2. 上海拜羡生物科技有限公司, 上海 201318
2. Shanghai Baixian Biotechnology Co., Ltd, Shanghai 201318, China
我国是肝脏疾病高发国家。多种因素会使肝脏遭受不可逆转的损伤,最终导致肝衰竭[1]。肝移植是终末期肝衰竭最有效的治疗方式[2],然而由于供体极度匮乏,部分患者在等待移植过程中死亡。研究表明,通过移植具有功能的肝细胞可以帮助患者度过等待期,甚至缓解肝衰竭相关症状而无需器官移植[3-4]。因此,体外获得有功能的肝细胞具有潜在医用价值。常见的功能性肝细胞的获取方式包括肝组织分离、胚胎干细胞或多能干细胞定向分化、体细胞重编程等。肝组织分离肝细胞是通过酶消化、分散、梯度离心等步骤获得肝组织中的实质细胞,获得的细胞功能完全,但同样依赖正常的供肝来源[5-6]。将胚胎干细胞或多能干细胞在特定的培养条件下分化为功能性肝细胞是目前研究较多的方法[7-9],其优势是细胞来源充足,但是移植入体内后存在一定致瘤风险。其他方法如体细胞重编程等所得肝细胞样细胞通常功能较弱,或因经病毒改造而存在不可预知的风险,暂时不适合移植入人体内。
研究表明,肝脏分化命运受Notch信号通路、Hedgehog、Wnt、TGF-β、成纤维细胞生长因子(fibroblast growth factor,FGF)、骨形态发生蛋白(bone morphogenetic protein,BMP)、Onecut转录因子等调控[10-13]。Kuver等[14]曾将小鼠胆囊上皮细胞诱导为能表达部分肝细胞特异性基因的细胞。Lee等[15]将外源胆囊上皮细胞移植入肝脏受损的小鼠体内,发现受体小鼠的肝脏微环境促使外源胆囊上皮细胞分化为肝细胞样细胞。我们前期建立了人胆囊上皮细胞(human gallbladder epithelial cell,hGBEC)培养体系,该细胞可在体外形成类器官,并表达肝脏干细胞标志物上皮细胞黏附分子(epithelial cell adhesion molecule,EpCAM)、性别决定区Y框蛋白(sex determining region Y box protein,SOX)9、富含亮氨酸重复序列G蛋白偶联受体5(leucine-rich repeat-containing G protein-coupled receptor 5,LGR5)、肝细胞核因子4α(hepatocyte nuclear factor 4α,HNF4α)等[16],说明其具有分化潜能。根据肝胆发育同源的特性,有望由hGBEC分化得到具有功能的肝细胞样细胞。本研究通过使用小分子化合物改变细胞分化过程中关键信号通路等方式筛选分化体系,建立了由hGBEC分化为肝细胞样细胞的培养方案,以期为后续临床应用提供候选功能性肝细胞。
1 材料和方法 1.1 主要试剂胶原酶Ⅰ购自瑞士Roche公司;基质胶购自美国Corning公司;DMEM/F12培养基、GlutaMAX添加剂、HEPES缓冲液、青霉素-链霉素溶液、N2添加剂、B27添加剂、ITS添加剂购自美国Gibco公司;N-乙酰半胱氨酸、烟酰胺购自美国Sigma公司;表皮生长因子(epidermal growth factor,EGF)、肝细胞生长因子(hepatocyte growth factor,HGF)、R-脊椎蛋白-1、胃泌素、抑癌蛋白M(oncostatin M,OSM)购自美国PeproTech公司;S1067、S2714、forskolin、S7359、S1143、维生素K(vitamin K,VK)、A83-01购自美国Selleck公司;兔抗EpCAM、兔抗细胞角蛋白19(cytokeratin 19,CK19)、兔抗SOX9、兔抗HNF4α、兔抗α1-抗胰蛋白酶(α1-antitrypsin,AAT)抗体购自英国Abcam公司,山羊抗白蛋白(albumin,ALB)抗体购自美国Bethyl公司,兔抗细胞色素P450 3A4(cytochrome P450 family 3 subfamily A member 4,CYP3A4)、兔抗延胡索酰乙酰乙酸水解酶(fumarylacetoacetic acid hydrolase,FAH)抗体购自美国Proteintech公司,荧光二抗购自美国Invitrogen公司;DNA酶Ⅰ、TRIzol试剂盒、DNA maker、PCR试剂盒购自日本TaKaRa公司;反转录试剂盒购自美国Promega公司;qPCR试剂盒购自德国Qiagen公司;DMSO、琼脂糖、牛血清白蛋白(bovine serum albumin,BSA)购自生工生物工程(上海)股份有限公司;糖原过碘酸希夫(periodic acid-Schiff,PAS)染色液购自北京索莱宝科技有限公司;BODIPY-493荧光探针购自北京多荧科技发展有限公司;DAPI购自上海碧云天生物技术有限公司;人ALB ELISA检测试剂盒购自美国Bethyl公司。
肝细胞基础培养基(hepatic basal medium,HBM)和人肝细胞生长培养基(human liver expansion medium,HLEM)均以DMEM/F12培养基为基础,添加其他添加剂。HBM中各添加剂及最终浓度如下:1×GlutaMAX添加剂,1×HEPES缓冲液,1×青霉素-链霉素溶液,1×N2添加剂,1×B27添加剂。HLEM中各添加剂及最终浓度如下:1×GlutaMAX,1×HEPES缓冲液,1×青霉素-链霉素溶液,1×N2添加剂,1×B27添加剂,1 mmol/L N-乙酰半胱氨酸,10 mmol/L烟酰胺,50 ng/mL EGF,25 ng/mL HGF,25 ng/mL R-脊椎蛋白-1,10 nmol/L胃泌素,5 μmol/L A83-01。
1.2 hGBEC的获取及培养原代hGBEC购自上海拜羡生物科技有限公司,分离和培养步骤如下:将胆囊纵向剪开,用PBS将胆囊中残留的胆汁清洗干净,放在培养皿中,剪去胆囊外壁;用剪刀将胆囊上皮剪碎,转移至离心管中,加入适量消化液(300 U/mL胶原酶Ⅰ+0.3 mg/mL DNA酶Ⅰ+1×ITS细胞培养添加物+0.1% BSA),在37 ℃水浴锅中消化60 min,每15 min摇晃1次离心管;消化结束后,用70 μm滤网过滤,收集滤液,加入PBS至50 mL,4 ℃下300×g离心7 min;弃上清,再加入PBS重悬细胞,4 ℃下300×g离心7 min;弃上清,用基质胶重悬细胞,将细胞悬液接种于24孔板,每孔50 μL,放于CO2细胞培养箱10 min使基质胶固化,再加入HLEM进行培养。
1.3 分化条件的筛选由于Notch、Hedgehog、Wnt、TGF-β等信号通路对肝细胞发育和命运决定至关重要[10-13],本研究选择DMSO及可干预相关信号通路的OSM、S7359、S1143、S1067、S2714、forskolin、VK共8个分子,对三维培养至第3~5代的细胞进行诱导分化,筛选合适的分化条件。
以HBM为基础添加诱导分子,所有分子的终浓度均为10 mmol/L。第1轮筛选时,采用多分子组合和单分子2种添加方式。多分子组合添加方式分为9组:A组添加全部8个分子;B~I组均添加7个分子,在8个分子中分别减去OSM、S7359、S1143、S1067、S2714、forskolin、DMSO、VK。单分子添加方式分为8组,培养基中只加入单个分子,L~S组分别加入OSM、S7359、S1143、S1067、S2714、forskolin、DMSO、VK。所有细胞均诱导分化14 d。另外用HBM和HLEM培养相同时间作为对照(J、K组)。Con组为三维培养7 d的第5代hGBEC。
根据第1轮筛选结果制订第2轮筛选方案,分别添加S1067、S2714、OSM、S7359、S1143、forskolin和VK 7个分子(A组)、只添加S1067和S2714(B组)、添加OSM、S7359、S1143、forskolin、VK 5个分子(C组)、以S1067和S2714作为固定添加物并分别添加OSM、S7359、S1143、forskolin、VK中任1个分子(D~H组),诱导分化14 d。
1.4 PCR和qPCR检测关键基因的表达收集待检测的细胞,按照TRIzol试剂盒说明书提取细胞总RNA。用紫外分光光度计测量RNA浓度,按照反转录试剂盒说明书将RNA反转录为cDNA。以cDNA作为模板,按照PCR试剂盒和qPCR试剂盒说明书进行PCR扩增。引物序列如表 1所示。PCR反应条件:95 ℃ 5 min;95 ℃ 5 s、58 ℃ 30 s、72℃ 30 s,检测β-肌动蛋白表达时为20个循环,检测其余基因表达时为30个循环;72 ℃ 5 min。qPCR反应条件:95 ℃ 30 s;95 ℃ 10 s、60 ℃ 30 s、95 ℃ 10 s、65 ℃ 60 s、97 ℃ 1 s、37 ℃ 30 s,共40个循环。
|
|
表 1 引物序列 Tab 1 Primer sequences |
1.5 免疫荧光染色检测相关蛋白的表达
待鉴定的细胞用4%多聚甲醛溶液室温过夜固定;用75%、80%、95%、100%、100%梯度乙醇脱水,每阶段30 min;二甲苯透明化10 min;浸58 ℃蜡液3次,每次30 min;将细胞转移至金属模具中,加入适量蜡液,凝固后形成蜡块。蜡块以2 μm厚度连续切片,将切片置于40 ℃烘片机上过夜烘干;将切片脱蜡复水,用酸性修复液修复抗原,高压灭菌锅设置为121 ℃,2 min开始修复,修复结束后自然放凉至室温;用免疫疏水笔圈出载玻片上细胞的部位,用BSA封闭液室温孵育30 min;加入一抗工作液,4 ℃过夜孵育;用PBS清洗3次,每次5 min;加入荧光二抗工作液,37 ℃孵育40 min;用PBS清洗3次,每次5 min;用DAPI复染细胞核。封片后在荧光显微镜下观察拍照。
1.6 BODIPY-493染色检测细胞脂肪合成能力吸取培养中的细胞悬液1 mL至低吸附细胞培养板,加入2 µmol/L BODIPY-493染色剂1 µL、DAPI染色液1 µL,在细胞培养箱中孵育30 min;孵育结束后,将细胞转移至离心管中,用预冷的PBS清洗3次,最后加入培养基转移到原孔中。在绿色激发光下观察细胞中绿色脂滴的分布。
1.7 PAS染色检测细胞糖原贮存能力根据PAS试剂使用说明书将实验步骤修改如下:石蜡切片常规脱蜡复水,并用疏水笔圈出细胞的部位;圈内滴加氧化剂完全覆盖细胞,室温孵育15 min;自来水浸洗2次,双蒸水浸洗2次,每次30 s;甩去多余水分,圈内滴加希夫试剂覆盖细胞,室温避光孵育15 min,结束后用自来水冲洗2 min;甩去多余水分,滴加Mayer苏木精复染2 min;结束后自来水冲洗5 min;常规方法脱水、封片。封片剂干透后在显微镜下观察并拍照。
1.8 ELISA检测细胞ALB分泌能力采用ELISA法检测细胞培养上清液中ALB的浓度,根据试剂盒说明书进行操作。
1.9 统计学处理用GraphPad Prism 9软件进行统计学分析。所有实验重复至少3次,数据以x±s表示,两组间比较采用独立样本t检验,多组间比较采用单因素方差分析。检验水准(α)为0.05。
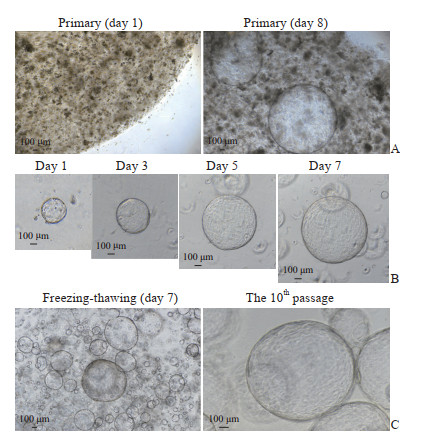
2 结果 2.1 hGBEC在体外可形成类器官将原代hGBEC接种在基质胶中,基质胶为细胞提供三维生长空间。细胞接种1 d后,在显微镜下可见已形成类器官样的球形结构,第8天可见中间透亮、整体封闭、体积较大的类器官,直径可达1.5~2.0 mm(图 1A)。由于基质胶内生长空间有限,7~10 d内需传代1次,传代后的细胞可形成类器官(图 1B)。冻存复苏后的细胞也可保持完整的球形结构,并稳定传至第10代(图 1C)。以上结果说明hGBEC能在体外以三维类器官的方式长期培养。
|
图 1 hGBEC在体外形成类器官 Fig 1 Organoids formed from hGBECs in vitro Typical cell growth was photographed with inverted phase contrast microscope. A: Primary hGBECs formed organoid-structures in the Matrigel, with a diameter of 1.5-2.0 mm on day 8; B: The cells formed organoids after passaging, and the lumen gradually enlarged; C: After cryopreservation and recovery, hGBECs could form organoids with transparent middle, regular shape and large volume, and stably propagating to the 10th passage. hGBEC: Human gallbladder epithelial cell. |
2.2 hGBEC形成的类器官表达肝脏干细胞标志物
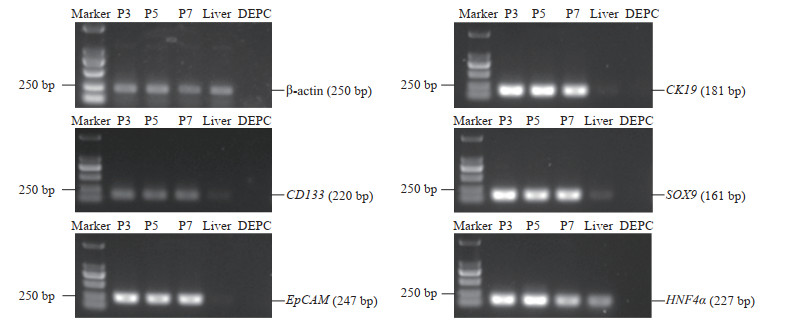
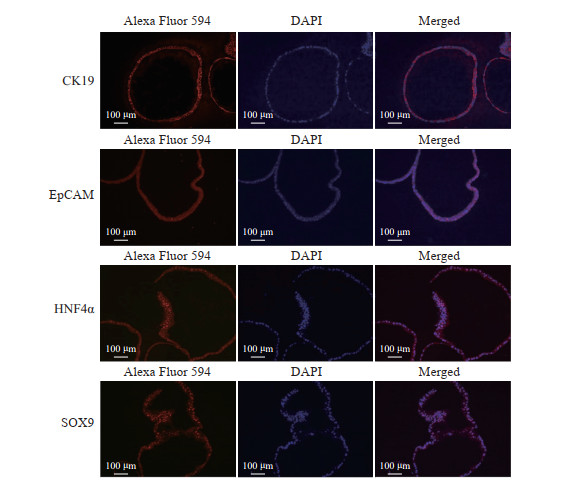
提取不同代次的类器官RNA经反转录得到cDNA,进行PCR鉴定。在mRNA水平,类器官可以表达干细胞相关基因CD133,肝脏干细胞相关基因EpCAM、CK19,以及肝前体细胞相关基因SOX9、HNF4α(图 2)。免疫荧光染色结果进一步证明,类器官在蛋白水平上表达肝脏干细胞和肝前体细胞标志物(图 3)。上述结果提示hGBEC具有分化为肝细胞的潜力。
|
图 2 PCR检测hGBEC中干性相关基因的表达 Fig 2 Expression of stem cell related genes in hGBECs detected by PCR Different passages of hGBECs expressed stem cell marker gene (CD133), liver stem cell marker genes (EpCAM and CK19), and liver precursor cell marker genes (SOX9 and HNF4α). P3, P5 and P7 represent the 3rd, 5th and 7th passage of hGBECs. PCR: Polymerase chain reaction; hGBEC: Human gallbladder epithelial cell; DEPC: Diethyl pyrocarbonate water (negative control); EpCAM: Epithelial cell adhesion molecule; CK19: Cytokeratin19; SOX9: Sex determining region Y box protein 9; HNF4α: Hepatocyte nuclear factor 4α. |
|
图 3 免疫荧光染色鉴定hGBEC中干性标志物的表达 Fig 3 Expression of stem cell markers in hGBECs identified by immunofluorescence staining hGBEC: Human gallbladder epithelial cell; CK19: Cytokeratin 19; EpCAM: Epithelial cell adhesion molecule; HNF4α: Hepatocyte nuclear factor 4 α; SOX9: Sex determining region Y box protein 9; DAPI: 4', 6-diamidino-2-phenylindole. |
2.3 hGBEC可诱导分化为肝细胞样细胞
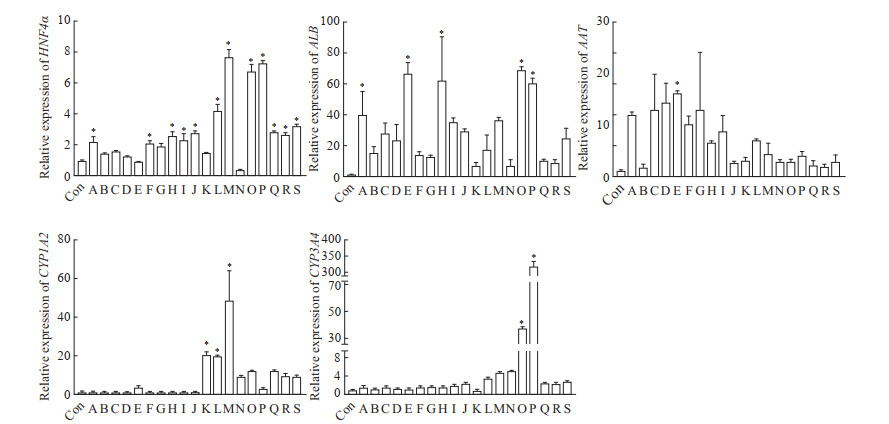
在第1轮筛选中,qPCR结果显示,多个分子共同诱导时,其中OSM主要促进AAT的表达,而对其他肝细胞功能标志物基因表达的影响不明显。只添加单种分子诱导时,TGF-β信号通路抑制剂S1067、Notch信号通路抑制剂S2714对肝细胞功能标志物基因的表达有明显促进作用(图 4)。
|
图 4 hGBEC经不同分化条件诱导后肝细胞相关基因的表达 Fig 4 Expression of hepatocyte function genes of hGBECs affected by different conditions The expression of genes was detected by quantitative polymerase chain reaction. Group Con: The 5th passage of hGBECs at day 7; Group A: hGBECs cultured with OSM, S7359, S1143, S1067, S2714, forskolin, DMSO and VK; Group B: hGBECs cultured with S7359, S1143, S1067, S2714, forskolin, DMSO and VK; Group C: hGBECs cultured with OSM, S1143, S1067, S2714, forskolin, DMSO and VK; Group D: hGBECs cultured with OSM, S7359, S1067, S2714, forskolin, DMSO and VK; Group E: hGBECs cultured with OSM, S7359, S1143, S2714, forskolin, DMSO and VK; Group F: hGBECs cultured with OSM, S7359, S1143, S1067, forskolin, DMSO and VK; Group G: hGBECs cultured with OSM, S7359, S1143, S1067, S2714, DMSO and VK; Group H: hGBECs cultured with OSM, S7359, S1143, S1067, S2714, forskolin and VK; Group Ⅰ: hGBECs cultured with OSM, S7359, S1143, S1067, S2714, forskolin and DMSO; Group J: hGBECs cultured with HBM; Group K: hGBECs cultured with HLEM; Group L: hGBECs cultured with OSM; Group M: hGBECs cultured with S7359; Group N: hGBECs cultured with S1143; Group O: hGBECs cultured with S1067; Group P: hGBECs cultured with S2714; Group Q: hGBECs cultured with forskolin; Group R: hGBECs cultured with DMSO; Group S: hGBECs cultured with VK. *P < 0.05 vs Con group. n=3, x±s. hGBEC: Human gallbladder epithelial cell; HNF4α: Hepatocyte nuclear factor 4α; ALB: Albumin; AAT: α1-antitrypsin; CYP1A2: Cytochrome P450 family 1 subfamily A member 2; CYP3A4: Cytochrome P450 family 3 subfamily A member 4; OSM: Oncostatin M; DMSO: Dimethyl sulfoxide; VK: Vitamin K; HBM: Hepatic basal medium; HLEM: Human liver expansion medium. |
2.4 抑制TGF-β和Notch信号通路可促进hGBEC分化的肝细胞样细胞功能成熟
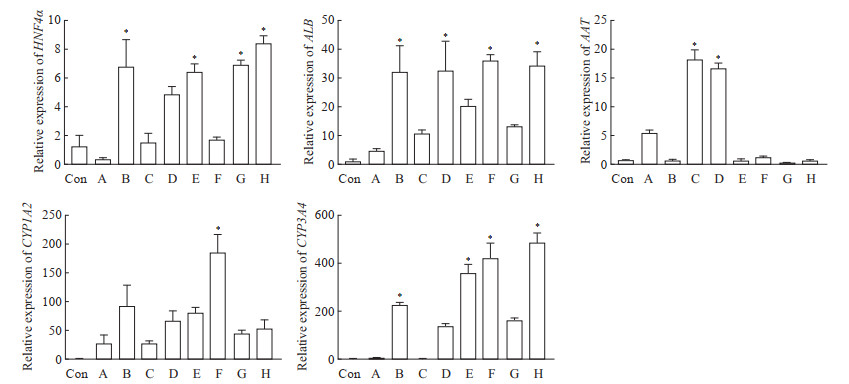
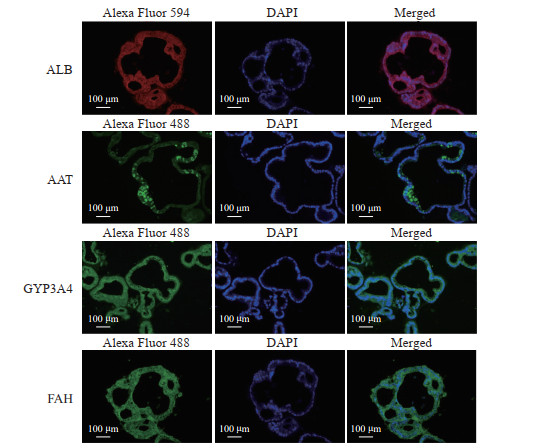
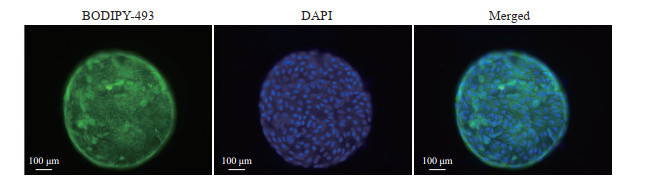
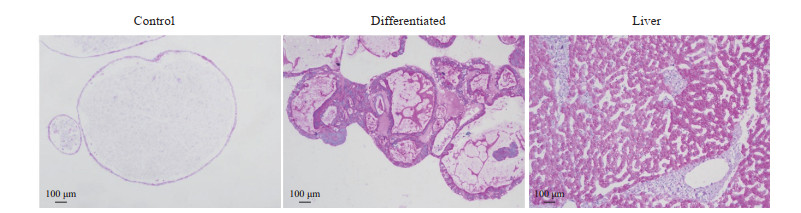
在第2轮筛选中,qPCR结果显示,添加S1067和S2714能有效促进肝细胞功能标志物基因HNF4α、ALB、AAT、CYP1A2和CYP3A4上调;在添加S1067和S2714基础上再加入其他单种分子时,同样可促进部分基因的表达;而在添加S1067和S2714基础上加入OSM时,能有效诱导AAT和ALB的表达,对其他肝细胞功能标志物基因表达无显著影响(图 5)。免疫荧光染色结果显示,在诱导分化后的细胞中,ALB、AAT、CYP3A4、FAH分布在细胞质中(图 6)。BODIPY-493染色结果显示,诱导分化后的细胞内可观察到脂滴分布(图 7)。PAS染色结果显示,诱导分化后的细胞同肝组织相似呈紫红色,未诱导分化的细胞则只显示少量紫红色(图 8)。ELISA检测结果显示,未诱导的细胞与诱导分化后的细胞培养上清液中ALB的含量分别为(0.03±0.00)pg·cell-1·d-1、(0.09±0.02)pg·cell-1·d-1,差异有统计学意义(P<0.05)。原代肝细胞的ALB分泌量为(0.91±0.08)pg·cell-1·d-1。上述结果表明,由hGBEC分化得到的肝细胞样细胞能够表达肝细胞功能标志物,并具有脂质合成、糖原贮存和ALB分泌等肝脏基本功能。
|
图 5 抑制TGF-β和Notch信号通路诱导hGBEC细胞表达肝细胞功能标志物 Fig 5 Expression of hepatocyte functional markers in hGBECs induced by inhibiting TGF-β and Notch signal pathways The expression of functional genes in hepatocytes was detected by quantitative polymerase chain reaction. Group Con: The 5th passage of hGBECs; Group A: hGBECs cultured with S1067, S2714, OSM, S7359, S1143, forskolin and VK; Group B: hGBECs cultured with S1067 and S2714; Group C: hGBECs cultured with OSM, S7359, S1143, forskolin and VK; Group D: hGBECs cultured with S1067, S2714 and OSM; Group E: hGBECs cultured with S1067, S2714 and S7359; Group F: hGBECs cultured with S1067, S2714 and S1143; Group G: hGBECs cultured with S1067, S2714 and forskolin; Group H: hGBECs cultured with S1067, S2714 and VK. *P < 0.05 vs Con group. n=3, x±s. TGF-β: Transforming growth factor β; hGBEC: Human gallbladder epithelial cell; HNF4α: Hepatocyte nuclear factor 4α; ALB: Albumin; AAT: α1-antitrypsin; CYP1A2: Cytochrome P450 family 1 subfamily A member 2; CYP3A4: Cytochrome P450 family 3 subfamily A member 4; OSM: Oncostatin M; VK: Vitamin K. |
|
图 6 免疫荧光染色显示hGBEC分化后的肝细胞样细胞中肝细胞功能标志物的分布 Fig 6 Distribution of liver functional markers in hGBEC-differentiated hepatocyte-like cells detected by immunofluorescence staining hGBEC: Human gallbladder epithelial cell; ALB: Albumin; AAT: α1-antitrypsin; CYP3A4: Cytochrome P450 family 3 subfamily A member 4; FAH: Fumarylacetoacetic acid hydrolase; GFP: Green fluorescence protein; DAPI: 4', 6-diamidino-2-phenylindole. |
|
图 7 BODIPY-493染色显示hGBEC分化后的肝细胞样细胞内脂滴的分布 Fig 7 Distribution of lipid droplets in hGBEC-differentiated hepatocyte-like cells displayed by BODIPY-493 staining hGBEC: Human gallbladder epithelial cell; DAPI: 4', 6-diamidino-2-phenylindole. |
|
图 8 过碘酸希夫染色显示hGBEC分化后的肝细胞样细胞内储存大量糖原 Fig 8 Periodic acid-Schiff staining showing a large amount of glycogen in hGBEC-differentiated hepatocyte-like cells hGBEC: Human gallbladder epithelial cell. |
3 讨论
胆囊组织是肝脏胆管结构的一部分,在发育过程中与肝脏同样起始于内胚层的肝芽组织[17]。在发育早期,胆囊与肝脏表达共同的早期前体细胞标志物叉头框蛋白A2(forkhead box A2,FOXA2)、GATA结合蛋白4(GATA binding protein 4,GATA4)等[18]。个体发育成熟后,部分胆囊上皮细胞表达肝脏干细胞标志物如LGR5、EpCAM、SOX17等,具备肝脏干细胞分化潜能[19-20]。我们前期建立了hGBEC的培养体系,该细胞可以囊状类器官的生长模式长期培养,表达肝脏经典干性标志物CK19、EpCAM、HNF4α、SOX9[16]。
胆囊上皮细胞分化为有功能肝细胞样细胞具有天然优势。第一,胆囊上皮细胞来源丰富,可由临床胆囊切除手术获得,保证了细胞充足和多样性的来源。第二,胆囊上皮细胞在发育上与肝脏同源,更易分化为肝细胞[17]。第三,胆囊上皮细胞可在体外扩增培养且不具有成瘤能力。我们前期做了细胞的体内安全性评价实验,通过将1×107个胆囊上皮细胞移植入裸鼠体内进行长期观察,结果显示所有受体动物均未成瘤(待发表资料)。第四,异体细胞排斥一直是临床移植面临的重要问题。通过建立不同胆囊来源的细胞库,有望构建不同抗原配型的生物库,扩大受众范围。
通过既往关于肝细胞、胆管细胞的发育路径,我们选择对发育中重要信号通路有影响的化合物和蛋白分子,研究其最佳组合条件,以获得具有肝细胞功能的体外肝细胞样细胞。经过多次组合筛选和重复实验,最终确定抑制TGF-β和Notch信号通路的化合物组合诱导hGBEC分化为肝细胞样细胞效果最好。因此,后期实验中用这个组合诱导以获得体外肝细胞样细胞,并对肝细胞样细胞进行肝细胞功能鉴定。肝细胞样细胞中存在脂滴和糖原的分布,说明肝细胞样细胞具有肝细胞脂质合成、糖原合成与贮存的基本功能。虽然肝细胞样细胞的ALB分泌能力较未分化的细胞有所提升,但对比原代肝细胞及其他研究人员发表的结果[21],本研究获得的肝细胞样细胞的ALB分泌能力仍较低。本研究得到的肝细胞样细胞目前只具备肝细胞基本功能,还需要进一步调整方案获得功能更加完全的肝细胞样细胞,并进一步检测目的细胞的药物代谢能力、验证其在体内的存活及功能等情况。
综上所述,hGBEC作为候选功能肝细胞来源具有良好的应用前景,通过抑制TGF-β和Notch信号通路可诱导其分化为具有部分肝功能的肝细胞样细胞,有望成为治疗肝脏疾病或研究药物的起始细胞。后续研究将进一步优化培养方案获得功能成熟的肝细胞样细胞,并深入探索相关机制。
| [1] |
MALHI H, GORES G J. Cellular and molecular mechanisms of liver injury[J]. Gastroenterology, 2008, 134: 1641-1654. DOI:10.1053/j.gastro.2008.03.002 |
| [2] |
AEHLING N F, SEEHOFER D, BERG T. [Liver transplantation—current trends][J]. Dtsch Med Wochenschr, 2020, 145: 1124-1131. DOI:10.1055/a-0982-0737 |
| [3] |
FORBES S J, GUPTA S, DHAWAN A. Cell therapy for liver disease: from liver transplantation to cell factory[J]. J Hepatol, 2015, 62(1 Suppl): S157-S169. |
| [4] |
LEE C A, SINHA S, FITZPATRICK E, DHAWAN A. Hepatocyte transplantation and advancements in alternative cell sources for liver-based regenerative medicine[J]. J Mol Med (Berl), 2018, 96: 469-481. DOI:10.1007/s00109-018-1638-5 |
| [5] |
BARTLETT D C, NEWSOME P N. Hepatocyte cell therapy in liver disease[J]. Expert Rev Gastroenterol Hepatol, 2015, 9: 1261-1272. DOI:10.1586/17474124.2015.1073106 |
| [6] |
ZHANG K, ZHANG L, LIU W, MA X, CEN J, SUN Z, et al. In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity[J/OL]. Cell Stem Cell, 2018, 23: 806-819.e4. DOI: 10.1016/j.stem.2018.10.018.
|
| [7] |
BROLÉN G, SIVERTSSON L, BJÖRQUIST P, ERIKSSON G, EK M, SEMB H, et al. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage[J]. J Biotechnol, 2010, 145: 284-294. DOI:10.1016/j.jbiotec.2009.11.007 |
| [8] |
HUPPERT S S, CAMPBELL K M. Emerging advancements in liver regeneration and organogenesis as tools for liver replacement[J]. Curr Opin Organ Transplant, 2016, 21: 581-587. DOI:10.1097/MOT.0000000000000365 |
| [9] |
FENG S S, WU J Y, QIU W L, YANG L, DENG X G, ZHOU Y, et al. Large-scale generation of functional and transplantable hepatocytes and cholangiocytes from human endoderm stem cells[J/OL]. Cell Rep, 2020, 33: 108455. DOI: 10.1016/j.celrep.2020.108455.
|
| [10] |
YOU N, LIU W, ZHONG X, DOU K, TAO K. Possibility of the enhanced progression of fetal liver stem/progenitor cells therapy for treating end-stage liver diseases by regulating the Notch signaling pathway[J]. Arch Med Res, 2012, 43: 585-587. DOI:10.1016/j.arcmed.2012.09.004 |
| [11] |
FITAMANT J, KOTTAKIS F, BENHAMOUCHE S, TIAN H, CHUVIN N, PARACHONIAK C, et al. YAP inhibition restores hepatocyte differentiation in advanced HCC, leading to tumor regression[J]. Cell Rep, 2015, 10: 1692-1707. DOI:10.1016/j.celrep.2015.02.027 |
| [12] |
PERUGORRIA M J, OLAIZOLA P, LABIANO I, ESPARZA-BAQUER A, MARZIONI M, MARIN J J G, et al. Wnt-β-catenin signalling in liver development, health and disease[J]. Nat Rev Gastroenterol Hepatol, 2019, 16: 121-136. DOI:10.1038/s41575-018-0075-9 |
| [13] |
CLOTMAN F, JACQUEMIN P, PLUMB-RUDEWIEZ N, PIERREUX C E, VAN DER SMISSEN P, DIETZ H C, et al. Control of liver cell fate decision by a gradient of TGF beta signaling modulated by Onecut transcription factors[J]. Genes Dev, 2005, 19: 1849-1854. DOI:10.1101/gad.340305 |
| [14] |
KUVER R, SAVARD C E, LEE S K, HAIGH W G, LEE S P. Murine gallbladder epithelial cells can differentiate into hepatocyte-like cells in vitro[J/OL]. Am J Physiol Gastrointest Liver Physiol, 2007, 293: G944-G955. DOI: 10.1152/ajpgi.00263.2006.
|
| [15] |
LEE S P, SAVARD C E, KUVER R. Gallbladder epithelial cells that engraft in mouse liver can differentiate into hepatocyte-like cells[J]. Am J Pathol, 2009, 174: 842-853. DOI:10.2353/ajpath.2009.080262 |
| [16] |
陈智闻, 陈费, 刘畅, 刘清桂, 王紫君, 王敏君, 等. 人胆囊类器官培养体系的建立与鉴定[J]. 海军军医大学学报, 2023, 44: 402-408. CHEN Z W, CHEN F, LIU C, LIU Q G, WANG Z J, WANG M J, et al. Establishment and identification of human gallbladder organoid culture system[J]. Acad J Naval Med Univ, 2023, 44: 402-408. |
| [17] |
CARDINALE V, WANG Y, CARPINO G, MENDEL G, ALPINI G, GAUDIO E, et al. The biliary tree: a reservoir of multipotent stem cells[J]. Nat Rev Gastroenterol Hepatol, 2012, 9: 231-240. DOI:10.1038/nrgastro.2012.23 |
| [18] |
ZONG Y, STANGER B Z. Molecular mechanisms of liver and bile duct development[J]. Wiley Interdiscip Rev Dev Biol, 2012, 1: 643-655. DOI:10.1002/wdev.47 |
| [19] |
SPENCE J R, LANGE A W, LIN S C J, KAESTNER K H, LOWY A M, KIM I, et al. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells[J]. Dev Cell, 2009, 17: 62-74. DOI:10.1016/j.devcel.2009.05.012 |
| [20] |
CARPENTIER R, SUÑER R E, VAN HUL N, KOPP J L, BEAUDRY J, CORDI S, et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells[J/OL]. Gastroenterology, 2011, 141: 1432-1438.e4. DOI: 10.1053/j.gastro.2011.06.049.
|
| [21] |
HUANG P, ZHANG L, GAO Y, HE Z, YAO D, WU Z, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes[J]. Cell Stem Cell, 2014, 14: 370-384. DOI:10.1016/j.stem.2014.01.003 |
 2023, Vol. 44
2023, Vol. 44


