2. 郑州大学药学院药物分析教研室, 郑州 450000;
3. 郑州大学附属郑州中心医院神经内科, 郑州 450001
2. Department of Pharmaceutical Analysis, School of Pharmacy, Zhengzhou University, Zhengzhou 450000, Henan, China;
3. Department of Neurology, Zhengzhou Central Hospital Affiliated to Zhengzhou University, Zhengzhou 450001, Henan, China
急性脑梗死(acute cerebral infarction,ACI)是临床常见的心、脑血管和神经系统疾病,发病率和病死率高,逐渐成为公共卫生面临的主要挑战之一[1]。ACI是脑血流短暂或永久减少的结果,仅涉及脑大动脉的范围[2]。目前,重组组织型纤溶酶原激活剂(recombinant tissue plasminogen activator,rt-PA)被认为是临床治疗ACI最有效的药物之一,但不良反应较多[3]。我国指南建议ACI患者发病3 h内应尽快静脉给予rt-PA溶栓治疗,发病6 h的ACI患者若不能使用rt-PA,可考虑静脉给予尿激酶[4]。替罗非班(tirofiban)是一种高选择性的糖蛋白Ⅱb/Ⅲa受体短效非肽抑制剂,可改善ACI患者的神经功能和体内炎症反应[5],但其作用机制尚未明确。沉默信息调节因子2相关酶1(silent information regulator factor 2-related enzyme 1,SIRT1)是一种依赖于烟酰胺腺嘌呤二核苷酸的Ⅲ类组蛋白脱乙酰酶,在所有细胞类型中都有表达。它以广泛的转录因子为靶标,参与许多生理和病理过程如氧化应激、炎症和凋亡等[6-8]。研究表明,SIRT1通过调节血管内皮生长因子(vascular endothelial growth factor,VEGF)的表达参与脑缺血再灌注损伤过程[9]。本研究通过构建ACI大鼠模型,观察替罗非班能否通过影响SIRT1/VEGF信号通路对ACI大鼠起保护作用。
1 材料和方法 1.1 实验动物75只体重220~270 g的雄性SD大鼠购自珠海百试通生物科技有限公司[动物生产许可证号为SCXK(粤)2020-0051]。将SD大鼠(4只/笼)在(24±1)℃、光/暗循环(12 h/12 h)条件下饲养,予自由饮食、饮水。本研究通过郑州市第七人民医院动物伦理委员会审批,实验操作均遵循动物实验3R原则。
1.2 试剂与仪器替罗非班(纯度98%)购自上海源叶生物科技有限公司,SIRT1特异性抑制剂EX-527和ECL发光试剂盒购自上海碧云天生物技术有限公司,丙二醛(硫代巴比妥酸法)、谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)(比色法)、超氧化物歧化酶(superoxide dismutase,SOD)(微板法)检测试剂盒及氯化三苯基四氮唑(triphenyl tetrazolium chloride,TTC)染色、H-E染色试剂盒购自北京索莱宝科技有限公司,兔源SIRT1、VEGF、GAPDH一抗及HRP偶联的二抗、TUNEL检测试剂盒购自英国Abcam公司。BX61型光学显微镜购自日本Olympus公司,Gel Doc XR+凝胶成像系统购自美国BIO-RAD公司。
1.3 实验方法 1.3.1 分组及给药大鼠随机分为假手术组、模型组、替罗非班组、SIRT1抑制剂组、替罗非班+SIRT1抑制剂组,每组15只。除假手术组外,其他4组大鼠均采用大脑中动脉闭塞法(middle cerebral artery occlusion,MCAO)诱发ACI。替罗非班组大鼠术前3 d经尾静脉注射60 μg/kg替罗非班[10];SIRT1抑制剂组大鼠术前3 d予5 mg/kg EX-527腹腔注射[11];替罗非班+SIRT1抑制剂组大鼠术前3 d经尾静脉注射60 μg/kg替罗非班的同时予5 mg/kg EX-527腹腔注射;假手术组和模型组大鼠术前3 d分别通过尾静脉和腹腔注射等体积的生理盐水。
1.3.2 ACI模型的建立[12]使用戊巴比妥钠(40 mg/kg)麻醉大鼠后,仰卧位固定,使体核温度保持在37 ℃。将大鼠右侧颈部切开,充分露出右侧颈总动脉,分离并结扎颈外动脉及其分支。经颈动脉残端将3-0尼龙线插入颈内动脉,并延伸至大脑前动脉以阻断大脑中动脉。2 h后取出尼龙线使血流恢复,然后缝合皮肤、消毒,单笼饲养。假手术组除未阻断大脑中动脉外,其余手术操作与其他4组相同。各组大鼠于再灌注24 h后处死并进行取材和指标检测。
1.3.3 神经功能评分使用双盲法进行神经功能评分[13]。评分标准:0分,无神经损伤症状;1分,患侧前爪不能完全伸出;2分,行走时患侧前爪向内旋转;3分,行走时患侧前爪向内倾斜;4分,患侧足不能自主活动,失去意识;5分,患侧肢体完全不能动。得分>3分说明ACI模型建立成功。
1.3.4 TTC染色检测脑梗死体积百分数神经功能评分完成后,每组取5只大鼠脑组织,切成2 mm厚的冠状切片,置于2% TTC溶液中在37 ℃孵育20 min,去除过量的TTC溶液,用4%多聚甲醛溶液固定。拍照并使用ImageJ 1.8.0软件分析脑梗死体积百分数。
1.3.5 血清丙二醛、GSH-Px、SOD的测定收集各组大鼠的外周血2 mL,分别按照试剂盒说明书操作,检测丙二醛、GSH-Px、SOD水平。
1.3.6 H-E染色检测脑组织海马区病理变化每组取5只大鼠脑组织,用4%多聚甲醛溶液固定过夜,经石蜡包埋后,用切片机切成4 μm厚的切片,经H-E染色后进行二甲苯透明、树胶封片。在光学显微镜下观察大鼠脑组织海马区病理变化并拍照。
1.3.7 TUNEL法检测神经元凋亡情况取脑组织海马区切片,用二甲苯脱蜡,乙醇梯度脱水至水化,按照TUNEL检测试剂盒说明书进行染色操作,然后在光学显微镜下随机选择5个视野拍照并进行细胞计数。正常细胞核呈蓝色,棕黄色为阳性细胞,按公式计算细胞凋亡率:细胞凋亡率(%)=阳性细胞数/总细胞数×100%。
1.3.8 蛋白质印迹法检测SIRT1/VEGF通路相关蛋白表达每组取5只大鼠脑组织海马区,置于-80 ℃保存。取出后研磨成匀浆,利用预冷的RIPA裂解缓冲液提取总蛋白质,采用BCA法测定蛋白质浓度,10% SDS-PAGE分离蛋白质后转膜,然后用5%脱脂奶粉溶液孵育2 h以阻断非特异性结合位点。加入SIRT1一抗(稀释比例为1∶1 000)、VEGF一抗(稀释比例为1∶1 000)、GAPDH一抗(稀释比例为1∶3 000),在4 ℃孵育过夜。用TBST洗涤2次后,加入HRP偶联的二抗在室温下孵育2 h,通过ECL发光试剂盒检测蛋白质显色情况。以GAPDH为内参照,采用凝胶成像系统分析目的蛋白的相对表达量。
1.4 统计学处理应用SPSS 25.0软件进行统计学分析。计量资料以x±s表示,多组间比较采用单因素方差分析,多重比较采用SNK-q检验。检验水准(α)为0.05。
2 结果 2.1 替罗非班降低大鼠脑梗死体积百分数及神经功能评分TTC染色结果显示,假手术组大鼠脑组织呈均一红色,模型组、替罗非班组、SIRT1抑制剂组、替罗非班+SIRT1抑制剂组大鼠脑组织存在白色梗死区域(图 1A)。由图 1B可见,与假手术组比较,模型组大鼠脑梗死体积百分数升高(P<0.05);与模型组比较,替罗非班组、替罗非班+SIRT1抑制剂组大鼠脑梗死体积百分数降低,SIRT1抑制剂组大鼠脑梗死体积百分数升高(P均<0.05);与替罗非班组比较,替罗非班+SIRT1抑制剂组大鼠脑梗死体积百分数升高(P<0.05)。由图 1C可见,与假手术组相比,模型组大鼠的神经功能评分升高(P<0.05);与模型组比较,替罗非班组、替罗非班+SIRT1抑制剂组大鼠的神经功能评分降低,SIRT1抑制剂组大鼠的神经功能评分升高(P均<0.05);与替罗非班组比较,替罗非班+SIRT1抑制剂组大鼠的神经功能评分升高(P<0.05)。

|
图 1 各组大鼠脑组织TTC染色、脑梗死体积百分数及神经功能评分 Fig 1 Brain tissue TTC staining, cerebral infarction volume percentage and neurological function score of rats in each group A: Representative TTC staining pictures of brain tissue; B: Comparison of cerebral infarction volume percentages in each group; C: Comparison of neurological function scores in each group. Sham group: The rats undergoing simulated surgery were injected with normal saline via tail vein 3 d before surgery; Model group: ACI model rats were injected intraperitoneally with normal saline 3 d before modeling; Tirofiban group: ACI model rats were injected with tirofiban (60 μg/kg) via tail vein 3 d before modeling; SIRT1 inhibitor group: ACI model rats were injected intraperitoneally with SITR1-specific inhibitor EX-527 (5 mg/kg) 3 d before modeling; Tirofiban+SITR1 inhibitor group: ACI model rats were injected with tirofiban (60 μg/kg) via tail vein and intraperitoneally administered with EX-527 (5 mg/kg) 3 d before modeling. *P < 0.05 vs sham group; △P < 0.05 vs model group; ▲P < 0.05 vs tirofiban group. n=5 (B) or 15 (C), x±s. TTC: Triphenyl tetrazolium chloride; ACI: Acute cerebral infarction; SITR1: Silent information regulator factor 2-related enzyme 1. |
2.2 替罗非班抑制大鼠氧化应激
与假手术组相比,模型组大鼠血清丙二醛水平升高,GSH-Px、SOD水平降低(P均<0.05);与模型组相比,替罗非班组、替罗非班+SIRT1抑制剂组大鼠血清丙二醛水平降低,GSH-Px、SOD水平升高,而SIRT1抑制剂组大鼠血清丙二醛水平升高,GSH-Px、SOD水平降低(P均<0.05);与替罗非班组比较,替罗非班+SIRT1抑制剂组大鼠血清丙二醛水平升高,GSH-Px、SOD水平降低(P均<0.05)。见图 2。

|
图 2 各组大鼠血清MDA、GSH-Px、SOD水平比较 Fig 2 Comparison of MDA, GSH-Px and SOD levels in serum of rats in each group A: MDA level detected by thiobarbituric acid method; B: GSH-Px level detected by colorimetry; C: SOD level detected by microplate test. Sham group: The rats undergoing simulated surgery were injected normal saline via tail vein 3 d before surgery; Model group: ACI model rats were injected intraperitoneally normal saline 3 d before modeling; Tirofiban group: ACI model rats were injected tirofiban (60 μg/kg) via tail vein 3 d before modeling; SIRT1 inhibitor group: ACI model rats were injected intraperitoneally SIRT1-specific inhibitor EX-527 (5 mg/kg) 3 d before modeling; Tirofiban+SIRT1 inhibitor group: ACI model rats were injected with tirofiban (60 μg/kg) via tail vein and intraperitoneally administered with EX-527 (5 mg/kg) 3 d before modeling. *P < 0.05 vs sham group; △P < 0.05 vs model group; ▲P < 0.05 vs tirofiban group. n=5, x±s. MDA: Malondialdehyde; GSH-Px: Glutathione peroxidase; SOD: Superoxide dismutase; ACI: Acute cerebral infarction; SIRT1: Silent information regulator factor 2-related enzyme 1. |
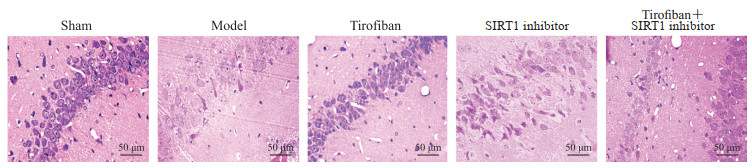
2.3 替罗非班减轻大鼠脑组织海马区病理损伤程度
H-E染色结果显示,假手术组大鼠脑组织海马区细胞结构完整,分布均匀,未见明显异常;模型组大鼠海马区大部分细胞排列紊乱,细胞间隙增大、细胞核破裂、凝缩变成固缩核,细胞坏死严重;替罗非班组大鼠海马区有少量细胞肿胀及坏死,病理损伤与模型组比较有所缓解;SIRT1抑制剂组大鼠海马区病理损伤程度较模型组明显加重;替罗非班+SIRT1抑制剂组大鼠脑组织海马区细胞排列紊乱,细胞间隙增大,大量细胞坏死,病变程度较替罗非班组严重,但较模型组有所缓解。见图 3。
|
图 3 各组大鼠脑组织海马区苏木精-伊红染色 Fig 3 Hematoxylin-eosin staining of hippocampus of rats in each group Sham group: The rats undergoing simulated surgery were injected with normal saline via tail vein 3 d before surgery; Model group: ACI model rats were injected intraperitoneally with normal saline 3 d before modeling; Tirofiban group: ACI model rats were injected with tirofiban (60 μg/kg) via tail vein 3 d before modeling; SIRT1 inhibitor group: ACI model rats were injected intraperitoneally with SIRT1-specific inhibitor EX-527 (5 mg/kg) 3 d before modeling; Tirofiban+SIRT1 inhibitor group: ACI model rats were injected with tirofiban (60 μg/kg) via tail vein and intraperitoneally administered with EX-527 (5 mg/kg) 3 d before modeling. ACI: Acute cerebral infarction; SIRT1: Silent information regulator factor 2-related enzyme 1. |
2.4 替罗非班抑制大鼠脑组织海马区的神经元凋亡
TUNEL染色结果显示,与假手术组相比,模型组大鼠脑组织海马区神经元凋亡率升高(P<0.05);与模型组比较,替罗非班组、替罗非班+SIRT1抑制剂组大鼠脑组织海马区神经元凋亡率降低,SIRT1抑制剂组大鼠脑组织海马区神经元凋亡率升高(P均<0.05);与替罗非班组比较,替罗非班+SIRT1抑制剂组大鼠脑组织海马区神经元凋亡率升高(P<0.05)。见图 4。

|
图 4 TUNEL染色检测各组大鼠脑组织海马区神经元凋亡情况 Fig 4 Neuronal apoptosis in brain hippocampal regions in rats of each group detected by TUNEL staining A: Representative TUNEL staining pictures of brain hippocampal regions of rats in each group; B: Comparison of neuronal apoptosis rate in each group. Sham group: The rats undergoing simulated surgery were injected with normal saline via tail vein 3 d before surgery; Model group: ACI model rats were injected intraperitoneally with normal saline 3 d before modeling; Tirofiban group: ACI model rats were injected with tirofiban (60 μg/kg) via tail vein 3 d before modeling; SIRT1 inhibitor group: ACI model rats were injected intraperitoneally with SIRT1-specific inhibitor EX-527 (5 mg/kg) 3 d before modeling; Tirofiban+SIRT1 inhibitor group: ACI model rats were injected with tirofiban (60 μg/kg) via tail vein and intraperitoneally administered with EX-527 (5 mg/kg) 3 d before modeling. *P < 0.05 vs sham group; △P < 0.05 vs model group; ▲P < 0.05 vs tirofiban group. n=5, x±s. TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling; ACI: Acute cerebral infarction; SIRT1: Silent information regulator factor 2-related enzyme 1. |
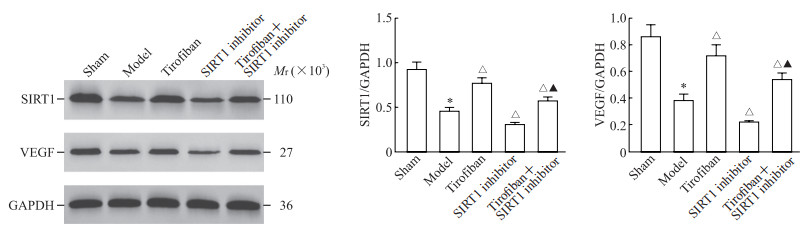
2.5 替罗非班激活SIRT1/VEGF信号通路
蛋白质印迹法检测结果显示,与假手术组相比,模型组大鼠海马组织中SIRT1、VEGF蛋白表达水平均降低(P均<0.05);与模型组比较,替罗非班组、替罗非班+SIRT1抑制剂组大鼠海马组织中SIRT1、VEGF蛋白表达水平均升高,SIRT1抑制剂组大鼠海马组织中SIRT1、VEGF蛋白表达水平均降低(P均<0.05);与替罗非班组比较,替罗非班+SIRT1抑制剂组大鼠海马组织中SIRT1、VEGF蛋白表达水平均降低(P均<0.05)。见图 5。
|
图 5 蛋白质印迹法检测各组大鼠海马组织中SIRT1、VEGF蛋白表达 Fig 5 Expression of SIRT1 and VEGF proteins in hippocampal tissue of rats in each group detected by Western blotting Sham group: The rats undergoing simulated surgery were injected with normal saline via tail vein 3 d before surgery; Model group: ACI model rats were injected intraperitoneally with normal saline 3 d before modeling; Tirofiban group: ACI model rats were injected with tirofiban (60 μg/kg) via tail vein 3 d before modeling; SIRT1 inhibitor group: ACI model rats were injected intraperitoneally with SIRT1-specific inhibitor EX-527 (5 mg/kg) 3 d before modeling; Tirofiban+inhibitor group: ACI model rats were injected with tirofiban (60 μg/kg) via tail vein and intraperitoneally administered with EX-527 (5 mg/kg) 3 d before modeling. *P < 0.05 vs sham group; △P < 0.05 vs model group; ▲P < 0.05 vs tirofiban group. n=5, x±s. SIRT1: Silent information regulator factor 2-related enzyme 1; VEGF: Vascular endothelial growth factor; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; ACI: Acute cerebral infarction. |
3 讨论
大脑是高灌注、高耗氧量、高代谢和低能量储备的重要器官。发生ACI时,血管内血流中断导致缺氧缺血、梗死区缺氧、脑组织能量耗尽,最终导致脑组织坏死或软化。研究表明,MCAO可导致大鼠的行为、神经化学和组织学异常,该模型能够模拟人类ACI的许多特征[14-15]。本研究通过MCAO构建ACI模型,考察替罗非班对ACI的神经保护作用,结果证实替罗非班可抑制氧化应激损伤、神经元凋亡,调节SIRT1、VEGF蛋白表达。
脑缺血是导致神经功能损害的重要原因。神经功能缺损评分是评价脑损伤的常用指标[16]。本研究结果表明,闭塞大鼠大脑中动脉可导致神经功能缺损评分升高,而替罗非班可改善神经功能、减轻脑缺血的发展;ACI模型大鼠出现神经元受损,替罗非班可改善脑损伤、缩小梗死灶。以上结果提示替罗非班能抑制MCAO诱导的神经元损伤,对脑缺血有保护作用。
人体大约20%的氧气供给被脑组织利用。大量证据表明,脑氧化应激在ACI的发病中起着重要作用。SOD是抵御线粒体基质中超氧阴离子的主要防线[17]。GSH-Px是一种细胞内元件,在保护细胞免受氧化损伤方面起着至关重要的作用[18]。丙二醛是脂质过氧化的重要产物,可以间接反映细胞内的脂质过氧化水平[19]。缺血的大脑皮质抗氧化酶活性升高,脂质过氧化产物含量降低,表明脑抗氧化能力提高。本研究结果显示替罗非班提高了SOD和GSH-Px水平、降低了丙二醛含量,与既往研究[14]一致,表明替罗非班能够通过抑制氧化应激减轻脑损伤。
SIRT1参与氧化应激、自噬、神经保护和线粒体功能等多种生物学过程[20]。SIRT1在脑缺血再灌注大鼠脑组织中低表达,激活其表达能改善神经功能障碍[21]。VEGF由大脑中许多神经血管细胞产生和分泌,被认为是缺血后血管生成的关键因子[22]。缺血性脑卒中发生时SIRT1表达降低,下游VEGF表达减少,阻碍脑血管生成,从而使神经功能受损[9]。EX-527是SIRT1的特异性抑制剂[23]。本研究中EX-527抑制ACI大鼠SIRT1表达的同时降低了VEGF蛋白水平,增加了脑组织病理损伤、神经元凋亡,激活了大鼠体内的氧化应激反应;抑制SIRT1减弱了替罗非班对缺血再灌注损伤的保护作用,提示替罗非班通过激活SIRT1/VEGF信号通路对ACI大鼠的神经元损伤起到保护作用。
综上所述,替罗非班可能通过激活SIRT1/VEGF信号通路抑制ACI过程中的氧化应激及神经元损伤,发挥对脑的保护作用。
| [1] |
ZHEN X Y, ZHENG Y, HONG X N, CHEN Y, GU P, TANG J R, et al. Physiological ischemic training promotes brain collateral formation and improves functions in patients with acute cerebral infarction[J/OL]. Front Neurol, 2016, 7: 235. DOI: 10.3389/fneur.2016.00235.
|
| [2] |
ZHANG J C, WU Y, WENG Z L, ZHOU T, FENG T, LIN Y. Glycyrrhizin protects brain against ischemia-reperfusion injury in mice through HMGB1-TLR4-IL-17A signaling pathway[J]. Brain Res, 2014, 1582: 176-186. DOI:10.1016/j.brainres.2014.07.002 |
| [3] |
DONG M X, HU Q C, SHEN P, PAN J X, WEI Y D, LIU Y Y, et al. Recombinant tissue plasminogen activator induces neurological side effects independent on thrombolysis in mechanical animal models of focal cerebral infarction: a systematic review and meta-analysis[J/OL]. PLoS One, 2016, 11: e0158848. DOI: 10.1371/journal.pone.0158848.
|
| [4] |
中华医学会神经病学分会, 中华医学会神经病学分会脑血管病学组. 中国急性缺血性脑卒中诊治指南2018[J]. 中华神经科杂志, 2018, 51: 666-682. DOI:10.3760/cma.j.issn.1006-7876.2018.09.004 |
| [5] |
田雨, 胡晓. 替罗非班在急性脑梗死中应用的研究进展[J]. 中国医药, 2021, 16: 763-767. |
| [6] |
LEE I C, HO X Y, GEORGE S E, GOH C W, SUNDARAM J R, PANG K K L, et al. Oxidative stress promotes SIRT1 recruitment to the GADD34/PP1α complex to activate its deacetylase function[J]. Cell Death Differ, 2018, 25: 255-267. DOI:10.1038/cdd.2017.152 |
| [7] |
VELAGAPUDI R, EL-BAKOUSH A, LEPIARZ I, OGUNRINADE F, OLAJIDE O A. AMPK and SIRT1 activation contribute to inhibition of neuroinflammation by thymoquinone in BV2 microglia[J]. Mol Cell Biochem, 2017, 435: 149-162. DOI:10.1007/s11010-017-3064-3 |
| [8] |
ZHANG X S, LU Y, LI W, TAO T, PENG L, WANG W H, et al. Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice[J]. Br J Pharmacol, 2021, 178: 1114-1132. DOI:10.1111/bph.15346 |
| [9] |
ZHENG X W, SHAN C S, XU Q Q, WANG Y, SHI Y H, WANG Y, et al. Buyang Huanwu decoction targets SIRT1/VEGF pathway to promote angiogenesis after cerebral ischemia/reperfusion injury[J]. Front Neurosci, 2018, 12: 911. DOI:10.3389/fnins.2018.00911 |
| [10] |
刘玲梅, 张梅, 刘艳红, 周欣, 叶帆, 何瑞波, 等. 替罗非班对大鼠心肌缺血再灌注后无复流及NF-κB激活的影响[J]. 中国病理生理杂志, 2009, 25: 1892-1897. DOI:10.3321/j.issn:1000-4718.2009.10.005 |
| [11] |
曹自为, 张嵘, 李蒙, 管叶明, 汪青松. 丹参多酚酸通过SIRT1/HMGB1路径改善大鼠脑缺血/再灌注损伤的机制研究[J]. 中风与神经疾病杂志, 2021, 38: 4-8. |
| [12] |
YANG B B, ZOU M, ZHAO L, ZHANG Y K. Astaxanthin attenuates acute cerebral infarction via Nrf-2/ HO-1 pathway in rats[J/OL]. Curr Res Transl Med, 2021, 69: 103271. DOI: 10.1016/j.retram.2020.103271.
|
| [13] |
BEDERSON J B, PITTS L H, TSUJI M, NISHIMURA M C, DAVIS R L, BARTKOWSKI H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination[J]. Stroke, 1986, 17: 472-476. DOI:10.1161/01.STR.17.3.472 |
| [14] |
DUAN X H, WANG W L, LIU X Q, YAN H W, DAI R, LIN Q. Neuroprotective effect of ethyl acetate extract from Gastrodia elata against transient focal cerebral ischemia in rats induced by middle cerebral artery occlusion[J]. J Tradit Chin Med, 2015, 35: 671-678. DOI:10.1016/S0254-6272(15)30158-8 |
| [15] |
PARK D J, KANG J B, KOH P O. Epigallocatechin gallate alleviates neuronal cell damage against focal cerebral ischemia in rats[J]. J Vet Med Sci, 2020, 82: 639-645. DOI:10.1292/jvms.19-0703 |
| [16] |
HUANG Y, PAN L S, WU T. Improvement of cerebral ischemia-reperfusion injury by L-3-n-butylphthalide through promoting angiogenesis[J]. Exp Brain Res, 2021, 239: 341-350. DOI:10.1007/s00221-020-05978-6 |
| [17] |
SUN K, FAN J Y, HAN J Y. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage[J]. Acta Pharm Sin B, 2015, 5: 8-24. DOI:10.1016/j.apsb.2014.11.002 |
| [18] |
YAN W J, REN D Q, FENG X X, HUANG J W, WANG D B, LI T, et al. Neuroprotective and anti-inflammatory effect of pterostilbene against cerebral ischemia/reperfusion injury via suppression of COX-2[J/OL]. Front Pharmacol, 2021, 12: 770329. DOI: 10.3389/fphar.2021.770329.
|
| [19] |
YU J, WANG W N, MATEI N, LI X, PANG J W, MO J, et al. Ezetimibe attenuates oxidative stress and neuroinflammation via the AMPK/Nrf2/TXNIP pathway after MCAO in rats[J/OL]. Oxid Med Cell Longev, 2020, 2020: 4717258. DOI: 10.1155/2020/4717258.
|
| [20] |
HORIO Y, HAYASHI T, KUNO A, KUNIMOTO R. Cellular and molecular effects of sirtuins in health and disease[J]. Clin Sci (Lond), 2011, 121: 191-203. DOI:10.1042/CS20100587 |
| [21] |
SHI Y H, ZHANG X L, YING P J, WU Z Q, LIN L L, CHEN W, et al. Neuroprotective effect of astragaloside Ⅳ on cerebral ischemia/reperfusion injury rats through Sirt1/Mapt pathway[J/OL]. Front Pharmacol, 2021, 12: 639898. DOI: 10.3389/fphar.2021.639898.
|
| [22] |
YIN K J, HAMBLIN M, CHEN Y E. Angiogenesis-regulating microRNAs and ischemic stroke[J]. Curr Vasc Pharmacol, 2015, 13: 352-365. DOI:10.2174/15701611113119990016 |
| [23] |
QIN T T, LIU W H, HUO J F, LI L L, ZHANG X Y, SHI X L, et al. SIRT1 expression regulates the transformation of resistant esophageal cancer cells via the epithelial-mesenchymal transition[J]. Biomed Pharmacother, 2018, 103: 308-316. DOI:10.1016/j.biopha.2018.04.032 |
 2023, Vol. 44
2023, Vol. 44


