阿尔茨海默病(Alzheimer’s disease,AD)是一种常见的神经退行性疾病,对社会和众多家庭造成极大负担。国际AD组织估计2018年全球痴呆症患者约有5 000万人,到2050年将增至3倍[1]。截至2019年的数据显示,我国60岁以上人群中的痴呆症患病率为6.2%,患者数量超过1 500万人[2]。由于该病的隐匿性,当患者出现较为明显的症状时已处于疾病的中晚期阶段。目前AD的药物治疗仅能改善症状,无法逆转病程,且由于AD的病理生理机制尚未完全阐明,针对目前致病假说的疾病修饰药物研发进展缓慢。本文聚焦于近年来AD诊断标准的演变与发展、经典和新晋的AD生物标志物及目前药物研发的新方向和新进展,讨论基于生物标志物的AD早期精准诊断和针对新机制的药物研发面临的挑战和前景。
1 AD诊断标准的演变和发展自1984年由美国国立神经病学、语言障碍和卒中研究所-阿尔茨海默病及相关疾病学会(National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association,NINCDS-ADRDA)首次提出国际公认的AD诊断标准[3]以来,随着检测手段和对疾病认识的进步,AD的诊断标准经历了数次修订。
2007年发布的科研用国际工作组(International Working Group,IWG)标准将AD分为临床前期、前驱期、痴呆期3个阶段,并首次将AD生物学标志物纳入诊断标准[4],推动了AD的早期诊断。
2011年美国国立老化研究所-阿尔茨海默病学会(National Institute on Aging-Alzheimer’s Association,NIA-AA)发布的标准亦将生物标志物纳入诊断标准,并分为反映β淀粉样蛋白(amyloid β protein,Aβ)积聚的标志物和反映神经元变性或损伤的标志物,另外将AD病程分为AD临床前期、AD源性轻度认知功能障碍(mild cognitive impairment,MCI)、AD痴呆3个阶段[5-6]。这一标准的提出极大地推动了临床和科研中AD的早期诊断。
2014年发布的科研用IWG-2标准简化了AD诊断流程,明确指出有AD典型情境记忆损害和AD相关生物标志物即可诊断,亦主张联合多个生物标志物对AD痴呆进行预测,并重新将AD的生物标志物划分为诊断性标志物和进展性标志物[7]。这一标准明确了不同标志物的使用时机及对诊断和研究的意义,提升了生物标志物诊断的特异性。
2018年NIA-AA提出了著名的ATN框架,这一框架由Aβ、Tau蛋白和神经变性3类标志物组成,即便没有认知障碍的症状,只要出现Aβ和Tau蛋白阳性也被定义为AD[8](表 1)。随着对AD病理生理机制认识的加深,在此框架基础上衍生出了ATX(N)框架,其中“X”代表新纳入的生物标志物类别,如整合血管、炎症、突触等不同类别的生物标志物创建的ATV(N)、ATI(N)和ATS(N)框架,这为AD诊断标准的后续发展指明了方向[9]。
|
|
表 1 AD诊断的ATN框架 Tab 1 ATN framework of AD diagnosis |
2 基于生物标志物的AD精准诊断
在不依靠生物标志物的情况下,临床诊断为AD的痴呆患者中有10%~30%不存在AD病理学改变[尸检、正电子发射断层显像(positron emission tomography,PET)或脑脊液检测][10]。2018年发布的ABIDE研究结果显示,生物标志物检测可降低临床诊断MCI患者约37%误诊率和24%漏诊率,以及降低AD痴呆诊断22%误诊率和33%漏诊率[11]。Aβ的异常沉积和Tau蛋白的异常磷酸化是AD最特征的生物标志物,此外,近年来也不断开发出反映AD病理过程中神经炎症、神经变性、突触功能障碍及其他机制的生物标志物(图 1)。
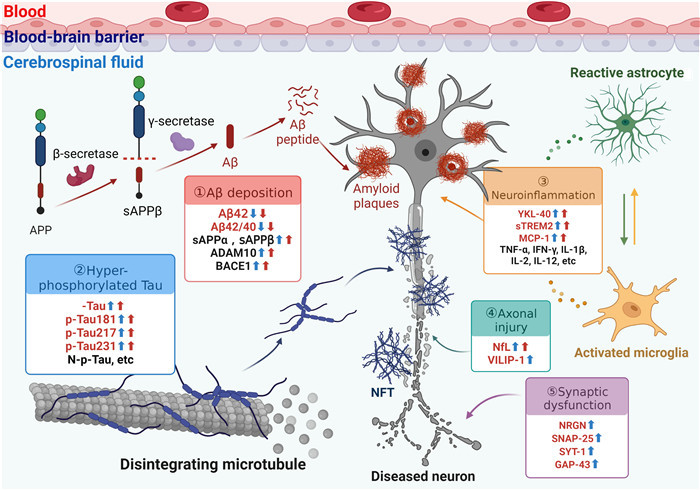
|
图 1 AD致病机制及相应体液生物标志物 Fig 1 Pathogenic mechanism of AD and corresponding fluid biomarkers AD: Alzheimer's disease; APP: Amyloid precursor protein; sAPP: Soluble amyloid precursor protein; Aβ: Amyloid β protein; ADAM10: A disintegrin and metalloproteinase 10; BACE1: β-site amyloid precursor protein cleaving enzyme 1; t-Tau: Total Tau; p-Tau: Phosphorylated Tau; N-p-Tau: N-terminal phosphorylated Tau; NFT: Neurofibrillary tangle; sTREM2: Soluble triggering receptor expressed on myeloid cells 2; MCP-1: Monocyte chemoattractant protein 1; TNF-α: Tumor necrosis factor α; IFN-γ: Interferon γ; IL: Interleukin; NfL: Neurofilament light chain; VILIP-1: Visinin like protein 1; NRGN: Neurogranin; SNAP-25: Synaptosomal associated protein 25; SYT-1: Synaptotagmin 1; GAP-43: Growth-associated protein 43. |
2.1 AD影像学生物标志物 2.1.1 PET
PET是一种分子成像技术,通过利用不同的示踪剂定位、定量地反映脑代谢功能和AD特征性病理蛋白沉积的改变,明确AD的诊断和分期。目前用于诊断AD的示踪剂主要有3类:(1)Aβ结合物,包括18F-florbetapir(18F-AV-45)、18F-florbetaben、18F-flutemetamol;(2)葡萄糖类似物,如18F-氟代脱氧葡萄糖(18F-fluoro-2-deoxyglucose,18F-FDG);(3)Tau蛋白结合物,如18F-flortaucipir,另有第2代Tau示踪剂18F-MK-6240正被广泛研究[12]。
Aβ-PET能够直观显示并在早期就检测到脑内不同区域尤其是颞叶基底部和额叶内侧部的Aβ病理性积聚,其与脑脊液Aβ42水平呈密切负相关[13-15]。虽然Aβ-PET不能用来区分AD亚型或临床表型[16],但能够以更高的灵敏度对Aβ积聚进行分期和监测,是目前诊断临床前AD患者的主要影像依据。
18F-FDG-PET可直观体现脑内葡萄糖代谢水平,间接反映神经元功能受损情况,能够先于颞顶和海马区域的萎缩显示出相应区域的代谢减低这一AD的特征性改变,有助于区分AD和认知正常者[17]。18F-FDG-PET测定的代谢减低程度和范围与AD认知障碍的总体严重程度大致相关,与Aβ-PET结合应用能更好地对Aβ阳性患者进行疾病分期分型及临床结局预测[18-19]。
Tau-PET可反映脑内Tau蛋白沉积的病理程度及空间分布,其示踪剂的区域积累常见于颞叶、顶叶等皮质中,与氟代脱氧葡萄糖低代谢有密切的对应关系[20-21]。来自瑞典的BioFINDER研究显示,Tau-PET在检测AD的皮质体积变化和早期认知障碍方面比Aβ-PET具有更高的灵敏度[22],并能够高度预测后续的认知能力下降和脑区萎缩[21],还可作为鉴别AD与其他神经退行性疾病Tau病理的一种强力生物标志物[17]。
针对神经炎症反应激活小胶质细胞这一机制,开发出了以分子量为18 000的转运蛋白(translocator protein,TSPO)为靶标的PET成像技术。相比健康人群,AD患者的TSPO表达显著上调,且其活化的小胶质细胞在AD早期即出现[23]。一项meta分析也表明TSPO-PET所显示的神经炎症增加与MCI及AD的进展相关[24]。因此,该技术也有望在AD临床病程中发挥早期诊断作用。
另外,结合使用Aβ-PET和Tau-PET能大大增强对AD病程进展的追踪[1],这提示利用不同类别生物标志物的多模态神经成像技术如双相Aβ-Tau-PET成像、混合PET-MRI及人工智能分析来早期、精确诊断AD是未来的重要研究方向。
2.1.2 MRI结构MRI通常被用来首先排除其他认知障碍疾病及疑似AD患者的临床评估。结构MRI能够清楚地显示内侧颞叶结构包括海马和内嗅皮质的萎缩,这是AD中最早、最突出的MRI特征,也有助于预测MCI向AD的进展[25],并且可以通过测量海马体积减少程度来区分AD与路易体痴呆(dementia with Lewy bodies,DLB)、帕金森病痴呆(Parkinson disease with dementia,PDD)[26]。
功能MRI通过测量脑局部血流量和容积变化的血氧水平依赖性信号来反映大脑神经元动态活动的变化。在任务态功能MRI中,与认知正常者相比,AD患者在完成记忆任务时缺失了海马或其他内侧颞叶结构的激活,却存在顶叶和后扣带回等区域编码期的代偿性激活现象[27],MCI患者在回忆过程中也表现出与AD患者相似的海马失活[25, 28]。目前任务态功能MRI的AD/MCI测试范式已拓展到视空间能力、注意力、语义知识和运动表现等方面[29-30]。默认模式网络(default mode network,DMN)是静息状态下仍有较强自发性神经活动的脑区集合网络。静息态功能MRI研究显示在AD和MCI患者的DMN中涉及记忆和信息处理相关的功能连接降低[31],在晚期AD患者中所有DMN区域的连通性均明显下降[32]。这意味着通过任务态或静息态fMRI指标能够区分AD/MCI和正常人群。
MRI弥散张量成像(diffusion tensor imaging,DTI)通过测量水分子的运动来量化评估白质的完整性及受损程度,主要指标包括平均弥散率(mean diffusion ratio,MD)和各向异性分数(fractional anisotropy,FA)[33-34]。一项meta分析显示,在AD中额颞顶枕区MD增加,在顶叶、内囊以外的白质区域FA降低;而在MCI中,仅有额枕区MD不增加,在顶枕叶以外的白质中FA均降低[35]。DTI指标也有助于区分AD和其他痴呆症患者,与AD相比,额颞叶痴呆的额区FA减少;与DLB相比,AD的颞顶区MD增加[36-37]。然而,由于扫描时间长,经常出现运动伪影,DTI在临床中的应用受到限制。
另外,磁共振波谱成像、动脉自旋标记等先进MRI技术有机会应用于AD/MCI的临床诊断及鉴别,但还需依赖相关影像技术的进一步发展[25]。
2.2 AD体液生物标志物 2.2.1 AD病理特异性脑脊液和血浆标志物脑脊液可以间接反映脑实质中的生物化学改变,是AD诊断中一个理想的生物标志物来源。而基于血液的生物标志物检测技术及新靶点因其便利性和适用性也在蓬勃发展。目前能够反映AD病理改变的主要生物标志物包括Aβ42、总Tau蛋白(total Tau,t-Tau)、磷酸化Tau蛋白(phosphorylated Tau,p-Tau)。脑脊液Aβ42降低(或Aβ42/Aβ40比值降低)和p-Tau(p-Tau181)升高是目前被认为最有效并被广泛接受的AD体液标志物[38-40],已被许多欧美国家用于临床。
Aβ是由淀粉样前体蛋白(amyloid precursor protein,APP)在β-分泌酶[又称β-淀粉样前体蛋白裂解酶1(β-site amyloid precursor protein cleaving enzyme 1,BACE1)]和γ-分泌酶的作用下不断裂解形成的多肽,人体内最常见的亚型是Aβ42和Aβ40。目前普遍认为脑脊液Aβ42在AD/MCI中减少并在AD痴呆期进一步降低,而Aβ40保持不变或减少,Aβ42/Aβ40比值可以预测AD[1, 15]。因脑脊液中Aβ42水平存在个体差异,单独使用很难起到明确诊断的作用,使用Aβ42/Aβ40比值或Aβ42/p-Tau比值能够更好地做出诊断[41-42]及预测MCI向AD的进展[43],还能够更好地区分AD与PDD、DLB[44]。血浆Aβ42和Aβ42/Aβ40比值也有可能用于筛查认知正常个体的淀粉样变性,但目前尚需更多的研究来证实[45-46]。
近年来,在APP裂解这一途径上又出现了众多新兴标志物。可溶性淀粉样蛋白前体蛋白(soluble amyloid precursor protein,sAPP)α和sAPPβ都是APP的裂解产物,在MCI和AD患者的脑脊液中升高,预测AD的准确度分别为0.653和0.778[47]。解整合素金属蛋白酶10(a disintegrin and metalloproteinase 10,ADAM10)是一种α-分泌酶,能够水解APP,阻止Aβ的产生并促进sAPPα的产生,而sAPPα具有神经保护作用[48]。ADAM10水平在MCI/AD患者的脑脊液、血液中均有升高[49-50]。BACE1水平在MCI/AD患者的血浆和脑脊液中升高,并且与神经变性和区域性脑萎缩的多个标志物相关[50-52]。
Tau蛋白是一种主要分布在神经元轴突上的微管相关蛋白。过度磷酸化会降低Tau与微管的亲和力,并且增加Tau的聚集和纤维化,这是形成AD神经纤维缠结的主要过程。脑脊液t-Tau的升高反映了神经元变性的程度,也可见于多系统萎缩、克雅氏病等其他神经变性疾病中,而脑脊液p-Tau仅在AD中升高[53]。在ATN框架中,p-Tau被认为是AD的特异性标志物,而t-Tau则被认为是神经变性的一般标志物。多数研究表明,脑脊液t-Tau/p-Tau在AD的所有阶段中都会升高,而在AD早期即趋于稳定,对AD的分期没有指导意义[54-57]。血浆t-Tau在MCI中增加,可以预测MCI向痴呆转化的风险,并提高AD诊断的准确性[58-60]。脑脊液和血浆Aβ42/t-Tau比值下降亦被认为是MCI进展为AD的潜在预测因子[61-62]。
在不同特定位置磷酸化的Tau蛋白可以更好地用来预测Tau病理的存在。(1)p-Tau181:脑脊液p-Tau181在AD中显著增加,而在其他大多数神经退行性疾病中并不显著增加[55, 63],这支持将p-Tau181作为AD特异性标志物使用。亦有研究显示p-Tau181能较好地区分MCI/AD患者与认知健康人群[64]。血浆p-Tau181水平从临床前期持续增加到MCI和痴呆期,表明其也可能作为疾病进展分期的标志物[65]。(2)p-Tau217:p-Tau217被认为是比p-Tau181更灵敏的标志物[66-67]。一项研究指出在AD这一连续过程中,脑脊液p-Tau217较其他p-Tau增加更明显,能以更高准确度鉴别AD与非AD并能与Aβ/Tau-PET相匹配[68]。血浆中p-Tau217从AD临床前期开始增加,在Tau-PET为阴性时就可以预测Aβ沉积,以区分AD患病风险的高低及鉴别AD与其他神经退行性疾病[69-70]。(3)p-Tau231:最近研究表明血浆p-Tau231与Aβ沉积有很强的关联,能够以更高的准确度区分AD与Aβ阴性的认知正常者、AD与非AD痴呆患者,并且在预测AD方面比p-Tau181更敏感[71-73]。
另外,氨基端Tau蛋白(N-terminal Tau,N-Tau)也是新近发现的生物标志物,其在AD患者的脑脊液中更为丰富,有可能将AD/MCI与对照组和其他痴呆症区分开来[74-77],包括N-Tau244、N-p-Tau181和N-p-Tau217等。在AD进程中,脑脊液N-p-Tau181和N-p-Tau217的升高比p-Tau181更早[77-78]。外周血浆中的N-Tau也对MCI/AD有预测作用[79]。
2.2.2 反映神经炎症的生物标志物在疾病早期,小胶质细胞和星形胶质细胞被激活并释放促炎因子,从而发生神经炎症反应,这与Aβ斑块和神经纤维缠结的形成密切相关[80-81]。YKL-40是一种潜在的炎症标志物,其在大脑中通过小胶质细胞和星形胶质细胞大量表达[82]。与认知正常者相比,MCI或更早期的AD患者脑脊液和血浆中YKL-40水平升高[83-84],尤其是脑脊液YKL-40水平与Tau病理有很强的相关性[85],反映了Tau病理相关神经变性的炎症过程。
髓细胞触发受体2(triggering receptor expressed on myeloid cells 2,TREM2)是一种跨膜糖蛋白免疫受体,由小胶质细胞表达,早期可能通过参与吞噬清除Aβ而发挥保护作用,在晚期却促进胶质细胞活化而加重AD进展[86]。TREM2在ADAM10和γ-分泌酶的共同作用下,于细胞外环境中产生能作为AD标志物的可溶性片段(soluble TREM2,sTREM2)[87]。脑脊液sTREM2水平在AD早期就有升高,并且与神经变性和Tau病理密切相关,亦随年龄增长而升高[88]。AD患者外周血中TREM2 mRNA和蛋白表达也增高[89]。
单核细胞趋化蛋白1(monocyte chemoattractant protein 1,MCP-1)是一种重要的促炎因子,由单核细胞、巨噬细胞等分泌。AD患者脑脊液和血浆中的MCP-1水平均高于健康对照组,其浓度会随AD进展而增加,并与简易智力状态检查量表评分降低相关,有可能用于AD进展的预测[90]。此外,许多研究观察到MCI/AD患者的TNF-α、干扰素γ、IL-12、IL-1β、IL-2等炎症因子水平较健康人升高[91],它们也可能是潜在的AD外周生物标志物。
2.2.3 反映轴突损伤或神经元变性的生物标志物神经元变性是AD的关键病理机制之一,轴突损伤作为其典型病理过程,主要标志物包括神经丝轻链(neurofilament light chain,NfL)和视锥样蛋白1(visinin like protein 1,VILIP-1)。
NfL是轴突中主要的细胞骨架蛋白——神经丝蛋白的重要组成部分,有维持轴突及神经元形态稳定、保证信号传递的功能。生理条件下轴突会释放少量NfL,病理条件下其释放则显著增加。研究发现其在Aβ和Tau蛋白阳性个体的脑脊液中升高,且与Tau负荷和整体认知下降相关[92],也与区域脑容量的损失相关[93-94]。当血浆Aβ42/Aβ40比值、p-Tau217与血浆NfL联合使用时,相比联合其他非特异血浆标志物,该组合最能预测异常的脑脊液Aβ42/Aβ40比值[95-97],这预示着血浆NfL有在社区中作为AD筛查简便工具的潜力。
VILIP-1作为神经元中的钙离子传感器在大脑中广泛表达,当神经元受损时被释放到脑脊液中。与正常人相比,AD患者脑脊液中VILIP-1水平更高,并且进展为AD的MCI患者脑脊液VILIP-1水平明显高于稳定型MCI患者[98]。但关于血浆VILIP-1在AD中的作用,目前还缺乏足够的数据。
2.2.4 反映突触功能障碍的生物标志物突触功能障碍发生在AD病程的相对早期阶段,是AD患者认知能力下降的另一个关键病理机制[99-100]。反映突触完整性和可塑性的生物标志物可能有助于AD的早期检测、神经变性程度和预后分析。神经粒蛋白(neurogranin,NRGN)是一种潜在的突触后标志物,在AD/MCI患者的脑脊液中升高,与Aβ和Tau病理呈正相关[101-102],能预测MCI到AD的进展[103]。脑脊液NRGN的升高对AD的诊断具有高度特异性,可用于AD同其他神经退行性疾病的鉴别[102]。但NRGN在AD患者血浆中的浓度无明显变化[104]。
突触前蛋白也是突触的重要组成部分,主要包括突触体相关蛋白25(synaptosomal associated protein 25,SNAP-25)、突触蛋白1(synaptotagmin 1,SYT-1)和生长相关蛋白43(growth-associated protein 43,GAP-43)。与健康对照组相比,所有阶段AD患者脑脊液SNAP-25、SYT-1和GAP-43水平均升高[105-107],其中GAP-43具有AD特异性[106]。另外还有诸如神经元五聚蛋白2(neuronal pentraxin-2,NPTX2)、神经元五聚蛋白受体(neuronal pentraxin receptor,NPTXR)、双载蛋白(amphiphysin,AMPH)、β突触核蛋白(β-synuclein,SNCB)和分泌核素1(secretogranin 1,SCG1)等新的突触前蛋白正在研究中。NPTX2和NPTXR水平在Aβ阳性个体脑脊液中随病程进展而不断下降[108-109],NPTX2的高水平与认知能力加速恶化显著相关[110]。AD脑脊液中SNCB、AMPH和SCG1的水平均升高[109, 111-112],同时血液中SNCB的水平也有升高[113]。然而,基于血液的突触前生物标志物研究目前仍较少。
3 AD药物的研发 3.1 AD药物治疗现状迄今为止,AD的药物治疗仍主要依靠认知增强疗法,而近30年来美国FDA仅批准过5种认知增强药物,包括胆碱酯酶抑制剂他克林、多奈哌齐、卡巴拉汀、加兰他敏及N-甲基-D-天冬氨酸受体拮抗剂盐酸美金刚,其中他克林由于严重不良反应已被多国停用。自2016年以来尚未有认知增强疗法新药在全球范围内获批,且认知增强治疗只能部分改善患者症状,无法延缓病情进展,难以满足临床需求。
3.2 AD疾病修饰疗法现阶段大多数AD药物研发管线致力于疾病修饰疗法,而其理论均基于前述各致病机制假说,尤其是以Aβ和Tau这2个被普遍认可的学说开展。截至2022年1月25日,在全球范围内进行的172项AD临床试验中涉及到143种药物,其中疾病修饰疗法药物占83.2%(119种),认知增强疗法药物和治疗神经精神症状的药物仅分别占9.8%(14种)和7.0%(10种)。在全部疾病修饰疗法试验中靶点为Aβ的药物有20种(16.8%),靶点为Tau的药物有13种(10.9%);而在进入Ⅲ期临床试验的21种疾病修饰疗法药物中有6种(28.6%)作用于Aβ,其中又以单克隆抗体药物为主[114]。截至发稿前,正处于Ⅲ期临床研究的AD疾病修饰疗法药物见表 2。
|
|
表 2 目前正处于Ⅲ期临床试验的AD疾病修饰疗法药物(截至发稿前) Tab 2 Current disease-modifying therapy drugs for AD in phase Ⅲ clinical trials (at press time) |
3.2.1 针对Aβ的药物研发
以Aβ单体、寡聚体、原纤维、斑块等不同形式的Aβ为靶点,通过人源性单克隆抗体的免疫治疗增加Aβ的清除或减少其生成,是近年发展最快的AD治疗手段。但诸如bapineuzumab、gantenerumab、solanezumab、crenezumab等众多单克隆抗体药物均因疗效不佳或不良反应严重而在Ⅲ期临床试验中失败,严重挫伤了AD药物的研发势头。令人振奋的是,美国渤健公司研发的aducanumab(商品名为Aduhelm)在2021年6月被美国FDA批准用于治疗早期AD,虽仍有争议,但作为2003年以来美国FDA批准的首个治疗AD的新药,它的上市给AD的治疗带来一丝曙光。目前仍在开展Ⅲ期临床试验的抗Aβ单克隆抗体药物主要有aducanumab、lecanemab、donanemab等。
aducanumab的作用靶点包括Aβ寡聚体、纤维及斑块。在Ⅰ期临床试验中发现其能够降低前驱和轻度AD患者的Aβ斑块,延缓认知功能下降[115]。2014年开展的2项Ⅲ期临床研究ENGAGE和EMERGE虽因中期无效性分析显示无法达到研究主要终点而在2019年3月被宣布终止,但后续发现在EMERGE中剂量为10 mg/kg的aducanumab能够显著改善患者认知能力,在ENGAGE中持续接受10 mg/kg aducanumab治疗的部分患者认知能力也得到了提高[116]。目前aducanumab仍在进行第4阶段的确认研究ENVISION,以期望得到更多的疗效数据。
lecanemab是美国渤健公司与日本卫材公司联手开发的另一款针对Aβ的单克隆抗体药物,可选择性结合可溶性Aβ原纤维,阻止Aβ沉积[117]。Ⅱ期临床试验结果显示lecanemab(10 mg/kg、每2周1次)组用于评估AD的各量表评分均明显下降并伴有显著的Aβ清除及标志物的变化,且安全性良好[118]。一项为期18个月的多中心、双盲、安慰剂对照、平行组设计的Ⅲ期临床试验Clarity AD在2022年11月底公布结果,显示达到研究主要终点及所有关键次要终点,证明lecanemab能够有效减少Aβ聚集,延缓疾病进展[119]。2022年12月22日,中国国家药品监督管理局接收了一类新药仑卡奈单抗注射液(lecanemab)的上市申请,并于2023年2月28日纳入优先审评和批准程序,预计将缩短审评时间。
donanemab是美国礼来公司开发的一款靶向Aβ特定亚型N3pG-Aβ的单克隆抗体,N3pG-Aβ更容易聚集成斑块,donanemab的靶向结合能促进Aβ沉积的清除[120]。Ⅱ期临床试验结果显示,donanemab能快速清除Aβ沉积,并且显著降低患者血浆中p-Tau217的水平,延缓早期AD患者的认知和日常生活能力的衰退速度,达到了研究主要终点[121]。基于此,donanemab在2021年6月获得美国FDA突破性疗法认证。目前donanemab共有3项正在进行中的Ⅲ期临床试验,分别研究其治疗早期症状性/临床前AD的安全性和有效性以及其与aducanumab清除斑块能力的优劣。
3.2.2 针对Tau蛋白的药物研发在以Aβ为靶点的药物研发屡屡受挫的背景下,针对Tau蛋白的药物研发重新引起人们的关注。目前以Tau为靶点的新药研发多处在Ⅰ、Ⅱ期临床试验阶段,其药理机制包括Tau蛋白翻译后修饰调节剂、Tau蛋白聚集抑制剂、微管稳定剂、Tau蛋白清除或表达抑制剂、基于Tau的主被动免疫疗法[122-123]。现阶段最有希望上市的靶向Tau的新药为口服Tau蛋白聚集抑制剂甲磺酸氢甲硫堇(hydromethylthionine mesylate,HMTM/TRx0237),其Ⅲ期临床研究LUCIDITY于2022年10月6日公布的初步数据显示,HMTM能够部分改善MCI和轻中度AD患者的认知功能,并且延缓病情进展[124]。目前国内已获批开展LUCIDITY补充研究Trx-237-801,以进一步评估HMTM的安全性和有效性。另一种靶向Tau的单克隆抗体E2814[125]也在去年开启Ⅱ/Ⅲ期临床试验Tau NexGen研究,旨在明确其在显性遗传性AD中的作用。
3.2.3 针对其他靶点的药物研发基于对衰老和AD发病机制更深入的理解,现有的研发管线已不再完全专注于Aβ和Tau,而是针对多样的创新靶点。例如针对减轻神经炎症这一途径开发的药物NE3107,作为一种细胞外调节蛋白激酶(extracellular signal-regulated kinase,ERK)小分子抑制剂,它可以选择性地抑制ERK、NF-κB和TNF刺激的全身性炎症,Ⅱ期临床试验数据显示接受NE3107治疗的AD患者在认知和生物标志物水平方面得到改善,目前已进入Ⅲ期临床试验[126]。另外,由我国上海绿谷制药有限公司自主研发的针对脑肠轴这一机制,通过调节肠道微生物从而减轻脑部神经炎症以改善认知障碍等AD症状的甘露特纳胶囊(GV-971)在国内完成Ⅲ期临床试验[127-128]后,于2019年在我国获批有条件上市,并于2020年10月开启国际多中心Ⅲ期临床试验,但因疫情及资金问题目前该研究已停止。
AGB101为针对神经保护途径的代表性药物,其本质是一款低剂量的抗癫痫药物左乙拉西坦的缓释配方,早期研究发现AD患者出现失智症状之前会出现海马体神经过度活跃,而AGB101能够让大脑的神经活动恢复正常,目前正在MCI人群中开展Ⅲ期临床试验[129]。
同样处于Ⅲ期临床试验的针对线粒体和能量代谢途径的抗AD药物有tricaprilin、semaglutide等。tricaprilin是一款中链甘油三酯的高纯度口服制剂,它通过诱导酮症状态来改善线粒体代谢,目标人群为轻中度AD患者[130];semaglutide是一款人胰高血糖素样肽1受体激动剂的口服制剂,原本用于2型糖尿病患者,分析其3项大型心血管结局试验的数据发现,使用semaglutide的2型糖尿病患者的痴呆发生率较对照组显著降低,目前正针对早期AD患者开展研究以评估其安全及有效性[131]。这类药物均通过改善脑内能量代谢来改善AD患者的认知功能。
此外,还有众多针对不同治疗机制的新型药物正在进行Ⅰ/Ⅱ期临床试验。利用间充质干细胞来源的细胞外囊泡包裹治疗药物以实现抗AD药物靶向递送[132]、利用基于纳米颗粒的抗衰老治疗[133]等新兴治疗手段也正蓬勃发展。
4 挑战和展望现阶段对于AD精准诊断和药物研发的焦点分别在于早筛诊断和疾病修饰治疗。目前针对早筛诊断的主要手段包括脑脊液Aβ和Tau水平检测及能直观反映病理变化的PET、MRI影像学检查,但这些方法因其高侵入性、高成本、接受程度低而在早筛诊断中受到限制。因此,开发基于外周体液的早筛标志物是更为理想的选择,其关键在于找到能够于外周表达、不受血脑屏障限制的外周体液生物标志物,并且符合AD早筛诊断的灵敏度和特异度要求。针对疾病修饰疗法的药物研发,处于研究后期的药物多基于Aβ沉积、Tau聚集、神经炎症、突触可塑性等理论假说,其中最受瞩目的当属以Aβ肽段为靶点的单克隆抗体药物,但近年来投入巨大时间和经济成本的Aβ单克隆抗体研发却屡屡受挫、收效甚微。究其根本,AD是病理生理机制未完全阐明的复杂疾病,药物研发可能靶向了错误的病理生理学机制;其二,在研究早期为减少不良反应而降低剂量,使得后期研究中的选定剂量低于有效剂量,或是在设计时未能选择合适的目标人群以及合适的试验终点,导致后期试验失败。但随着对AD发病机制的更深入理解,未来有望开发出基于外周体液的更简便、准确、灵敏的AD辅助诊断方法,助力AD药物研发,而一些新兴治疗技术如干细胞治疗也将为AD患者带来新的希望(图 2)。
|
图 2 AD早筛诊断和药物研发的未来展望 Fig 2 Future prospects of early diagnosis and drug development for AD AD: Alzheimer's disease; MSC-EV: Mesenchymal stem cell-derived extracellular vesicle; BBB: Blood-brain barrier. |
| [1] |
SCHELTENS P, DE STROOPER B, KIVIPELTO M, et al. Alzheimer's disease[J]. Lancet, 2021, 397(10284): 1577-1590. DOI:10.1016/s0140-6736(20)32205-4 |
| [2] |
WANG Q, GAO F, DAI L N, et al. Clinical research investigating Alzheimer's disease in China: current status and future perspectives toward prevention[J]. J Prev Alzheimers Dis, 2022, 9(3): 532-541. DOI:10.14283/jpad.2022.46 |
| [3] |
MCKHANN G, DRACHMAN D, FOLSTEIN M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease[J]. Neurology, 1984, 34(7): 939-944. DOI:10.1212/wnl.34.7.939 |
| [4] |
DUBOIS B, FELDMAN H H, JACOVA C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria[J]. Lancet Neurol, 2007, 6(8): 734-746. DOI:10.1016/S1474-4422(07)70178-3 |
| [5] |
MCKHANN G M, KNOPMAN D S, CHERTKOW H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease[J]. Alzheimers Dement, 2011, 7(3): 263-269. DOI:10.1016/j.jalz.2011.03.005 |
| [6] |
ALBERT M S, DEKOSKY S T, DICKSON D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease[J]. Alzheimers Dement, 2011, 7(3): 270-279. DOI:10.1016/j.jalz.2011.03.008 |
| [7] |
DUBOIS B, FELDMAN H H, JACOVA C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria[J]. Lancet Neurol, 2014, 13(6): 614-629. DOI:10.1016/S1474-4422(14)70090-0 |
| [8] |
KHACHATURIAN A S, HAYDEN K M, MIELKE M M, et al. Future prospects and challenges for Alzheimer's disease drug development in the era of the NIA-AA Research Framework[J]. Alzheimers Dement, 2018, 14(4): 532-534. DOI:10.1016/j.jalz.2018.03.003 |
| [9] |
HAMPEL H, CUMMINGS J, BLENNOW K, et al. Developing the ATX(N) classification for use across the Alzheimer disease continuum[J]. Nat Rev Neurol, 2021, 17(9): 580-589. DOI:10.1038/s41582-021-00520-w |
| [10] |
NELSON P T, HEAD E, SCHMITT F A, et al. Alzheimer's disease is not brain aging: neuropathological, genetic, and epidemiological human studies[J]. Acta Neuropathol, 2011, 121(5): 571-587. DOI:10.1007/s00401-011-0826-y |
| [11] |
DE WILDE A, VAN DER FLIER W M, PELKMANS W, et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE Project[J]. JAMA Neurol, 2018, 75(9): 1062-1070. DOI:10.1001/jamaneurol.2018.1346 |
| [12] |
PASCOAL T A, THERRIAULT J, BENEDET A L, et al. 18F-MK-6240 PET for early and late detection of neurofibrillary tangles[J]. Brain, 2020, 143(9): 2818-2830. DOI:10.1093/brain/awaa180 |
| [13] |
LEUZY A, CHIOTIS K, HASSELBALCH S G, et al. Pittsburgh compound B imaging and cerebrospinal fluid amyloid-β in a multicentre European memory clinic study[J]. Brain, 2016, 139(Pt 9): 2540-2553. DOI:10.1093/brain/aww160 |
| [14] |
GROTHE M J, BARTHEL H, SEPULCRE J, et al. In vivo staging of regional amyloid deposition[J]. Neurology, 2017, 89(20): 2031-2038. DOI:10.1212/WNL.0000000000004643 |
| [15] |
KNOPMAN D S, AMIEVA H, PETERSEN R C, et al. Alzheimer disease[J]. Nat Rev Dis Primers, 2021, 7(1): 33. DOI:10.1038/s41572-021-00269-y |
| [16] |
SEPULCRE J, GROTHE M J, SABUNCU M, et al. Hierarchical organization of tau and amyloid deposits in the cerebral cortex[J]. JAMA Neurol, 2017, 74(7): 813-820. DOI:10.1001/jamaneurol.2017.0263 |
| [17] |
OSSENKOPPELE R, RABINOVICI G D, SMITH R, et al. Discriminative accuracy of[18F] flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders[J]. JAMA, 2018, 320(11): 1151-1162. DOI:10.1001/jama.2018.12917 |
| [18] |
IACCARINO L, SALA A, PERANI D. Alzheimer's Disease Neuroimaging Initiative Predicting long-term clinical stability in amyloid-positive subjects by FDG-PET[J]. Ann Clin Transl Neurol, 2019, 6(6): 1113-1120. DOI:10.1002/acn3.782 |
| [19] |
NESTOR P J, ALTOMARE D, FESTARI C, et al. Clinical utility of FDG-PET for the differential diagnosis among the main forms of dementia[J]. Eur J Nucl Med Mol Imaging, 2018, 45(9): 1509-1525. DOI:10.1007/s00259-018-4035-y |
| [20] |
LEUZY A, CHIOTIS K, LEMOINE L, et al. Tau PET imaging in neurodegenerative tauopathies-still a challenge[J]. Mol Psychiatry, 2019, 24(8): 1112-1134. DOI:10.1038/s41380-018-0342-8 |
| [21] |
LA JOIE R, VISANI A V, BAKER S L, et al. Prospective longitudinal atrophy in Alzheimer's disease correlates with the intensity and topography of baseline tau-PET[J]. Sci Transl Med, 2020, 12(524): eaau5732. DOI:10.1126/scitranslmed.aau5732 |
| [22] |
OSSENKOPPELE R, SMITH R, OHLSSON T, et al. Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease[J]. Neurology, 2019, 92(6): e601-e612.. DOI:10.1212/WNL.0000000000006875 |
| [23] |
ZHANG L, HU K, SHAO T, et al. Recent developments on PET radiotracers for TSPO and their applications in neuroimaging[J]. Acta Pharm Sin B, 2021, 11(2): 373-393. DOI:10.1016/j.apsb.2020.08.006 |
| [24] |
BRADBURN S, MURGATROYD C, RAY N. Neuroinflammation in mild cognitive impairment and Alzheimer's disease: a meta-analysis[J]. Ageing Res Rev, 2019, 50: 1-8. DOI:10.1016/j.arr.2019.01.002 |
| [25] |
CHANDRA A, DERVENOULAS G, POLITIS M, et al. Magnetic resonance imaging in Alzheimer's disease and mild cognitive impairment[J]. J Neurol, 2019, 266(6): 1293-1302. DOI:10.1007/s00415-018-9016-3 |
| [26] |
DELLI PIZZI S, FRANCIOTTI R, BUBBICO G, et al. Atrophy of hippocampal subfields and adjacent extrahippocampal structures in dementia with Lewy bodies and Alzheimer's disease[J]. Neurobiol Aging, 2016, 40: 103-109. DOI:10.1016/j.neurobiolaging.2016.01.010 |
| [27] |
SPERLING R A, BATES J F, CHUA E F, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease[J]. J Neurol Neurosurg Psychiatry, 2003, 74(1): 44-50. DOI:10.1136/jnnp.74.1.44 |
| [28] |
PETRELLA J R, WANG L, KRISHNAN S, et al. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging[J]. Radiology, 2007, 245(1): 224-235. DOI:10.1148/radiol.2451061847 |
| [29] |
VIDONI E D, THOMAS G P, HONEA R A, et al. Evidence of altered corticomotor system connectivity in early-stage Alzheimer's disease[J]. J Neurol Phys Ther, 2012, 36(1): 8-16. DOI:10.1097/NPT.0b013e3182462ea6 |
| [30] |
VAN DAM N T, SANO M, MITSIS E M, et al. Functional neural correlates of attentional deficits in amnestic mild cognitive impairment[J]. PLoS One, 2013, 8(1): e54035. DOI:10.1371/journal.pone.0054035 |
| [31] |
ZHU D C, MAJUMDAR S, KOROLEV I O, et al. Alzheimer's disease and amnestic mild cognitive impairment weaken connections within the default-mode network: a multi-modal imaging study[J]. J Alzheimers Dis, 2013, 34(4): 969-984. DOI:10.3233/jad-121879 |
| [32] |
YU E, LIAO Z, MAO D, et al. Directed functional connectivity of posterior cingulate cortex and whole brain in Alzheimer's disease and mild cognitive impairment[J]. Curr Alzheimer Res, 2017, 14(6): 628-635. DOI:10.2174/1567205013666161201201000 |
| [33] |
MORI S, ZHANG J. Principles of diffusion tensor imaging and its applications to basic neuroscience research[J]. Neuron, 2006, 51(5): 527-539. DOI:10.1016/j.neuron.2006.08.012 |
| [34] |
MADDEN D J, BENNETT I J, BURZYNSKA A, et al. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging[J]. Biochim Biophys Acta, 2012, 1822(3): 386-400. DOI:10.1016/j.bbadis.2011.08.003 |
| [35] |
SEXTON C E, KALU U G, FILIPPINI N, et al. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease[J]. Neurobiol Aging, 2011, 32(12): 2322. e5-e18. DOI:10.1016/j.neurobiolaging.2010.05.019 |
| [36] |
ZHANG Y, SCHUFF N, DU A T, et al. White matter damage in frontotemporal dementia and Alzheimer's disease measured by diffusion MRI[J]. Brain, 2009, 132(Pt 9): 2579-2592. DOI:10.1093/brain/awp071 |
| [37] |
FIRBANK M J, WATSON R, MAK E, et al. Longitudinal diffusion tensor imaging in dementia with Lewy bodies and Alzheimer's disease[J]. Park Relat Disord, 2016, 24: 76-80. DOI:10.1016/j.parkreldis.2016.01.003 |
| [38] |
SHAW L M, ARIAS J, BLENNOW K, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer's disease[J]. Alzheimers Dement, 2018, 14(11): 1505-1521. DOI:10.1016/j.jalz.2018.07.220 |
| [39] |
MOLINUEVO J L, AYTON S, BATRLA R, et al. Current state of Alzheimer's fluid biomarkers[J]. Acta Neuropathol, 2018, 136(6): 821-853. DOI:10.1007/s00401-018-1932-x |
| [40] |
DUBOIS B, VILLAIN N, FRISONI G B, et al. Clinical diagnosis of Alzheimer's disease: recommendations of the International Working Group[J]. Lancet Neurol, 2021, 20(6): 484-496. DOI:10.1016/S1474-4422(21)00066-1 |
| [41] |
HANSSON O, LEHMANN S, OTTO M, et al. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer's disease[J]. Alzheimers Res Ther, 2019, 11(1): 34. DOI:10.1186/s13195-019-0485-0 |
| [42] |
AMFT M, ORTNER M, EICHENLAUB U, et al. The cerebrospinal fluid biomarker ratio Aβ42/40 identifies amyloid positron emission tomography positivity better than Aβ42 alone in a heterogeneous memory clinic cohort[J]. Alzheimers Res Ther, 2022, 14(1): 60. DOI:10.1186/s13195-022-01003-w |
| [43] |
BALDEIRAS I, SANTANA I, LEITÃ O M J, et al. Addition of the Aβ42/40 ratio to the cerebrospinal fluid biomarker profile increases the predictive value for underlying Alzheimer's disease dementia in mild cognitive impairment[J]. Alzheimers Res Ther, 2018, 10(1): 33. DOI:10.1186/s13195-018-0362-2 |
| [44] |
JANELIDZE S, ZETTERBERG H, MATTSSON N, et al. CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease[J]. Ann Clin Transl Neurol, 2016, 3(3): 154-165. DOI:10.1002/acn3.274 |
| [45] |
KESHAVAN A, PANNEE J, KARIKARI T K, et al. Population-based blood screening for preclinical Alzheimer's disease in a British birth cohort at age 70[J]. Brain, 2021, 144(2): 434-449. DOI:10.1093/brain/awaa403 |
| [46] |
NI W, JAGUST W, WANG D. Multiplex mass spectrometry analysis of amyloid proteins in human plasma for Alzheimer's disease diagnosis[J]. J Proteome Res, 2021, 20(8): 4106-4112. DOI:10.1021/acs.jproteome.1c00424 |
| [47] |
ARAKI W, KANEMARU K, HATTORI K, et al. Soluble APP-α and APP-β in cerebrospinal fluid as potential biomarkers for differential diagnosis of mild cognitive impairment[J]. Aging Clin Exp Res, 2022, 34(2): 341-347. DOI:10.1007/s40520-021-01935-7 |
| [48] |
ENDRES K, DELLER T. Regulation of alpha-secretase ADAM10 in vitro and in vivo: genetic, epigenetic, and protein-based mechanisms[J]. Front Mol Neurosci, 2017, 10: 56. DOI:10.3389/fnmol.2017.00056 |
| [49] |
DE OLIVEIRA T R, ERBERELI C R, MANZINE P R, et al. Early diagnosis of Alzheimer's disease in blood using a disposable electrochemical microfluidic platform[J]. ACS Sens, 2020, 5(4): 1010-1019. DOI:10.1021/acssensors.9b02463 |
| [50] |
PEREIRA VATANABE I, PERON R, MANTELLATTO GRIGOLI M, et al. ADAM10 plasma and CSF levels are increased in mild Alzheimer's disease[J]. Int J Mol Sci, 2021, 22(5): 2416. DOI:10.3390/ijms22052416 |
| [51] |
SHEN Y, WANG H, SUN Q, et al. Increased plasma beta-secretase 1 may predict conversion to Alzheimer's disease dementia in individuals with mild cognitive impairment[J]. Biol Psychiatry, 2018, 83(5): 447-455. DOI:10.1016/j.biopsych.2017.02.007 |
| [52] |
VERGALLO A, LEMERCIER P, CAVEDO E, et al. Plasma β-secretase1 concentrations correlate with basal forebrain atrophy and neurodegeneration in cognitively healthy individuals at risk for AD[J]. Alzheimers Dement, 2021, 17(4): 629-640. DOI:10.1002/alz.12228 |
| [53] |
SEINO Y, NAKAMURA T, KAWARABAYASHI T, et al. Cerebrospinal fluid and plasma biomarkers in neurodegenerative diseases[J]. J Alzheimers Dis, 2019, 68(1): 395-404. DOI:10.3233/jad-181152 |
| [54] |
MATTSSON N, ZETTERBERG H, HANSSON O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment[J]. JAMA, 2009, 302(4): 385-393. DOI:10.1001/jama.2009.1064 |
| [55] |
SKILLBÄ CK T, FARAHMAND B Y, ROSÉN C, et al. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia[J]. Brain, 2015, 138(9): 2716-2731. DOI:10.1093/brain/awv181 |
| [56] |
FAGAN A M, XIONG C, JASIELEC M S, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer's disease[J]. Sci Transl Med, 2014, 6(226): 226ra30. DOI:10.1126/scitranslmed.3007901 |
| [57] |
MATTSSON N, SCHÖLL M, STRANDBERG O, et al. 18F-AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer's disease[J]. EMBO Mol Med, 2017, 9(9): 1212-1223. DOI:10.15252/emmm.201707809 |
| [58] |
CHEN Y R, LIANG C S, CHU H, et al. Diagnostic accuracy of blood biomarkers for Alzheimer's disease and amnestic mild cognitive impairment: a meta-analysis[J]. Ageing Res Rev, 2021, 71: 101446. DOI:10.1016/j.arr.2021.101446 |
| [59] |
FOSSATI S, RAMOS CEJUDO J, DEBURE L, et al. Plasma tau complements CSF tau and P-tau in the diagnosis of Alzheimer's disease[J]. Alzheimers Dement (Amst), 2019, 11(1): 483-492. DOI:10.1016/j.dadm.2019.05.001 |
| [60] |
PASE M P, BEISER A S, HIMALI J J, et al. Assessment of plasma total tau level as a predictive biomarker for dementia and related endophenotypes[J]. JAMA Neurol, 2019, 76(5): 598. DOI:10.1001/jamaneurol.2018.4666 |
| [61] |
PARK J C, HAN S H, YI D, et al. Plasma tau/amyloid-β1-42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer's disease[J]. Brain, 2019, 142(3): 771-786. DOI:10.1093/brain/awy347 |
| [62] |
CHEN T B, LEE Y J, LIN S Y, et al. Plasma Aβ42 and total tau predict cognitive decline in amnestic mild cognitive impairment[J]. Sci Rep, 2019, 9: 13984. DOI:10.1038/s41598-019-50315-9 |
| [63] |
OSSENKOPPELE R, VAN DER KANT R, HANSSON O. Tau biomarkers in Alzheimer's disease: towards implementation in clinical practice and trials[J]. Lancet Neurol, 2022, 21(8): 726-734. DOI:10.1016/S1474-4422(22)00168-5 |
| [64] |
PROVOST K, IACCARINO L, SOLEIMANI-MEIGOONI D N, et al. Comparing ATN-T designation by tau PET visual reads, tau PET quantification, and CSF PTau181 across three cohorts[J]. Eur J Nucl Med Mol Imaging, 2021, 48(7): 2259-2271. DOI:10.1007/s00259-020-05152-8 |
| [65] |
MOSCOSO A, GROTHE M J, ASHTON N J, et al. Time course of phosphorylated-tau181 in blood across the Alzheimer's disease spectrum[J]. Brain, 2021, 144(1): 325-339. DOI:10.1093/brain/awaa399 |
| [66] |
BARTHÉLEMY N R, LI Y, JOSEPH-MATHURIN N, et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer's disease[J]. Nat Med, 2020, 26(3): 398-407. DOI:10.1038/s41591-020-0781-z |
| [67] |
JANELIDZE S, STOMRUD E, SMITH R, et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer's disease[J]. Nat Commun, 2020, 11(1): 1683. DOI:10.1038/s41467-020-15436-0 |
| [68] |
LEUZY A, JANELIDZE S, MATTSSON-CARLGREN N, et al. Comparing the clinical utility and diagnostic performance of CSF p-Tau181, p-Tau217, and p-Tau231 assays[J]. Neurology, 2021, 97(17): e1681-e1694. DOI:10.1212/WNL.0000000000012727 |
| [69] |
JANELIDZE S, BERRON D, SMITH R, et al. Associations of plasma phospho-Tau217 levels with tau positron emission tomography in early Alzheimer disease[J]. JAMA Neurol, 2021, 78(2): 149-156. DOI:10.1001/jamaneurol.2020.4201 |
| [70] |
PALMQVIST S, JANELIDZE S, QUIROZ Y T, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders[J]. JAMA, 2020, 324(8): 772-781. DOI:10.1001/jama.2020.12134 |
| [71] |
MILÀ-ALOMÀ M, ASHTON N J, SHEKARI M, et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's disease[J]. Nat Med, 2022, 28(9): 1797-1801. DOI:10.1038/s41591-022-01925-w |
| [72] |
ASHTON N J, PASCOAL T A, KARIKARI T K, et al. Plasma p-tau231:a new biomarker for incipient Alzheimer's disease pathology[J]. Acta Neuropathol, 2021, 141(5): 709-724. DOI:10.1007/s00401-021-02275-6 |
| [73] |
ASHTON N J, JANELIDZE S, MATTSSON-CARLGREN N, et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer's trial selection and disease monitoring[J]. Nat Med, 2022, 28(12): 2555-2562. DOI:10.1038/s41591-022-02074-w |
| [74] |
CICOGNOLA C, HANSSON O, SCHELTENS P, et al. Cerebrospinal fluid N-224 tau helps discriminate Alzheimer's disease from subjective cognitive decline and other dementias[J]. Alzheimers Res Ther, 2021, 13(1): 38. DOI:10.1186/s13195-020-00756-6 |
| [75] |
CHEN Z, MENGEL D, KESHAVAN A, et al. Learnings about the complexity of extracellular tau aid development of a blood-based screen for Alzheimer's disease[J]. Alzheimers Dement, 2019, 15(3): 487-496. DOI:10.1016/j.jalz.2018.09.010 |
| [76] |
CICOGNOLA C, BRINKMALM G, WAHLGREN J, et al. Novel tau fragments in cerebrospinal fluid: relation to tangle pathology and cognitive decline in Alzheimer's disease[J]. Acta Neuropathol, 2019, 137(2): 279-296. DOI:10.1007/s00401-018-1948-2 |
| [77] |
SUÁ REZ-CALVET M, KARIKARI T K, ASHTON N J, et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer's continuum when only subtle changes in Aβ pathology are detected[J]. EMBO Mol Med, 2020, 12(12): e12921. DOI:10.15252/emmm.202012921 |
| [78] |
KARIKARI T K, EMERŠIČ A, VRILLON A, et al. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer's disease diagnosis[J]. Alzheimers Dement, 2021, 17(5): 755-767. DOI:10.1002/alz.12236 |
| [79] |
CHHATWAL J P, SCHULTZ A P, DANG Y, et al. Plasma N-terminal tau fragment levels predict future cognitive decline and neurodegeneration in healthy elderly individuals[J]. Nat Commun, 2020, 11(1): 6024. DOI:10.1038/s41467-020-19543-w |
| [80] |
CALSOLARO V, EDISON P. Neuroinflammation in Alzheimer's disease: current evidence and future directions[J]. Alzheimers Dement, 2016, 12(6): 719-732. DOI:10.1016/j.jalz.2016.02.010 |
| [81] |
HENEKA M T, KUMMER M P, LATZ E. Innate immune activation in neurodegenerative disease[J]. Nat Rev Immunol, 2014, 14(7): 463-477. DOI:10.1038/nri3705 |
| [82] |
CONNOLLY K, LEHOUX M, O'ROURKE R, et al. Potential role of chitinase-3-like protein 1(CHI3L1/YKL-40) in neurodegeneration and Alzheimer's disease[J]. Alzheimers Dement, 2023, 19(1): 9-24. DOI:10.1002/alz.12612 |
| [83] |
VILLAR-PIQUÉ A, SCHMITZ M, HERMANN P, et al. Plasma YKL-40 in the spectrum of neurodegenerative dementia[J]. J Neuroinflammation, 2019, 16(1): 145. DOI:10.1186/s12974-019-1531-3 |
| [84] |
DHIMAN K, BLENNOW K, ZETTERBERG H, et al. Cerebrospinal fluid biomarkers for understanding multiple aspects of Alzheimer's disease pathogenesis[J]. Cell Mol Life Sci, 2019, 76(10): 1833-1863. DOI:10.1007/s00018-019-03040-5 |
| [85] |
ALCOLEA D, VILAPLANA E, PEGUEROLES J, et al. Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer's disease[J]. Neurobiol Aging, 2015, 36(6): 2018-2023. DOI:10.1016/j.neurobiolaging.2015.03.001 |
| [86] |
ULRICH J D, ULLAND T K, COLONNA M, et al. Elucidating the role of TREM2 in Alzheimer's disease[J]. Neuron, 2017, 94(2): 237-248. DOI:10.1016/j.neuron.2017.02.042 |
| [87] |
EWERS M, FRANZMEIER N, SUÁ REZ-CALVET M, et al. Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer's disease[J]. Sci Transl Med, 2019, 11(507): eaav6221. DOI:10.1126/scitranslmed.aav6221 |
| [88] |
SUÁREZ-CALVET M, MORENAS-RODRÍGUEZ E, KLEINBERGER G, et al. Early increase of CSF sTREM2 in Alzheimer's disease is associated with tau related-neurodegeneration but not with amyloid-β pathology[J]. Mol Neurodegener, 2019, 14(1): 1-14. DOI:10.1186/s13024-018-0301-5 |
| [89] |
GUVEN G, BILGIC B, SAMANCI B, et al. Peripheral TREM2 mRNA levels in early and late-onset Alzheimer disease's patients[J]. Mol Biol Rep, 2020, 47(8): 5903-5909. DOI:10.1007/s11033-020-05661-7 |
| [90] |
LEE W J, LIAO Y C, WANG Y F, et al. Plasma MCP-1 and cognitive decline in patients with Alzheimer's disease and mild cognitive impairment: a two-year follow-up study[J]. Sci Rep, 2018, 8: 1280. DOI:10.1038/s41598-018-19807-y |
| [91] |
LAI K S P, LIU C S, RAU A, et al. Peripheral inflammatory markers in Alzheimer's disease: a systematic review and meta-analysis of 175 studies[J]. J Neurol Neurosurg Psychiatry, 2017, 88(10): 876-882. DOI:10.1136/jnnp-2017-316201 |
| [92] |
PEREIRA J B, JANELIDZE S, OSSENKOPPELE R, et al. Untangling the association of amyloid-β and tau with synaptic and axonal loss in Alzheimer's disease[J]. Brain, 2021, 144(1): 310-324. DOI:10.1093/brain/awaa395 |
| [93] |
SÁ NCHEZ-BENAVIDES G, SUÁ REZ-CALVET M, MILÀ-ALOMÀ M, et al. Amyloid-β positive individuals with subjective cognitive decline present increased CSF neurofilament light levels that relate to lower hippocampal volume[J]. Neurobiol Aging, 2021, 104: 24-31. DOI:10.1016/j.neurobiolaging.2021.02.026 |
| [94] |
BENEDET A L, LEUZY A, PASCOAL T A, et al. Stage-specific links between plasma neurofilament light and imaging biomarkers of Alzheimer's disease[J]. Brain, 2020, 143(12): 3793-3804. DOI:10.1093/brain/awaa342 |
| [95] |
MATTSSON N, ANDREASSON U, ZETTERBERG H, et al. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease[J]. JAMA Neurol, 2017, 74(5): 557-566. DOI:10.1001/jamaneurol.2016.6117 |
| [96] |
CULLEN N C, LEUZY A, JANELIDZE S, et al. Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations[J]. Nat Commun, 2021, 12(1): 3555. DOI:10.1038/s41467-021-23746-0 |
| [97] |
JANELIDZE S, PALMQVIST S, LEUZY A, et al. Detecting amyloid positivity in early Alzheimer's disease using combinations of plasma Aβ42/Aβ40 and p-tau[J]. Alzheimers Dement, 2022, 18(2): 283-293. DOI:10.1002/alz.12395 |
| [98] |
MAVROUDIS I A, PETRIDIS F, CHATZIKONSTANTINOU S, et al. A meta-analysis on the levels of VILIP-1 in the CSF of Alzheimer's disease compared to normal controls and other neurodegenerative conditions[J]. Aging Clin Exp Res, 2021, 33(2): 265-272. DOI:10.1007/s40520-019-01458-2 |
| [99] |
JACKSON J, JAMBRINA E, LI J, et al. Targeting the synapse in Alzheimer's disease[J]. Front Neurosci, 2019, 13: 735. DOI:10.3389/fnins.2019.00735 |
| [100] |
HONG S, BEJA-GLASSER V F, NFONOYIM B M, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models[J]. Science, 2016, 352(6286): 712-716. DOI:10.1126/science.aad8373 |
| [101] |
PEREIRA J B, WESTMAN E, HANSSON O. Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer's disease[J]. Neurobiol Aging, 2017, 58: 14-29. DOI:10.1016/j.neurobiolaging.2017.06.002 |
| [102] |
PORTELIUS E, OLSSON B, HÖGLUND K, et al. Cerebrospinal fluid neurogranin concentration in neurodegeneration: relation to clinical phenotypes and neuropathology[J]. Acta Neuropathol, 2018, 136(3): 363-376. DOI:10.1007/s00401-018-1851-x |
| [103] |
WELLINGTON H, PATERSON R W, PORTELIUS E, et al. Increased CSF neurogranin concentration is specific to Alzheimer disease[J]. Neurology, 2016, 86(9): 829-835. DOI:10.1212/WNL.0000000000002423 |
| [104] |
DE VOS A, JACOBS D, STRUYFS H, et al. C-terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer's disease[J]. Alzheimers Dement, 2015, 11(12): 1461-1469. DOI:10.1016/j.jalz.2015.05.012 |
| [105] |
JIA L, ZHU M, KONG C, et al. Blood neuro-exosomal synaptic proteins predict Alzheimer's disease at the asymptomatic stage[J]. Alzheimers Dement, 2021, 17(1): 49-60. DOI:10.1002/alz.12166 |
| [106] |
SANDELIUS Å, PORTELIUS E, KÄLLÉN Å, et al. Elevated CSF GAP-43 is Alzheimer's disease specific and associated with tau and amyloid pathology[J]. Alzheimers Dement, 2019, 15(1): 55-64. DOI:10.1016/j.jalz.2018.08.006 |
| [107] |
MILÀ-ALOMÀ M, BRINKMALM A, ASHTON N J, et al. CSF synaptic biomarkers in the preclinical stage of Alzheimer disease and their association with MRI and PET: a cross-sectional study[J]. Neurology, 2021, 97(21): e2065-e2078. DOI:10.1212/WNL.0000000000012853 |
| [108] |
LIM B, TSOLAKI M, SOOSAIPILLAI A, et al. Liquid biopsy of cerebrospinal fluid identifies neuronal pentraxin receptor (NPTXR) as a biomarker of progression of Alzheimer's disease[J]. Clin Chem Lab Med, 2019, 57(12): 1875-1881. DOI:10.1515/cclm-2019-0428 |
| [109] |
NILSSON J, COUSINS K A Q, GOBOM J, et al. Cerebrospinal fluid biomarker panel of synaptic dysfunction in Alzheimer's disease and other neurodegenerative disorders[J]. Alzheimers Dement, 2023, 19(5): 1775-1784. DOI:10.1002/alz.12809 |
| [110] |
LIBIGER O, SHAW L M, WATSON M H, et al. Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer's disease[J]. Alzheimers Dement, 2021, 17(12): 1976-1987. DOI:10.1002/alz.12353 |
| [111] |
BERGSTRÖM S, REMNESTÅ L J, YOUSEF J, et al. Multi-cohort profiling reveals elevated CSF levels of brain-enriched proteins in Alzheimer's disease[J]. Ann Clin Transl Neurol, 2021, 8(7): 1456-1470. DOI:10.1002/acn3.51402 |
| [112] |
PARK S A, JUNG J M, PARK J S, et al. SWATH-MS analysis of cerebrospinal fluid to generate a robust battery of biomarkers for Alzheimer's disease[J]. Sci Rep, 2020, 10(1): 7423. DOI:10.1038/s41598-020-64461-y |
| [113] |
OECKL P, HALBGEBAUER S, ANDERL-STRAUB S, et al. Targeted mass spectrometry suggests beta-synuclein as synaptic blood marker in Alzheimer's disease[J]. J Proteome Res, 2020, 19(3): 1310-1318. DOI:10.1021/acs.jproteome.9b00824 |
| [114] |
CUMMINGS J, LEE G, NAHED P, et al. Alzheimer's disease drug development pipeline: 2022[J]. Alzheimers Dement (N Y), 2022, 8(1): e12295. DOI:10.1002/trc2.12295 |
| [115] |
SEVIGNY J, PING C, BUSSIÈRE T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease[J]. Nature, 2016, 537(7618): 50-56. DOI:10.1038/nature19323 |
| [116] |
HAEBERLEIN S B, AISEN P S, BARKHOF F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease[J]. J Prev Alzheimers Dis, 2022, 9(2): 197-210. DOI:10.14283/jpad.2022.30 |
| [117] |
LOGOVINSKY V, SATLIN A, LAI R, et al. Safety and tolerability of BAN2401:a clinical study in Alzheimer's disease with a protofibril selective Aβ antibody[J]. Alzheimers Res Ther, 2016, 8(1): 14. DOI:10.1186/s13195-016-0181-2 |
| [118] |
SWANSON C J, ZHANG Y, DHADDA S, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Aβ protofibril antibody[J]. Alzheimers Res Ther, 2021, 13(1): 80. DOI:10.1186/s13195-021-00813-8 |
| [119] |
VAN DYCK C H, SWANSON C J, AISEN P, et al. Lecanemab in early Alzheimer's disease[J]. N Engl J Med, 2023, 388(1): 9-21. DOI:10.1056/nejmoa2212948 |
| [120] |
DEMATTOS R B, LU J, TANG Y, et al. A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer's disease mice[J]. Neuron, 2012, 76(5): 908-920. DOI:10.1016/j.neuron.2012.10.029 |
| [121] |
MINTUN M A, LO A C, DUGGAN EVANS C, et al. Donanemab in early Alzheimer's disease[J]. N Engl J Med, 2021, 384(18): 1691-1704. DOI:10.1056/NEJMoa2100708 |
| [122] |
GUO Y, LI S, ZENG L H, et al. Tau-targeting therapy in Alzheimer's disease: critical advances and future opportunities[J]. Ageing Neur Dis, 2022, 2(3): 11. DOI:10.20517/and.2022.16 |
| [123] |
CONGDON E E, SIGURDSSON E M. Tau-targeting therapies for Alzheimer disease[J]. Nat Rev Neurol, 2018, 14(7): 399-415. DOI:10.1038/s41582-018-0013-z |
| [124] |
WISCHIK C M, BENTHAM P, GAUTHIER S, et al. Oral Tau aggregation inhibitor for Alzheimer's disease: design, progress and basis for selection of the 16 mg/day dose in a phase 3, randomized, placebo-controlled trial of hydromethylthionine mesylate[J]. J Prev Alzheimers Dis, 2022, 9(4): 780-790. DOI:10.14283/jpad.2022.63 |
| [125] |
ROBERTS M, SEVASTOU I, IMAIZUMI Y, et al. Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer's disease[J]. Acta Neuropathol Commun, 2020, 8(1): 13. DOI:10.1186/s40478-020-0884-2 |
| [126] |
READING C L, AHLEM C N, MURPHY M F. NM101 phase Ⅲ study of NE3107 in Alzheimer's disease: rationale, design and therapeutic modulation of neuroinflammation and insulin resistance[J]. Neurodegener Dis Manag, 2021, 11(4): 289-298. DOI:10.2217/nmt-2021-0022 |
| [127] |
XIAO S, CHAN P, WANG T, et al. A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer's dementia[J]. Alzheimers Res Ther, 2021, 13(1): 62. DOI:10.1186/s13195-021-00795-7 |
| [128] |
WANG T, KUANG W, CHEN W, et al. A phase Ⅱrandomized trial of sodium oligomannate in Alzheimer's dementia[J]. Alzheimers Res Ther, 2020, 12(1): 110. DOI:10.1186/s13195-020-00678-3 |
| [129] |
ROSENZWEIG LIPSON S, BARTON R, GALLAGHER M, et al. HOPE4MCI trial: first trial targeting reduction of hippocampal overactivity to treat mild cognitive impairment due to Alzheimer's disease with AGB101[J]. Alzheimers Dement, 2021, 17(S9): e057813. DOI:10.1002/alz.057813 |
| [130] |
HENDERSON S T, MORIMOTO B H, CUMMINGS J L, et al. A placebo-controlled, parallel-group, randomized clinical trial of AC-1204 in mild-to-moderate Alzheimer's disease[J]. J Alzheimers Dis, 2020, 75(2): 547-557. DOI:10.3233/jad-191302 |
| [131] |
MAHAPATRA M K, KARUPPASAMY M, SAHOO B M. Therapeutic potential of semaglutide, a newer GLP-1 receptor agonist, in abating obesity, non-alcoholic steatohepatitis and neurodegenerative diseases: a narrative review[J]. Pharm Res, 2022, 39(6): 1233-1248. DOI:10.1007/s11095-022-03302-1 |
| [132] |
YIN T, LIU Y, JI W, et al. Engineered mesenchymal stem cell-derived extracellular vesicles: a state-of-the-art multifunctional weapon against Alzheimer's disease[J]. Theranostics, 2023, 13(4): 1264-1285. DOI:10.7150/thno.81860 |
| [133] |
CHU J J, JI W B, ZHUANG J H, et al. Nanoparticles-based anti-aging treatment of Alzheimer's disease[J]. Drug Deliv, 2022, 29(1): 2100-2116. DOI:10.1080/10717544.2022.2094501 |
 2023, Vol. 44
2023, Vol. 44


