cell Prolif, 2020, 53(3): e12761. DOI:10.1111/cpr.12761 |
| [21] |
HIDER R C, KONG X L. Glutathione: a key component of the cytoplasmic labile iron pool[J]. BioMetals, 2011, 24(6): 1179-1187. DOI:10.1007/s10534-011-9476-8 |
| [22] |
PATEL S J, FREY A G, PALENCHAR D J, et al. A PCBP1-BolA2 chaperone complex delivers iron for cytosolic [2Fe-2S] cluster assembly[J]. Nat Chem Biol, 2019, 15(9): 872-881. DOI:10.1038/s41589-019-0330-6 |
| [23] |
KAGAN V E, MAO G, QU F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis[J]. Nat Chem Biol, 2017, 13(1): 81-90. DOI:10.1038/nchembio.2238 |
| [24] |
GUILLOU H, ZADRAVEC D, MARTIN P G P, et al. The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice[J]. Prog Lipid Res, 2010, 49(2): 186-199. DOI:10.1016/j.plipres.2009.12.002 |
| [25] |
KAGAN V E, TYURINA Y Y, SUN W Y, et al. Redox phospholipidomics of enzymatically generated oxygenated phospholipids as specific signals of programmed cell death[J]. Free Radic Biol Med, 2020, 147: 231-241. DOI:10.1016/j.freeradbiomed.2019.12.028 |
| [26] |
DIXON S J, STOCKWELL B R. The role of iron and reactive oxygen species in cell death[J]. Nat Chem Biol, 2014, 10(1): 9-17. DOI:10.1038/nchembio.1416 |
| [27] |
FRIEDMANN ANGELI J P, SCHNEIDER M, PRONETH B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice[J]. Nat Cell Biol, 2014, 16(12): 1180-1191. DOI:10.1038/ncb3064 |
| [28] |
INGOLD I, BERNDT C, SCHMITT S, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis[J]. Cell, 2018, 172(3): 409-422.e21. DOI:10.1016/j.cell.2017.11.048 |
| [29] |
HAYANO M, YANG W S, CORN C K, et al. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation[J]. Cell Death Differ, 2016, 23(2): 270-278. DOI:10.1038/cdd.2015.93 |
| [30] |
MURPHY T H, MIYAMOTO M, SASTRE A, et al. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress[J]. Neuron, 1989, 2(6): 1547-1558. DOI:10.1016/0896-6273(89)90043-3 |
| [31] |
GAO M, MONIAN P, QUADRI N, et al. Glutaminolysis and transferrin regulate ferroptosis[J]. Mol Cell, 2015, 59(2): 298-308. DOI:10.1016/j.molcel.2015.06.011 |
| [32] |
XIE Y, ZHU S, SONG X, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity[J]. Cell Rep, 2017, 20(7): 1692-1704. DOI:10.1016/j.celrep.2017.07.055 |
| [33] |
JENNIS M, KUNG C P, BASU S, et al. An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model[J]. Genes Dev, 2016, 30(8): 918-930. DOI:10.1101/gad.275891.115 |
| [34] |
FRIEDMANN ANGELI J P, CONRAD M. Selenium and GPX4, a vital symbiosis[J]. Free Radic Biol Med, 2018, 127: 153-159. DOI:10.1016/j.freeradbiomed.2018.03.001 |
| [35] |
ELGUINDY M M, NAKAMARU-OGISO E. Apoptosis-inducing factor (AIF) and its family member protein, AMID, are rotenone-sensitive NADH: ubiquinone oxidoreductases (NDH-2)[J]. J Biol Chem, 2015, 290(34): 20815-20826. DOI:10.1074/jbc.M115.641498 |
| [36] |
BERSUKER K, HENDRICKS J M, LI Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis[J]. Nature, 2019, 575(7784): 688-692. DOI:10.1038/s41586-019-1705-2 |
| [37] |
KRAFT V A N, BEZJIAN C T, PFEIFFER S, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling[J]. ACS Cent Sci, 2020, 6(1): 41-53. DOI:10.1021/acscentsci.9b01063 |
| [38] |
SOULA M, WEBER R A, ZILKA O, et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers[J]. Nat Chem Biol, 2020, 16(12): 1351-1360. DOI:10.1038/s41589-020-0613-y |
| [39] |
GALLUZZI L, VITALE I, AARONSON S A, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018[J]. Cell Death Differ, 2018, 25(3): 486-541. DOI:10.1038/s41418-017-0012-4 |
| [40] |
LINKERMANN A, STOCKWELL B R, KRAUTWALD S, et al. Regulated cell death and inflammation: an auto-amplification loop causes organ failure[J]. Nat Rev Immunol, 2014, 14(11): 759-767. DOI:10.1038/nri3743 |
| [41] |
DONG H, QIANG Z, CHAI D, et al. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1[J]. Aging, 2020, 12(13): 12943-12959. DOI:10.18632/aging.103378 |
| [42] |
XU Y, LI X, CHENG Y, et al. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion[J]. FASEB J, 2020, 34(12): 16262-16275. DOI:10.1096/fj.202001758R |
| [43] |
QUINLAN G J, EVANS T W, GUTTERIDGE J M C. Iron and the redox status of the lungs[J]. Free Radic Biol Med, 2002, 33(10): 1306-1313. DOI:10.1016/S0891-5849(02)00903-6 |
| [44] |
ALI M K, KIM R Y, KARIM R, et al. Role of iron in the pathogenesis of respiratory disease[J]. Int J Biochem Cell Biol, 2017, 88: 181-195. DOI:10.1016/j.biocel.2017.05.003 |
| [45] |
HUPPERT L A, MATTHAY M A, WARE L B. Pathogenesis of acute respiratory distress syndrome[J]. Semin Respir Crit Care Med, 2019, 40(1): 31-39. DOI:10.1055/s-0039-1683996 |
| [46] |
HOROWITZ R I, FREEMAN P R, BRUZZESE J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases[J]. Respir Med Case Rep, 2020, 30: 101063. DOI:10.1016/j.rmcr.2020.101063 |
| [47] |
PACHT E R, TIMERMAN A P, LYKENS M G, et al. Deficiency of alveolar fluid glutathione in patients with sepsis and the adult respiratory distress syndrome[J]. Chest, 1991, 100(5): 1397-1403. DOI:10.1378/chest.100.5.1397 |
| [48] |
AGGARWAL S, DIMITROPOULOU C, LU Q, et al. Glutathione supplementation attenuates lipopolysaccharide-induced mitochondrial dysfunction and apoptosis in a mouse model of acute lung injury[J]. Front Physio, 2012, 3: 161. DOI:10.3389/fphys.2012.00161 |
| [49] |
KELLNER M, NOONEPALLE S, LU Q, et al. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)[J]. Adv Exp Med Biol, 2017, 967: 105-137. DOI:10.1007/978-3-319-63245-2_8 |
 2024, Vol. 45
2024, Vol. 45


