2. 上海健康医学院附属周浦医院康复医学科, 上海 201318
2. Department of Rehabilitation Medicine, Zhoupu Hospital Affiliated to Shanghai University of Medicine & Health Sciences, Shanghai 201318, China
缺血性脑卒中是由脑血管阻塞引起的脑组织损伤和功能障碍,具有高发病率、高致残率和高病死率等特点[1]。脑血供恢复后会引发更严重的缺血再灌注损伤,其损伤机制与氨基酸毒性作用、自由基损伤、炎症反应和细胞凋亡等有关[2-3]。神经系统受损后,表达离子钙结合适配器分子1(ionized calcium-binding adaptor molecule 1,Iba-1)的小胶质细胞会释放大量促炎症因子,如TNF-α和IL-1β,引起神经炎症反应和细胞凋亡,加重神经元损伤[4-5]。
作为一种缺血预处理方法,丰富环境预处理是在损伤前将动物饲养在丰富环境中,增加其运动、感觉、社交刺激和自发性运动,诱导脑缺血耐受,从而产生脑保护作用[6]。前期研究证明丰富环境能够影响核因子E2相关因子2(nuclear factor erythroid 2-related factor 2,Nrf2)-抗氧化响应元件(antioxidant response element,ARE)、NF-κB通路和小胶质细胞激活,进而抑制脑缺血损伤后的炎症反应和细胞凋亡[7-9]。本研究建立了短暂性大脑中动脉栓塞(transient middle cerebral artery occlusion,tMCAO)大鼠模型,通过观察丰富环境预处理对模型大鼠神经功能和脑组织病理变化的影响,并检测梗死周围皮质TNF-α、IL-1β的表达及细胞凋亡情况,探讨丰富环境预处理对缺血再灌注损伤大鼠的脑保护作用及可能机制。
1 材料和方法 1.1 实验动物及分组清洁级SD雄性成年大鼠45只,体重(140±20)g,购于上海中医药大学实验动物中心[动物生产许可证号为SCXK(沪)2017-0005]。所有大鼠称重编号,适应性饲养3 d,按随机数字表法分为假手术组、模型组和丰富环境预处理组,每组15只。
1.2 主要试剂与仪器尼龙线栓(深圳瑞沃德生命科技有限公司),激光多普勒血流仪(英国Devon公司),凝胶电泳仪(美国Bio-Rad公司),共聚焦显微镜(德国Leica公司);TUNEL检测试剂盒(瑞士Roche公司);Iba-1抗体、TNF-α抗体和β肌动蛋白抗体(货号分别为ab178847、ab205587和ab8226,英国Abcam公司),IL-1β抗体(货号为12703,美国Cell Signaling Technology公司)。
1.3 标准环境和丰富环境的搭建假手术组和模型组大鼠置于标准环境笼中饲养,丰富环境预处理组大鼠置于丰富环境笼中饲养,3组大鼠均全天干预,共21 d。2种饲养环境均提供12 h/12 h昼夜光照,室温(23±2)℃,环境相对湿度56%。大鼠自由进食、进水。
参照文献[8]设置丰富环境笼(图 1),长80 cm、宽50 cm、高30 cm,笼中放置爬梯、迷宫、管道、爬梯、塑料玩偶等,每笼饲养7只大鼠。每3 d更换1次笼内物品的摆放位置,每周更换1次笼内物品。

|
图 1 大鼠丰富环境饲养笼 Fig 1 Enriched environment cage for rat feeding |
标准环境鼠笼长40 cm、宽30 cm、高20 cm,仅放置垫料、饲料和水,每笼饲养5只大鼠。
1.4 模型制备与评估模型组和丰富环境预处理组大鼠参照改良Longa线栓法[10]建立tMCAO模型,步骤如下:大鼠乙醚吸入麻醉后,依次钝性分离出右侧颈总动脉、颈外动脉及颈内动脉;结扎颈总动脉近心端,用动脉夹暂时夹闭颈内动脉起始处,从颈总动脉插入线栓到大脑中动脉起始处(感觉到有轻微阻力);缺血90 min后,再次麻醉大鼠,拔出线栓,缝合手术切口。假手术组大鼠无插入线栓步骤,其余操作相同。
用随机数字表法从每组取3只大鼠,麻醉后俯卧位固定,沿头皮矢状面剪开额顶处皮肤2.5 cm,用棉签摩擦暴露的筋膜。以冠状缝与矢状缝结合点为原点,冠状缝向后5 mm、矢状缝向右3 mm,使用牙科钻将该区域颅骨磨薄,以穿透颅骨但未打破硬脑膜为宜[11]。固定激光多普勒血流仪探头,记录右侧大脑中动脉供血区域血流值。每只大鼠分别于术前、插入线栓后、术后做3次脑血流检测,以插入线栓后脑血流降至术前60%以下为造模成功。用于检测脑血流的3只大鼠不纳入后续实验。
其余大鼠苏醒后用Longa评分法[10]判定造模效果,评分为1~3分的大鼠为造模成功,纳入实验。
1.5 大鼠神经功能评估分别于造模后1、2、3 d由不知晓实验分组情况的研究人员进行改良神经功能缺损评分(modified neurological severity score,mNSS)评估各组大鼠的神经功能,mNSS量表包括运动、感觉、反射和平衡4个方面,得分范围为0~18分,分数越高表示神经损伤越严重。
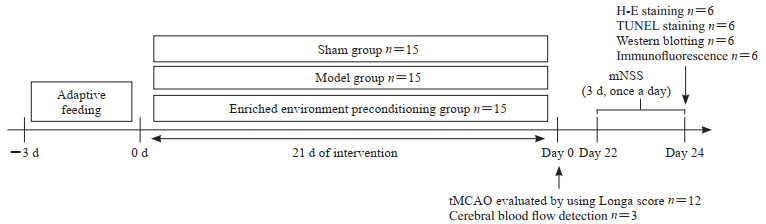
1.6 取材和检测造模后3 d,各组大鼠腹腔注射10%水合氯醛(3.5 mL/kg),断头取脑组织。根据大鼠脑立体定位图[12],切取造模大鼠的梗死周围组织,假手术组取对应部位组织。实验流程见图 2。
|
图 2 实验时间线 Fig 2 Experiment timeline tMCAO: Transient middle cerebral artery occlusion; H-E: Hematoxylin-eosin; TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay; mNSS: Modified neurological severity score. |
1.6.1 H-E染色
取各组大鼠脑组织置于4%多聚甲醛溶液中过夜,石蜡包埋、切片(厚度为5 μm),常规H-E染色,用光学显微镜观察病理学变化。
1.6.2 TUNEL检测切片烤干、脱蜡复水后,加入蛋白酶K常温孵育30 min,PBS漂洗后加入TUNEL反应液,置于37 ℃湿盒反应60 min后,在光学显微镜下观察凋亡细胞。苏木精轻度复染,返蓝,脱水,透明,干燥后封片,在光学显微镜下观察并拍照。每只大鼠随机选择5张切片,在光学显微镜下计数凋亡细胞数量。
1.6.3 免疫荧光染色切片在26 ℃用4%多聚甲醛溶液固定10 min,10%牛血清白蛋白溶液阻断1 h,加入Iba-1抗体(1∶1 000)4 ℃孵育过夜。用PBS冲洗3次后,用荧光偶联二抗(1∶300)孵育1 h。用共聚焦显微镜对每个区域的4个视野进行拍摄,阳性细胞呈红色或粉红色,记录视野内Iba-1阳性细胞数。
1.6.4 蛋白质印迹法另取各组大鼠脑组织加入蛋白质裂解液,4 ℃、14 650×g离心30 min,取上清,用BCA试剂盒检测各组蛋白质浓度。进行10% SDS-PAGE,转膜2 h后,用5%脱脂牛奶封闭2 h,TBST洗涤3次;分别加入TNF-α抗体(1∶900)、IL-1β抗体(1∶1 000)和β-肌动蛋白抗体(1∶5 000),4 ℃过夜孵育,TBST洗涤3次;加入HRP标记的二抗(羊抗兔IgG,1∶5 000)室温孵育60 min,滴加发光液,放入自动化学发光图像分析系统内成像;以β-肌动蛋白为内参,用ImageJ软件进行灰度值分析。
1.7 统计学处理应用SPSS 26.0软件进行数据处理。服从正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验,多组间比较采用单因素方差分析。检验水准(α)为0.05。
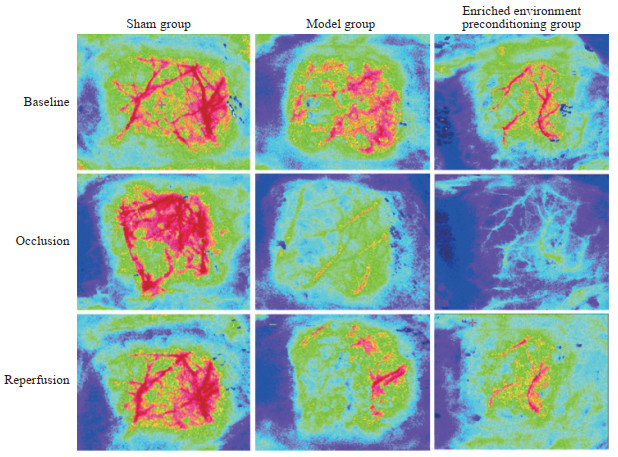
2 结果 2.1 脑血流变化插入线栓后,模型组大鼠脑血流值(39.33±2.08)和丰富环境预处理组大鼠脑血流值(35.67±3.22)相比术前(均为100)下降幅度均>60%,提示tMCAO大鼠模型制作成功。术后,模型组大鼠脑血流值(85.67±1.53)和丰富环境预处理组大鼠脑血流值(89.33±3.06)均明显恢复。两组大鼠在插入线栓后和术后的脑血流值差异均无统计学意义(P均>0.05)。见图 3。
|
图 3 激光多普勒超声检测各组大鼠梗死侧脑血流动态变化 Fig 3 Dynamic changes of cerebral blood flow in infarcted side of rats in each group detected by laser Doppler flowmetry |
2.2 大鼠mNSS评估
模型组和丰富环境预处理组大鼠术后3 d内的mNSS均高于假手术组(P均<0.05)。术后第1、2天模型组与丰富环境预处理组大鼠mNSS差异无统计学意义(P均>0.05);术后第3天丰富环境预处理组大鼠mNSS低于模型组,差异有统计学意义(P<0.05)。见表 1。
|
|
表 1 各组大鼠术后不同时间点mNSS比较 Tab 1 Comparison of mNSS at different time points after operation in rats of each group |
2.3 大鼠梗死周围皮质组织病理学变化
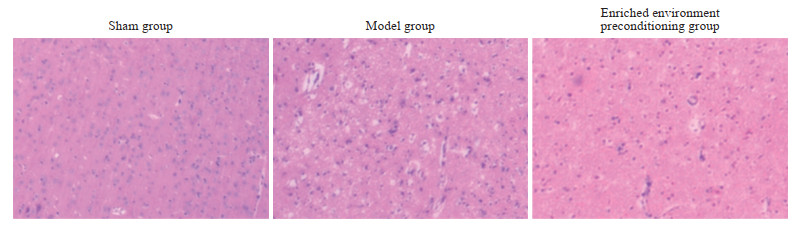
光镜下可见假手术组大鼠未发生梗死,大脑皮质组织结构清晰,神经元排列规则,核仁清晰。模型组大鼠梗死周围皮质组织结构疏松呈空泡状、核固缩、核仁消失、细胞质深染等病理改变,未见明显的神经元丢失。与模型组相比,丰富环境预处理组大鼠梗死周围皮质组织病理损伤减轻,组织水肿和核固缩减轻、组织空泡减少。见图 4。
|
图 4 各组大鼠梗死周围皮质组织病理学变化(苏木精-伊红染色,400×) Fig 4 Histopathological changes of peri-infarct cortex in rats of each group (hematoxylin-eosin staining, 400×) |
2.4 大鼠梗死周围皮质细胞凋亡情况
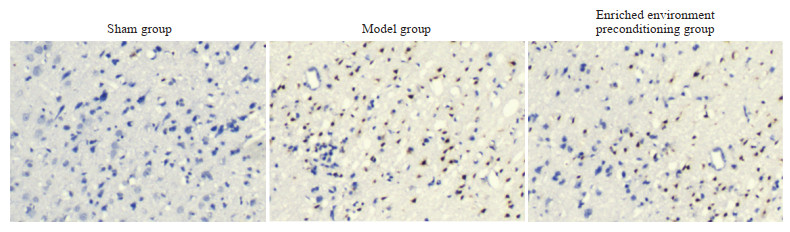
由图 5可见,假手术组大鼠梗死周围皮质仅可见少数棕黄色TUNEL阳性凋亡细胞,数量为2.50±1.38;模型组和丰富环境预处理组大鼠梗死周围皮质的TUNEL阳性细胞较多,数量分别为61.67±5.32和41.67±4.23。与模型组相比,丰富环境预处理组大鼠梗死周围皮质TUNEL阳性细胞数量减少(P<0.05)。
|
图 5 各组大鼠梗死周围皮质细胞凋亡情况(TUNEL染色,400×) Fig 5 Apoptosis of peri-infarct cortical cells in rats of each group (TUNEL staining, 400×) TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay. |
2.5 大鼠梗死周围皮质小胶质细胞激活及炎症反应情况
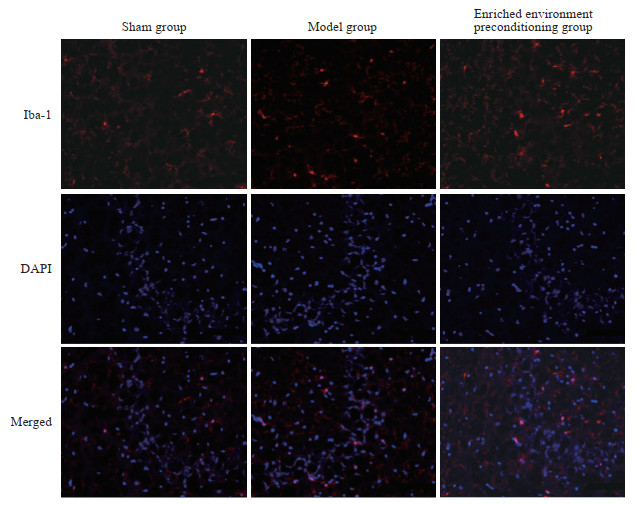
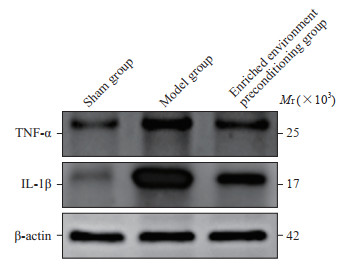
由图 6可见,模型组和丰富环境预处理组大鼠梗死周围皮质小胶质细胞被激活,Iba-1阳性细胞数量[(24.67±1.86)和(18.83±2.64)个/视野]与假手术组[(1.50±1.05)个/视野]相比均增加(P均<0.05),但丰富环境预处理组大鼠梗死周围皮质Iba-1阳性细胞数量少于模型组(P<0.05)。由图 7可见,模型组和丰富环境预处理组大鼠梗死周围皮质TNF-α表达(0.72±0.04、0.52±0.04)和IL-1β表达(1.34±0.07、1.16±0.04)与假手术组(TNF-α:0.38±0.02;IL-1β:0.67±0.03)相比均升高(P均<0.05),但丰富环境预处理组大鼠梗死周围皮质TNF-α和IL-1β表达低于模型组(P均<0.05)。
|
图 6 各组大鼠梗死周围皮质Iba-1荧光表达(免疫荧光染色,200×) Fig 6 Fluorescence expression of Iba-1 in peri-infarct cortex of rats in each group (immunofluorescence staining, 200×) Iba-1: Ionized calcium-binding adaptor molecule 1; DAPI: 4', 6-diamidino-2-phenylindole. |
|
图 7 各组大鼠梗死周围皮质TNF-α和IL-1β表达的蛋白质印迹法检测结果 Fig 7 Expression of TNF-α and IL-1β in peri-infarct cortex of rats in each group detected by Western blotting TNF-α: Tumor necrosis factor α; IL-1β: Interleukin 1β. |
3 讨论
Murry等[13]于1986年首次提出缺血预处理(ischemic preconditioning,IPC)的概念。IPC指在缺血损伤发生前进行多次干预,以诱导机体对缺血损伤产生保护作用。IPC概念的提出提供了一个全新的研究方向,目前已有多项基于该理论的脑保护研究开展,如缺血预适应[14]、运动预适应[15]、药物预适应[16]和丰富环境预适应[17]等。
丰富环境作为一种行为干预方法安全有效、可操作性强,以自愿或无心理压力的方式为受试者提供运动、感觉和认知等多种刺激[18]。研究证明,丰富环境预处理可增加大鼠缺血视网膜脑源性神经营养因子(brain derived-neurotrophic factor,BDNF)水平,从而减少急性缺血引起的视网膜损伤[19];改善缺血缺氧大鼠认知水平,提高大脑皮质生长相关蛋白43(growth associated-protein 43,GAP-43)和海马血管内皮生长因子(vascular endothelial growth factor,VEGF)、酪氨酸激酶B(tyrosine kinase B,TrkB)的表达水平[20-21];减少tMCAO小鼠脑梗死体积,降低海马IL-1β的表达,增加胶质纤维酸性蛋白(glial fibrillary acidic protein,GFAP)的表达[22]。
炎症反应在缺血性卒中发生、发展中的各个方面扮演着重要角色,包括损伤的急性期和恢复期[23-24]。TNF-α和IL-1β是调节炎症反应的关键因子。在缺血再灌注损伤急性期,激活的小胶质细胞释放TNF-α、IL-1β和其他促炎因子[5]。研究证明,缺血性卒中患者脑脊液和血清中的TNF-α水平与脑梗死体积呈正相关[25]。IL-1β是IL-1参与缺血性损伤的主要表型,其表达增多会加重卒中后大鼠的脑损伤[26]。González Fleitas等[19]发现,3周的丰富环境预处理可抑制视网膜缺血大鼠的小胶质细胞激活,减轻炎症反应引起的视网膜损伤。有实验证明5周的丰富环境预处理使大脑中动脉栓塞小鼠的认知功能改善,脑组织中炎症因子IL-1β表达降低[22]。本研究结果显示在缺血再灌注3 d后,模型组大鼠梗死周围皮质TNF-α和IL-1β的表达升高,而3周的丰富环境预处理可以抑制小胶质细胞的激活,降低TNF-α和IL-1β的表达,发挥脑保护作用。
研究表明,促炎因子TNF-α和IL-1β与神经元细胞膜表面受体结合后,可以促进和启动细胞的程序化凋亡[27]。在脑缺血再灌注损伤过程中,炎症反应和细胞凋亡会加重脑组织损伤,最终导致神经元细胞的不可逆损伤甚至坏死[28-29]。Chen等[17]发现,4周的丰富环境预处理可以抑制大脑中动脉栓塞大鼠半暗带细胞凋亡,促凋亡因子Bax表达水平降低,抗凋亡因子Bcl-2表达水平升高。本研究中TUNEL检测结果显示,丰富环境预处理组大鼠梗死周围皮质凋亡细胞数量少于模型组,表明丰富环境预处理可减少缺血再灌注损伤大鼠的细胞凋亡。
丰富环境预处理调控缺血再灌注损伤后神经保护作用的具体分子机制仍不明确。本研究结果显示,丰富环境预处理可以改善大鼠脑缺血再灌注损伤后3 d神经功能障碍和梗死周围皮质损伤,这可能与丰富预处理降低了TNF-α和IL-1β蛋白表达、抑制了细胞凋亡有关。丰富环境对脑的保护作用机制有多方面、多靶点、多层次的特点[30-31],神经炎症和细胞凋亡之间作用复杂,但都在缺血再灌注损伤中发挥着重要作用,今后仍需深入研究。
| [1] |
WANG W, JIANG B, SUN H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480687 adults[J]. Circulation, 2017, 135(8): 759-771. DOI:10.1161/CIRCULATIONAHA.116.025250 |
| [2] |
JAYARAJ R L, AZIMULLAH S, BEIRAM R, et al. Neuroinflammation: friend and foe for ischemic stroke[J]. J Neuroinflammation, 2019, 16(1): 142. DOI:10.1186/s12974-019-1516-2 |
| [3] |
SHEN L, GAN Q, YANG Y, et al. Mitophagy in cerebral ischemia and ischemia/reperfusion injury[J]. Front Aging Neurosci, 2021, 13: 687246. DOI:10.3389/fnagi.2021.687246 |
| [4] |
ZUO C, CAO H, FENG F, et al. Repetitive transcranial magnetic stimulation exerts anti-inflammatory effects via modulating glial activation in mice with chronic unpredictable mild stress-induced depression[J]. Int Immunopharmacol, 2022, 109: 108788. DOI:10.1016/j.intimp.2022.108788 |
| [5] |
JIANG C T, WU W F, DENG Y H, et al. Modulators of microglia activation and polarization in ischemic stroke (review)[J]. Mol Med Rep, 2020, 21(5): 2006-2018. DOI:10.3892/mmr.2020.11003 |
| [6] |
SU Q, PU H, HU C. Neuroprotection by combination of resveratrol and enriched environment against ischemic brain injury in rats[J]. Neurol Res, 2016, 38(1): 60-68. DOI:10.1080/01616412.2015.1133027 |
| [7] |
GUO Y S, YUAN M, HAN Y, et al. Effects of enriched environment on microglia and functional white matter recovery in rats with post stroke cognitive impairment[J]. Neurochem Int, 2022, 154: 105295. DOI:10.1016/j.neuint.2022.105295 |
| [8] |
ZHANG X, YUAN M, YANG S, et al. Enriched environment improves post-stroke cognitive impairment and inhibits neuroinflammation and oxidative stress by activating Nrf2-ARE pathway[J]. Int J Neurosci, 2021, 131(7): 641-649. DOI:10.1080/00207454.2020.1797722 |
| [9] |
ZHANG Y, XU D, QI H, et al. Enriched environment promotes post-stroke neurogenesis through NF-κB-mediated secretion of IL-17A from astrocytes[J]. Brain Res, 2018, 1687: 20-31. DOI:10.1016/j.brainres.2018.02.030 |
| [10] |
LONGA E Z, WEINSTEIN P R, CARLSON S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats[J]. Stroke, 1989, 20(1): 84-91. DOI:10.1161/01.str.20.1.84 |
| [11] |
刘宇, 孟然, 吉训明, 等. 激光多普勒血流仪动态观察镁对脑缺血再灌注损伤保护作用[J]. 中国病理生理杂志, 2011, 27(9): 1811-1815. DOI:10.3969/j.issn.1000-4718.2011.09.029 |
| [12] |
TOGA A W, SANTORI E M, HAZANI R, et al. A 3D digital map of rat brain[J]. Brain Res Bull, 1995, 38(1): 77-85. DOI:10.1016/0361-9230(95)00074-O |
| [13] |
MURRY C E, JENNINGS R B, REIMER K A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium[J]. Circulation, 1986, 74(5): 1124-1136. DOI:10.1161/01.cir.74.5.1124 |
| [14] |
FAN X, WANG H, ZHANG L, et al. Neuroprotection of hypoxic/ischemic preconditioning in neonatal brain with hypoxic-ischemic injury[J]. Rev Neurosci, 2021, 32(1): 23-34. DOI:10.1515/revneuro-2020-0024 |
| [15] |
OTSUKA S, SETOYAMA K, TAKADA S, et al. Preconditioning exercise in rats attenuates early brain injury resulting from subarachnoid hemorrhage by reducing oxidative stress, inflammation, and neuronal apoptosis[J]. Mol Neurobiol, 2021, 58(11): 5602-5617. DOI:10.1007/s12035-021-02506-7 |
| [16] |
WANG L, WANG A, GUO H, et al. Neuroprotective effects of long-term metformin preconditioning on rats with ischemic brain injuries[J]. Eur Neurol, 2021, 84(3): 212-218. DOI:10.1159/000514431 |
| [17] |
CHEN X, ZHANG X. Effect of prior exposure to enriched environment on cellular apoptosis after experimental stroke[J]. Mol Biol Rep, 2022, 49(7): 6541-6551. DOI:10.1007/s11033-022-07494-y |
| [18] |
YUAN M, ZHANG X X, FU X C, et al. Enriched environment alleviates post-stroke cognitive impairment through enhancing α7-nAChR expression in rats[J]. Arq Neuro-Psiquiatr, 2020, 78(10): 603-610. DOI:10.1590/0004-282x20200081 |
| [19] |
GONZÁ LEZ FLEITAS M F, ARANDA M L, DIEGUEZ H H, et al. Pre-ischemic enriched environment increases retinal resilience to acute ischemic damage in adult rats[J]. Exp Eye Res, 2019, 178: 198-211. DOI:10.1016/j.exer.2018.10.007 |
| [20] |
DURÁ N-CARABALI L E, ODORCYK F K, GREGGIO S, et al. Pre- and early postnatal enriched environmental experiences prevent neonatal hypoxia-ischemia late neurodegeneration via metabolic and neuroplastic mechanisms[J]. J Neurochem, 2021, 157(6): 1911-1929. DOI:10.1111/jnc.15221 |
| [21] |
DURÁ N-CARABALI L E, ARCEGO D M, SANCHES E F, et al. Preventive and therapeutic effects of environmental enrichment in Wistar rats submitted to neonatal hypoxia-ischemia[J]. Behav Brain Res, 2019, 359: 485-497. DOI:10.1016/j.bbr.2018.11.036 |
| [22] |
GONÇ ALVES L V, HERLINGER A L, FERREIRA T A A, et al. Environmental enrichment cognitive neuroprotection in an experimental model of cerebral ischemia: biochemical and molecular aspects[J]. Behav Brain Res, 2018, 348: 171-183. DOI:10.1016/j.bbr.2018.04.023 |
| [23] |
SHEKHAR S, CUNNINGHAM M W, PABBIDI M R, et al. Targeting vascular inflammation in ischemic stroke: recent developments on novel immunomodulatory approaches[J]. Eur J Pharmacol, 2018, 833: 531-544. DOI:10.1016/j.ejphar.2018.06.028 |
| [24] |
MO Y, SUN Y Y, LIU K Y. Autophagy and inflammation in ischemic stroke[J]. Neural Regen Res, 2020, 15(8): 1388-1396. DOI:10.4103/1673-5374.274331 |
| [25] |
ZAREMBA J, SKROBANSKI P, LOSY J. Tumour necrosis factor-alpha is increased in the cerebrospinal fluid and serum of ischaemic stroke patients and correlates with the volume of evolving brain infarct[J]. Biomed Pharmacother, 2001, 55(5): 258-263. DOI:10.1016/S0753-3322(01)00058-0 |
| [26] |
BOUTIN H, LEFEUVRE R A, HORAI R, et al. Role of IL-1α and IL-1β in ischemic brain damage[J]. J Neurosci, 2001, 21(15): 5528-5534. DOI:10.1523/jneurosci.21-15-05528.2001 |
| [27] |
CHEN J X, HUANG X Y, WANG P, et al. Effects and mechanism of arachidonic acid against TNF-α induced apoptosis of endothelial cells[J]. Clin Hemorheol Microcirc, 2021, 77(3): 259-265. DOI:10.3233/ch-200946 |
| [28] |
ZHI S M, FANG G X, XIE X M, et al. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling[J]. Eur Rev Med Pharmacol Sci, 2020, 24(3): 1524-1536. DOI:10.26355/eurrev_202002_20211 |
| [29] |
AKHTER R, SHAO Y, FORMICA S, et al. TREM2 alters the phagocytic, apoptotic and inflammatory response to Aβ42 in HMC3 cells[J]. Mol Immunol, 2021, 131: 171-179. DOI:10.1016/j.molimm.2020.12.035 |
| [30] |
YUAN M, GUO Y S, HAN Y, et al. Effectiveness and mechanisms of enriched environment in post-stroke cognitive impairment[J]. Behav Brain Res, 2021, 410: 113357. DOI:10.1016/j.bbr.2021.113357 |
| [31] |
HUA M, MIN J. Postoperative cognitive dysfunction and the protective effects of enriched environment: a systematic review[J]. Neurodegener Dis, 2021, 20(4): 113-122. DOI:10.1159/000513196 |
 2023, Vol. 44
2023, Vol. 44


