2. 海军军医大学(第二军医大学)转化医学研究中心干细胞与再生医学研究室, 上海 200433
2. Laboratory of Stem Cell and Regenerative Medicine, Center of Translational Medicine, Naval Medical University(Second Military Medical University), Shanghai 200433, China
肝细胞癌(hepatocellular carcinoma,HCC)是导致癌症相关死亡的主要原因,全世界每年约有200万HCC患者死亡[1-2]。铁死亡不同于通常的细胞坏死,是一种受调控的坏死过程[3]。尽管目前铁死亡在肿瘤发生、发展中的确切机制仍不清晰,但已经有大量的研究表明促进癌细胞的铁死亡可以抑制肿瘤的生长[4]。HCC患者对铁死亡药物的耐受促进了HCC的恶性发展[5]。了解铁死亡相关基因对HCC患者预后的影响,以及关键基因在HCC细胞中的特异性表达,将为HCC的治疗提供潜在靶点。
本研究通过癌症基因组图谱(The Cancer Genome Atlas,TCGA)数据库和铁死亡相关基因数据库,获得HCC铁死亡相关预后基因,利用HCC单细胞测序数据对铁死亡相关预后基因在HCC不同细胞群体和不同发育阶段的特异性表达及在细胞间的相互作用进行解析,明确铁死亡在HCC中的作用,为探讨更加精准的靶向治疗策略提供数据支撑。
1 材料和方法 1.1 数据收集下载TCGA数据库中HCC患者样本数据,共374份肿瘤样本和50份正常组织对照样本,将miRNA表达值标准化,转换为log2(TPM+1),其中TPM(transcripts per million)为每106个转录本的数量,拷贝数扩增和删除阈值通过GISTIC2.0软件确定;铁死亡相关基因在FerrDb数据库(http://www.zhounan.org/ferrdb)下载[6];HCC单细胞测序数据从基因表达汇编(Gene Expression Omnibus,GEO)数据库中下载(GSE125449)。
1.2 差异表达基因和预后基因筛选使用limma包对TCGA HCC转录组数据和对照数据进行比较。|log2FC|>1[FC为差异倍数(fold change)]和校准P<0.05定义为差异表达基因,利用在线工具Venny 2.1(https://bioinfogp.cnb.csic.es/tools/venny/)找到与铁死亡相关的差异表达基因[6]。为进一步探讨铁死亡相关差异表达基因的临床指导价值,将相关差异表达基因与TCGA数据库中样本的临床资料进行匹配,然后使用生存包进行单因素Cox回归分析和对数秩检验来评价预后差异,将P<0.05的相关差异表达基因定义为预后基因。
1.3 风险预后模型的建立将差异表达基因的表达数据与临床数据结合,使用glmnet包构建预后模型,并使用最小绝对收缩和选择算子(least absolute shrinkage and selection operator,LASSO)-惩罚Cox回归分析排除拟合倾向的基因。采用LASSO回归分析在TCGA数据库中构建HCC的风险预后模型,将得到的系数和基因表达值相乘后求和得到风险评分。根据风险评分的中位数,将TCGA数据库中肝癌表达数据分为高危组和低危组,并使用Kaplan-Meier生存曲线和ROC曲线评估预后模型的预测能力。
1.4 单细胞测序分析下载GSE125449并使用Seurat包进行分析,用t-分布随机近邻嵌入(t-distributed stochastic neighbor embedding,tSNE)聚类降维分析不同聚类中预后基因的表达,不同的细胞亚群用SingleR包进行注释。利用Monocle包对细胞的发育阶段进行拟时序分析,明确铁死亡预后相关基因在不同发育阶段细胞的表达情况。最后利用CellChat包分析铁死亡相关预后基因IL-33在不同细胞间的交互情况。
1.5 基因富集分析利用DAVID网站(https://david.ncifcrf.gov/)对基因进行富集分析,用气泡图显示京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)信号通路及基因本体(Gene Ontology,GO)细胞组分、分子功能和生物过程中显著富集的基因。
1.6 统计学处理应用R4.0软件对数据进行统计分析,采用非配对t检验比较组间差异,检验水准(α)为0.05。
2 结果 2.1 HCC患者铁死亡相关预后基因分析比较TCGA数据库中HCC组织和正常组织之间的基因表达差异,共发现了1 727个差异表达基因,与388个铁死亡相关基因取交集获得54个HCC患者铁死亡相关差异表达基因。54个铁死亡相关差异表达基因在不同样本中表达的基因热图见图 1A。对54个差异表达基因进行单因素回归分析,鉴定出25个基因为HCC患者预后基因,其中14个基因为危险因素、11个基因为保护因素(图 1B)。
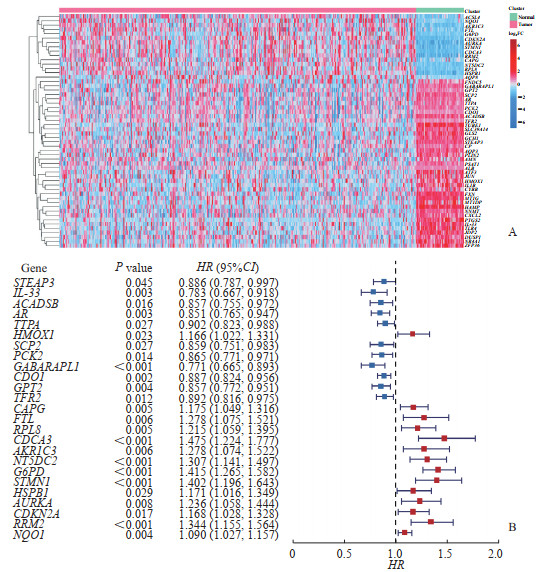
|
图 1 HCC患者铁死亡相关差异表达基因热图及预后单因素回归分析 Fig 1 Heat mapping of ferroptosis-related differentially expressed genes and univariate regression analysis for prognosis in HCC patients A: Heat mapping of 54 ferroptosis-related differentially expressed genes; B: Univariate regression analysis of ferroptosis-related differentially expressed genes for prognosis in HCC. HCC: Hepatocellular carcinoma; HR: Hazard ratio; CI: Confidence interval. |
2.2 基于铁死亡相关差异表达基因的风险预后模型的建立与评价
采用LASSO回归分析构建铁死亡相关差异表达基因的风险预后模型,当基因数目为4时模型的平均方差最小(图 2A),因此最终选择4个基因构建风险预后模型。这4个基因分别为含5'-核苷酸酶结构域2(5'-nucleotidase domain containing 2,NT5DC2)、葡萄糖-6-磷酸脱氢酶(glucose-6-phosphate dehydrogenase,G6PD)、微管解聚蛋白1(stathmin1,STMN1)和IL-33。其中,IL-33为良性预后因素,回归系数为-0.024 355 59,NT5DC2、G6PD、STMN1的回归系数分别为0.055 323 29、0.225 399 53和0.077 707 64。根据这4个基因的表达水平和回归系数,以它们乘积求和的方式构建风险评分。将HCC患者根据风险评分中位数分为高危组和低危组,结果发现高危组患者总生存期低于低危组患者(P<0.000 1,图 2B、2C)。ROC曲线分析结果显示,该模型预测HCC患者3、5年生存率的AUC分别为0.664 7、0.711 9(图 2D)。
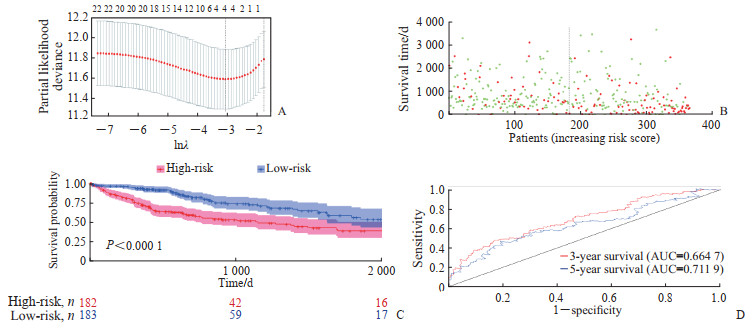
|
图 2 HCC患者铁死亡相关差异表达基因危险预后模型建立与评价 Fig 2 Establishment and evaluation of risk prognostic model for ferroptosis-related differentially expressed genes in HCC patients A: Determination of λ in LASSO Cox regression analysis; B: Distribution of high-risk and low-risk patients and their survival time in the TCGA database of HCC patients (green indicates low-risk, red indicates high-risk); C: Survival curves of high-risk and low-risk prognostic groups of HCC patients in TCGA database; D: The prognostic performance of the HCC risk score in the TCGA database was verified by ROC curve. HCC: Hepatocellular carcinoma; LASSO: Least absolute shrinkage and selection operator; TCGA: The Cancer Genome Atlas; ROC: Receiver operating characteristic; AUC: Area under curve. |
2.3 基于单细胞测序数据解析铁死亡相关HCC风险基因的细胞分布
利用Seurat包,tSNE降维分析后共聚类出16个细胞亚群,利用SingleR包对细胞亚群进行注释(图 3A)。其中亚群0注释为T细胞和自然杀伤(natural killer,NK)细胞,亚群1注释为T细胞,亚群2注释为B细胞,亚群3注释为内皮细胞,亚群4注释为肝细胞和上皮细胞,亚群5注释为单核细胞、巨噬细胞和树突状细胞,亚群6注释为肝细胞,亚群7注释为组织干细胞、平滑肌细胞和成纤维细胞,亚群8注释为上皮细胞,亚群9注释为成纤维细胞、平滑肌细胞和组织干细胞,亚群10注释为T细胞和NK细胞,亚群11注释为组织干细胞,亚群12注释为内皮细胞,亚群13注释为肝细胞和上皮细胞,亚群14注释为肝细胞,亚群15注释为前B细胞(CD34-)。在各个细胞亚群中,NT5DC2主要在上皮和内皮细胞中表达,IL-33主要在内皮细胞中表达,而G6PD和STMN1在多个细胞类型中均有表达(图 3B)。
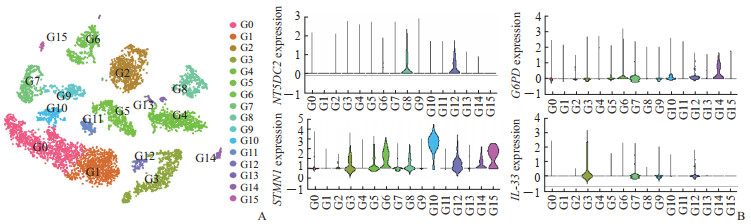
|
图 3 单细胞测序数据解析铁死亡相关HCC风险基因的细胞分布 Fig 3 Cellular distribution of ferroptosis-related HCC risk genes analyzed by single cell sequencing data A: The tSNE diagram showed the different cell types; B: Expression levels of risk genes in different cell types. G0: T cells and natural killer cells; G1: T cells; G2: B cells; G3: Endothelial cells; G4: Hepatocytes and epithelial cells; G5: Monocytes, macrophages, and dendritic cells; G6: Hepatocytes; G7: Tissue stem cells, smooth muscle cells, and fibroblasts; G8: Epithelial cells; G9: Fibroblasts, smooth muscle cells, and tissue stem cells; G10: T cells and natural killer cells; G11: Tissue stem cells; G12: Endothelial cells; G13: Hepatocytes and epithelial cells; G14: Hepatocytes; G15: Pre-B cells (CD34-). HCC: Hepatocellular carcinoma; tSNE: t-distributed stochastic neighbor embedding; NT5DC2: 5'-nucleotidase domain containing 2; G6PD: Glucose-6-phosphate dehydrogenase; STMN1: Stathmin 1; IL-33: Interleukin 33. |
2.4 拟时序分析解析铁死亡相关HCC风险基因在不同发育阶段的表达
整个HCC细胞群可分为3个不同发育阶段,16个细胞亚群分别处于不同的发育阶段,其中内皮细胞的2个亚群3和12分别处于阶段1和阶段3两个完全不同的发育阶段(图 4A)。不同细胞亚群在发育时间演化上的顺序表明,内皮细胞亚群3相对于亚群12处于发育的更早期阶段(图 4B)。在不同的发育状态中,铁死亡相关HCC预后基因IL-33主要处于发育阶段1,而NT5DC2、G6PD、STMN1在3个发育阶段下均有表达(图 4C)。综合以上数据,铁死亡相关HCC预后基因IL-33主要在内皮细胞发育的相对原始阶段中表达,而在发育晚期阶段的内皮细胞中几乎不表达。
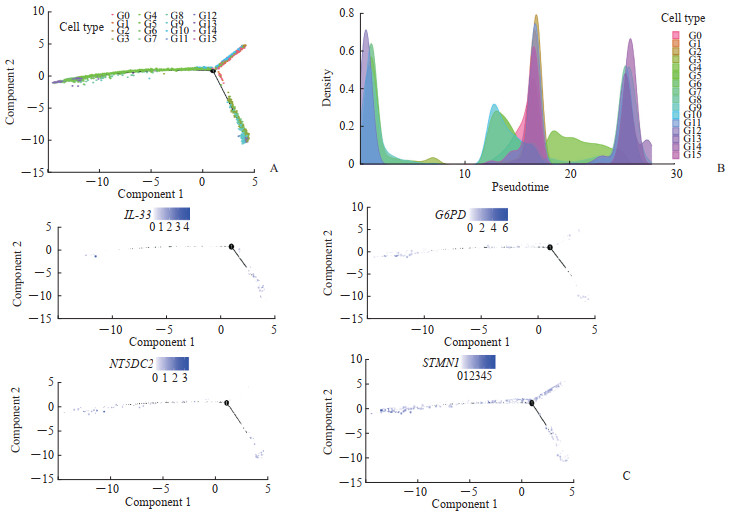
|
图 4 铁死亡相关HCC风险基因在不同发育阶段的表达 Fig 4 Expression of ferroptosis-related HCC risk genes at different developmental stages A: Skeletal diagram of cell trajectories at different developmental stages; B: Cell density plot in the timeline; C: Skeletal diagram of risk genes (IL-33, G6PD, NT5DC2, and STMN1) expression at different developmental stages (shades of color indicate gene expression levels in different cells). G0: T cells and natural killer cells; G1: T cells; G2: B cells; G3: Endothelial cells; G4: Hepatocytes and epithelial cells; G5: Monocytes, macrophages, and dendritic cells; G6: Hepatocytes; G7: Tissue stem cells, smooth muscle cells, and fibroblasts; G8: Epithelial cells; G9: Fibroblasts, smooth muscle cells, and tissue stem cells; G10: T cells and natural killer cells; G11: Tissue stem cells; G12: Endothelial cells; G13: Hepatocytes and epithelial cells; G14: Hepatocytes; G15: Pre-B cells (CD34-). HCC: Hepatocellular carcinoma; IL-33: Interleukin 33; G6PD: Glucose-6-phosphate dehydrogenase; NT5DC2: 5'-nucleotidase domain containing 2; STMN1: Stathmin 1. |
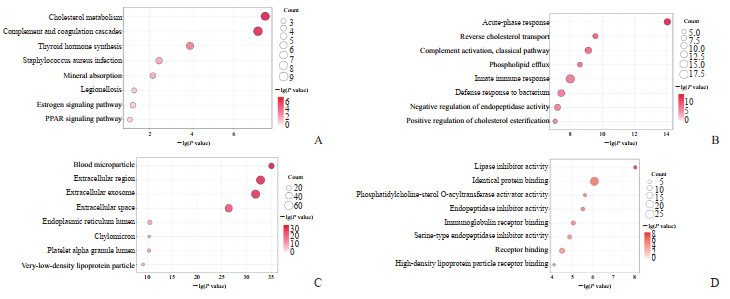
对拟时相关差异表达基因进行KEGG富集分析发现,不同细胞的差异表达基因主要富集在胆固醇代谢、补体和凝血级联反应等信号通路中(图 5A)。GO富集分析显示,拟时相关基因主要与急性期反应、细胞外区域和脂肪酶抑制剂活性等相关(图 5B~5D)。
|
图 5 拟时相关差异表达基因富集分析 Fig 5 Pseudotime-related differentially expressed gene enrichment analysis A: Bubble plot of the results of KEGG analysis; B: Results of GO biological process analysis; C: Results of GO cellular component analysis; D: Results of GO molecular function analysis. KEGG: Kyoto Encyclopedia of Genes and Genomes; GO: Gene Ontology; PPAR: Peroxisome proliferator activated receptor. |
2.5 铁死亡相关HCC风险基因IL-33在细胞间的交互情况
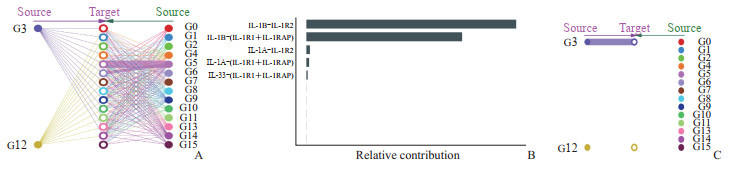
结果证明IL-1家族在HCC不同细胞亚群之间广泛作用,而不同发育阶段的内皮细胞(细胞亚群3和12)在IL-1家族的细胞间交互中表现出不同的作用(图 6A)。IL-33与其受体的结合在整个IL-1家族贡献中占比最低(图 6B),并且IL-33介导的细胞间交互只发生在细胞亚群3中(图 6C),进一步证实铁死亡相关HCC预后基因IL-33仅在发育早期阶段下的内皮细胞中发挥作用,发育晚期阶段内皮细胞不再通过IL-33与周围细胞建立联系。结果表明发育早期阶段的内皮细胞可能通过IL-33介导的炎症反应诱导内皮细胞的铁死亡,改善HCC患者的预后。
|
图 6 铁死亡相关HCC风险基因IL-33在不同细胞亚群的细胞间交互情况 Fig 6 Cell-to-cell interaction of ferroptosis-related HCC risk gene IL-33 in different cell subsets A: Interactions of the IL-1 family in different cell subsets of HCC; B: Contribution ratios of different IL-1 family members in cell interactions; C: Interaction of IL-33 in different cell subsets of HCC. HCC: Hepatocellular carcinoma; IL-33: Interleukin 33; G0: T cells and natural killer cells; G1: T cells; G2: B cells; G3: Endothelial cells; G4: Hepatocytes and epithelial cells; G5: Monocytes, macrophages, and dendritic cells; G6: Hepatocytes; G7: Tissue stem cells, smooth muscle cells, and fibroblasts; G8: Epithelial cells; G9: Fibroblasts, smooth muscle cells, and tissue stem cells; G10: T cells and natural killer cells; G11: Tissue stem cells; G12: Endothelial cells; G13: Hepatocytes and epithelial cells; G14: Hepatocytes; G15: Pre-B cells (CD34-); IL-1: Interleukin 1; IL-1β: Interleukin 1β; IL-1R2: Interleukin 1 receptor type 2; IL-1R1: Interleukin 1 receptor type 1; IL-1RAP: Interleukin 1 receptor accessory protein; IL-1α: Interleukin 1α; IL-1RL1: Interleukin 1 receptor like 1. |
3 讨论
我国的HCC发病率居于全球的前列[7]。近年来高通量测序技术在揭示癌症发生、发展过程中基因的异常及癌症诊断和预后相关的生物标志物方面起到了至关重要的作用[8]。在临床实践中,患者及疾病的病因、临床表现和治疗等具有可变性,单个预测因子很少能对预后做出充分的评估,因此构建稳定的风险预后模型对于预测患者个体疾病的发生、发展,以及指导医师对疾病的进一步干预有着重要意义。本研究结合TCGA数据库和铁死亡相关基因数据库,建立了有效的铁死亡相关HCC预后模型,确认4个铁死亡相关差异表达基因NT5DC2、G6PD、STMN1和IL-33为HCC的预后基因,并通过生存曲线分析和ROC曲线分析证实了铁死亡相关HCC预后模型的可靠性。在4个铁死亡相关HCC风险基因中,IL-33作为IL-1家族的成员,能够与受体结合并有效驱动辅助性T细胞产生细胞因子,调节局部免疫微环境,参与多重生物学过程[9],并在多种疾病的发生、发展中发挥重要作用[10-11]。利用HCC单细胞测序数据发现,NT5DC2主要在上皮和内皮细胞中表达,IL-33主要在内皮细胞中表达,而G6PD和STMN1在多个细胞类型中均有表达。拟时序分析和细胞间交互分析表明,IL-33主要在发育早期阶段的内皮细胞特异性表达,并与相应的受体结合发挥作用。
IL-33主要参与辅助性T细胞细胞介导的免疫应答、调节肥大细胞的功能,还作为一种细胞内的核因子调节基因转录,可引起并调控多种炎症反应,可能在慢性炎症和自身免疫性疾病中发挥作用。IL-33在肿瘤中的作用表现出两面性,如在乳腺癌的肺转移发生过程中,成纤维细胞来源的IL-33通过改变免疫微环境促进其发生[12];而在EL-4淋巴瘤细胞系中过表达IL-33抑制了肿瘤的生长[13]。本研究中,IL-33作为一个良性的预后因素,在HCC早期阶段的内皮细胞中发挥重要作用,推测IL-33可能通过与受体的结合促进局部的炎症反应,进而介导早期内皮细胞等细胞的铁死亡,抑制HCC的进展,改善HCC的长期预后。本研究结果为了解IL-33在HCC中的作用提供了数据支撑,但相关的结论仍需要进一步的实验验证。
| [1] |
ASRANI S K, DEVARBHAVI H, EATON J, et al. Burden of liver diseases in the world[J]. J Hepatol, 2019, 70(1): 151-171. DOI:10.1016/j.jhep.2018.09.014 |
| [2] |
PAIK J M, GOLABI P, YOUNOSSI Y, et al. Changes in the global burden of chronic liver diseases from 2012 to 2017:the growing impact of NAFLD[J]. Hepatology, 2020, 72(5): 1605-1616. DOI:10.1002/hep.31173 |
| [3] |
ZHENG J, CONRAD M. The metabolic underpinnings of ferroptosis[J]. Cell Metab, 2020, 32(6): 920-937. DOI:10.1016/j.cmet.2020.10.011 |
| [4] |
ALBORZINIA H, FLóREZ A F, KRETH S, et al. MYCN mediates cysteine addiction and sensitizes neuroblastoma to ferroptosis[J]. Nat Cancer, 2022, 3(4): 471-485. DOI:10.1038/s43018-022-00355-4 |
| [5] |
YAO F, DENG Y, ZHAO Y, et al. A targetable LIFR-NF-κB-LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis[J]. Nat Commun, 2021, 12(1): 7333. DOI:10.1038/s41467-021-27452-9 |
| [6] |
ZHOU N, BAO J. FerrDb: a manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations[J]. Database (Oxford), 2020, 2020: baaa021. DOI:10.1093/database/baaa021 |
| [7] |
XIA C, DONG X, LI H, et al. Cancer statistics in China and United States, 2022:profiles, trends, and determinants[J]. Chin Med J, 2022, 135(5): 584-590. DOI:10.1097/CM9.0000000000002108 |
| [8] |
WU P, XIAO Y, GUO T, et al. Identifying miRNA-mRNA pairs and novel miRNAs from hepatocelluar carcinoma miRNomes and TCGA database[J]. J Cancer, 2019, 10(11): 2552-2559. DOI:10.7150/jca.28167 |
| [9] |
CHAN A H, SCHRODER K. Inflammasome signaling and regulation of interleukin-1 family cytokines[J]. J Exp Med, 2020, 217(1): e20190314. DOI:10.1084/jem.20190314 |
| [10] |
XIE D, LIU H, XU F, et al. IL33(interleukin 33)/ST2(interleukin 1 receptor-like 1) axis drives protective microglial responses and promotes white matter integrity after stroke[J]. Stroke, 2021, 52(6): 2150-2161. DOI:10.1161/STROKEAHA.120.032444 |
| [11] |
TONACCI A, QUATTROCCHI P, GANGEMI S. IL33/ST2 axis in diabetic kidney disease: a literature review[J]. Medicina, 2019, 55(2): 50. DOI:10.3390/medicina55020050 |
| [12] |
SHANI O, VOROBYOV T, MONTERAN L, et al. Fibroblast-derived IL33 facilitates breast cancer metastasis by modifying the immune microenvironment and driving type 2 immunity[J]. Cancer Res, 2020, 80(23): 5317-5329. DOI:10.1158/0008-5472.CAN-20-2116 |
| [13] |
KIM J, KIM W, MOON U J, et al. Intratumorally establishing type 2 innate lymphoid cells blocks tumor growth[J]. J Immunol, 2016, 196(5): 2410-2423. DOI:10.4049/jimmunol.1501730 |
 2023, Vol. 44
2023, Vol. 44


