锰(Mn)是机体必需的微量元素之一,在机体的营养代谢、生长发育和系统免疫中发挥着重要作用[1-2],参与一百多种酶的激活,主要涉及糖、氨基酸、蛋白质、胆固醇的合成、脂肪代谢等过程[3-4]。在免疫调节中,Smialowicz等[5]发现在小鼠腹腔注射MnCl2可促进干扰素的产生,进而增强自然杀伤细胞的活性。Mn可增强环鸟苷酸-腺苷酸合酶(cyclic GMP-AMP synthase,cGAS)/环鸟苷酸-腺苷酸(cyclic GMP-AMP,cGAMP)/干扰素基因刺激因子(stimulator of interferon gene,STING)信号通路的敏感性,从而增强机体免疫反应[6]。
巨噬细胞来源于骨髓中的前体细胞,主要功能是吞噬细胞残片与病原体,并激活其他免疫细胞,增强机体抗感染、抗肿瘤等作用[7]。已证实天然抗性相关巨噬蛋白1(natural resistance-associated macrophage protein 1,NRAMP1)可调节细胞内的Mn2+水平[8],但Mn2+与巨噬细胞相互作用的分子机制尚不清楚。本研究对Mn2+调控的小鼠巨噬细胞进行转录组测序(mRNA-seq)和生物信息学分析,筛选出差异表达的基因并分析其生物学功能,为探究Mn2+对巨噬细胞调控作用的分子机制、寻找新的药物靶点提供理论依据。
1 材料和方法 1.1 主要仪器和试剂CR21型高速离心机(日本Hitachi公司),FA2104N电子天平(上海菁海仪器有限公司),二氧化碳培养箱(日本Sanyo公司),恒温金属浴(海门市其林贝尔仪器制造有限公司),Light Cycler 96实时荧光定量PCR仪(瑞士Roche公司),BCN-1360B洁净工作台(哈尔滨市东联电子技术开发有限公司)。NH4Cl-Tris红细胞裂解液(美国Biolegend公司),MnCl2(美国Sigma公司),NaCl(哈尔滨三联药业股份有限公司),FBS(北京赛默飞世尔生物化学制品有限公司),硫巯基乙醇酸盐(美国Sigma公司),青霉素-链霉素溶液、DMEM(美国HyClone公司),庆大霉素(上海现代哈森药业有限公司)。总RNA提取试剂盒(北京百泰克生物技术有限公司),反转录试剂盒[兰博利德(福州)生物技术有限公司],荧光定量PCR试剂盒(南京诺唯赞生物科技有限公司)。
1.2 小鼠腹腔巨噬细胞的提取成年野生型C57小鼠购自长春市亿斯实验动物技术有限公司[实验动物生产许可证号:SCXK(吉)2020-0002]。在小鼠腹腔注射1 mL硫巯基乙醇酸盐培养基。3 d后将小鼠颈椎脱臼处死,于75%乙醇中浸泡10 min。用灭菌的剪刀和镊子剪开小鼠外皮,暴露出腹膜,用酒精棉轻轻擦拭腹膜,使用20 mL注射器向小鼠腹腔注射15 mL DMEM,为了收集更多的巨噬细胞,需来回抽吸并轻轻抚摸小鼠腹部。将收集到的灌洗液以500×g离心5 min,加入1 mL NH4Cl-Tris裂解液裂解红细胞,2 min后加入10 mL 0.01 mol/L PBS(pH 7.4),500×g离心5 min,弃掉上清;补加10 mL PBS轻轻吹悬细胞,500×g离心5 min,洗涤细胞3次,然后对细胞进行计数。将收集到的细胞铺到6孔板中,在细胞培养箱中过夜培养后弃掉细胞上清液,用PBS洗细胞3次,加入DMEM进行饥饿处理1 h后分为实验组和对照组。实验组细胞加入250 μmol/mL MnCl2处理,对照组细胞加入250 μmol/mL NaCl处理,1 h后感染金黄色葡萄球菌Newman株(感染复数为1∶50),3 h后加入100 μg/mL庆大霉素处理细胞外的金黄色葡萄球菌,24 h后收集细胞进行检测。
1.3 mRNA-seq利用RNA提取试剂盒提取MnCl2和NaCl处理巨噬细胞的总RNA,操作过程按照试剂盒说明书进行。检测提取的总RNA样本的浓度和纯度,将符合要求的样本送生工生物工程(上海)股份有限公司进行测序。
1.4 差异表达基因分析使用FastQC软件(https://www.bioinformatics.babraham.ac.uk/projects/fastqc/)对获得的mRNA-seq数据进行评估和质控,使用Trimmomatic数据过滤软件去除低质量数据和核糖体RNA序列后获得合格的样本,用DEGseq软件包进行差异表达分析。筛选条件:q-value<0.05且差异倍数(fold change,FC)>2或<0.05。
1.5 差异表达基因的通路富集分析和基因功能注释筛选出MnCl2组与NaCl组的差异表达基因,通过基因本体(Gene Ontology,GO)和京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)进行基因功能和信号通路分析。
1.6 关键基因的qPCR验证筛选出与免疫相关且差异表达最显著的10个基因,利用反转录试剂盒将提取的合格样本RNA(每个样本取1 μg)合成cDNA。反应体系(20 μL):cDNA模板1 μL,正、反向引物各0.5 μL,2×ChamQTM Universal® SYBR qPCR Master Mix 10 μL,无RNA酶水8 μL。反应条件:95 ℃ 1 min;95 ℃ 5 s、58 ℃ 10 s、72 ℃ 15 s,40个循环。引物由生工生物工程(上海)股份有限公司合成,各引物序列见表 1。采用2-ΔΔCt法计算目的基因的相对表达量,利用GraphPad Prism 8.3.0和SPSS 19.0软件对qPCR检测结果进行统计和分析。
|
|
表 1 用于qPCR的引物序列 Tab 1 Primer sequences for qPCR |
2 结果 2.1 mRNA-seq数据分析
通过mRNA-seq分析MnCl2和NaCl处理的小鼠腹腔巨噬细胞的差异表达基因,共获得差异表达基因1 637个,其中在MnCl2处理组表达上调的基因有912个、表达下调的基因有725个(图 1)。
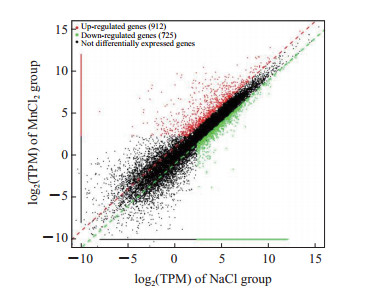
|
图 1 MnCl2和NaCl处理的小鼠腹腔巨噬细胞差异表达基因散点图 Fig 1 Scatter diagram of differentially expressed genes of mouse peritoneal macrophages treated with MnCl2 or NaCl TPM: Transcripts per million. |
2.2 差异表达基因功能和通路分析
GO富集分析结果(图 2A)显示,MnCl2和NaCl处理的小鼠腹腔巨噬细胞的差异表达基因功能主要富集在免疫吞噬、细胞增殖和分化、转录因子调控等生物活性。KEGG富集分析结果(图 2B)表明,这些差异表达基因主要参与的信号通路包括PI3K-Akt信号通路、NF-κB信号通路等,这可能预示着Mn2+通过激活巨噬细胞调控宿主的免疫应答。

|
图 2 MnCl2和NaCl处理的小鼠腹腔巨噬细胞的差异表达基因的GO(A)和KEGG(B)富集分析 Fig 2 GO (A) and KEGG (B) enrichment analyses of differentially expressed genes of mouse peritoneal macrophages treated with MnCl2 or NaCl GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes; TNF: Tumor necrosis factor; RIG-Ⅰ: Retinoic acid inducible gene Ⅰ; PPAR: Peroxisome proliferator-activated receptor; PI3K: Phosphatidylinositol 3-kinase; Akt: Protein kinase B; NOD: Nucleotide oligomerization domain; NF-κB: Nuclear factor κB; MAPK: Mitogen-activated protein kinase; JAK: Janus kinase; STAT: Signal transducer and activator of transcription; IgA: Immunoglobulin A; HIF-1: Hypoxia-inducible factor-1. |
2.3 关键差异表达基因的验证
筛选出与免疫相关且差异最显著的10个基因,分别为免疫球蛋白G Fc段受体Ⅰ(Fc receptor, immunoglobulin G, high affinity Ⅰ;Fcgr1)、含不育α基序结构域蛋白11(sterile alpha motif domain containing 11,Samd11)、活性调节的细胞骨架相关蛋白(activity regulated cytoskeleton associated protein,Arc)、G蛋白偶联受体35(G protein-coupled receptor 35,Gpr35)、富含脯氨酸的小蛋白2H(small proline-rich protein 2H,Sprr2h)、Wnt家族成员4(Wnt family member 4,Wnt4)、整合膜蛋白2A(integral membrane protein 2A,Itm2a)、无调性bHLH转录因子8(atonal bHLH transcription factor 8,Atoh8)、泛素结合酶E2C(ubiquitin conjugating enzyme E2C,Ube2c)及富含脯氨酸的小蛋白2B(small proline rich protein 2B,Sprr2b)。采用qPCR对上述10个关键差异表达基因进行验证,结果显示10个基因的差异表达趋势与测序结果一致,Fcgr1、Samd11、Arc、Gpr35及Sprr2h在MnCl2处理组表达上调,Wnt4、Itm2a、Atoh8、Ube2c及Sprr2b在MnCl2处理组表达下调(图 3)。

|
图 3 MnCl2和NaCl处理的小鼠腹腔巨噬细胞10个关键差异表达基因qPCR验证结果 Fig 3 Validation results of qPCR for 10 key differentially expressed genes in mouse peritoneal macrophages treated with MnCl2 or NaCl n=3, x±s. qPCR: Quantitative polymerase chain reaction; mRNA-seq: MicroRNA-sequencing; Fcgr1: Fc receptor, immunoglobulin G, high affinity Ⅰ; Samd11: Sterile α motif domain containing 11; Arc: Activity regulated cytoskeleton associated protein; Gpr35: G protein-coupled receptor 35; Sprr2h: Small proline-rich protein 2H; Wnt4: Wnt family member 4; Itm2a: Integral membrane protein 2A; Atoh8: Atonal bHLH transcription factor 8; Ube2c: Ubiquitin conjugating enzyme E2C; Sprr2b: Small proline rich protein 2B. |
3 讨论
Mn是哺乳动物体内含量最丰富的金属元素之一,在机体发育、神经功能调节和免疫调节等过程中发挥重要作用。在化学性质上,Mn2+与Mg2+非常相似[9],研究发现Mn2+可以取代Mg2+激活cGAS,并提升cGAS对双链DNA检测的灵敏性[10-11]。Mn2+在炎症反应及机体免疫中具有重要作用,Mokgobu等[12]研究表明,Mn2+可通过激活巨噬细胞和中性粒细胞促进过氧化氢的产生,发挥促氧化作用;但Daejin等[13]研究却显示出相反的结果,即Mn2+可激活巨噬细胞发挥抗氧化作用。因此,Mn2+在激活巨噬细胞后引发的免疫应答的分子机制有待于进一步深入研究。
巨噬细胞在吞噬细菌的过程中,首先识别细菌表面分子,进而引起细胞骨架重塑,在受体的帮助下形成吞噬杯,最后将细菌完全吞噬[14-15]。在qPCR筛选的10个差异表达基因中,Fcgr1和Arc可能参与巨噬细胞吞噬细菌的过程。Fcgr1可以特异性识别IgG,主要在巨噬细胞、树突状细胞、单核细胞和嗜酸性粒细胞中表达,其表达受细胞因子的调节,当菌体侵入机体后细胞因子会诱导中性粒细胞中Fcgr1的表达,促进中性粒细胞的吞噬作用[16-17]。本研究中mRNA-seq测序分析和qPCR检测结果均显示小鼠腹腔巨噬细胞受到Mn2+刺激后Fcgr1表达上调,表明Fcgr1可能参与了巨噬细胞的吞噬过程。Arc为神经元突触后致密物质的细胞骨架相关性蛋白,能够调节细胞骨架活性,进而促进细胞的增殖和分化[18-19]。本研究中小鼠腹腔巨噬细胞受到Mn2+刺激后Arc基因表达上调,原因可能在于Mn2+刺激触发了巨噬细胞对细菌表面分子的识别,引起细胞骨架重塑,最终使巨噬细胞吞噬能力增强。Atoh8是bHLH家族的一种转录因子,与许多肿瘤的发生有关,Atoh8敲低可抑制结肠癌细胞的增殖[20]。Sprr2B为角质形成细胞中交联包膜蛋白的成员[21],Yao等[22]报道Sprr2B在胃腺癌中高表达,并且是预后不良的独立预测因子。本研究中Mn2+刺激巨噬细胞后,Atoh8和Sprr2B基因表达下调,提示Mn2+可能通过调控Atoh8与Sprr2B基因的表达来调控机体免疫反应。
本研究对筛选出的Mn2+刺激的小鼠巨噬细胞差异表达基因进行了功能分析,结果显示这些差异表达基因主要参与免疫吞噬、细胞增殖和分化、转录因子调控等生物活性,其主要介导PI3K-Akt和NF-κB等信号通路活化,这提示Mn2+激活的巨噬细胞可能通过这些信号通路调控机体免疫应答。NF-κB是参与调节多种生物反应和免疫基因表达的转录调节因子,当细胞被外界刺激物激活后便与NF-κB抑制因子(inhibitor of NF-κB,IκB)解离,从而转移至核内与特异性启动子相结合,进而调控基因的表达[23-24]。PI3K-Akt-哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)通路在免疫应答中起着至关重要的作用。临床前数据表明,PI3K-Akt-mTOR通路的改变可能促进免疫抑制反应[25]。PI3K-Akt通路参与代谢转变,将效应CD8+ T细胞转化为记忆T细胞[26],并调节肿瘤相关巨噬细胞在免疫刺激和免疫抑制之间的转换。
综上所述,本研究利用mRNA-seq技术分析了Mn2+调控小鼠巨噬细胞的mRNA水平,并获得了与免疫相关的差异表达基因,表明Mn2+对巨噬细胞具有免疫调节作用,为揭示Mn2+调控巨噬细胞的功能提供了理论依据,也为后续研究Mn2+在机体免疫中的作用机制奠定了基础。
| [1] |
LV M, CHEN M, ZHANG R, ZHANG W, WANG C, ZHANG Y, et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy[J]. Cell Res, 2020, 30: 966-979. DOI:10.1038/s41422-020-00395-4 |
| [2] |
GUPTA U C, GUPTA S C. Sources and deficiency diseases of mineral nutrients in human health and nutrition: a review[J]. Pedosphere, 2014, 24: 13-38. DOI:10.1016/S1002-0160(13)60077-6 |
| [3] |
ASCHNER M, ERIKSON K. Manganese[J]. Adv Nutr, 2017, 8: 520-521. DOI:10.3945/an.117.015305 |
| [4] |
黄鑫, 李玲玲, 王君豪, 闵军霞, 王福俤. 锰离子转运蛋白的发现及功能机制研究进展[J]. 生命科学, 2018, 30: 603-614. DOI:10.13376/j.cbls/2018073 |
| [5] |
SMIALOWICZ R J, ROGERS R R, RIDDLE M M, ROWE D G, LUEBKE R W. In vitro augmentation of natural killer cell activity by manganese chloride[J]. J Toxicol Environ Health, 1986, 19: 243-254. DOI:10.1080/15287398609530924 |
| [6] |
卫潇茗, 王晨光, 张睿, 蒋争凡. 锰离子作为免疫调节剂的发现及应用展望[J]. 中国细胞生物学学报, 2020, 42: 1721-1731. |
| [7] |
YANG Q Y, GUO N N, ZHOU Y, CHEN J J, WEI Q C, HAN M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy[J]. Acta Pharm Sin B, 2020, 10: 2156-2170. DOI:10.1016/j.apsb.2020.04.004 |
| [8] |
MORIGUCHI R, OTAKI Y, HAZEKI S, SHIMADA T, MATSUMOTO A, KAKITA N, et al. High levels of tumor necrosis factor-α downregulate antimicrobial iron transport protein, Nramp1, in chronic hemodialysis patients: a key factor for infection risk[J]. Am J Nephrol, 2012, 35: 372-378. DOI:10.1159/000337742 |
| [9] |
WEDLER F C. Biological significance of manganese in mammalian systems[J]. Prog Med Chem, 1993, 30: 89-133. |
| [10] |
WANG C G, GUAN Y K, LV M Z, ZHANG R, GUO Z Y, WEI X M, et al. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses[J]. Immunity, 2018, 48: 675-687. DOI:10.1016/j.immuni.2018.03.017 |
| [11] |
WENTE S R, VILLALBA M, SCHRAMM V L, ROSEN O M. Mn2+-binding properties of a recombinant protein-tyrosine kinase derived from the human insulin receptor[J]. Proc Natl Acad Sci USA, 1990, 87: 2805-2809. DOI:10.1073/pnas.87.7.2805 |
| [12] |
MOKGOBU M I, ANDERSON R, STEEL H C, CHOLO M C, TINTINGER G R, THERON A J. Manganese promotes increased formation of hydrogen peroxide by activated human macrophages and neutrophils in vitro[J]. Inhal Toxicol, 2012, 24: 634-644. DOI:10.3109/08958378.2012.706657 |
| [13] |
DAEJIN K, GA B P, DAE Y H. Apoptotic signaling through reactive oxygen species in cancer cells[J]. World J Immunol, 2014, 4: 158-173. DOI:10.5411/wji.v4.i3.158 |
| [14] |
赵文心, 马岚, 杨雪婷, 李晶莹, 杜书嵩, 张雪薇, 等. 肌动蛋白亚型在巨噬细胞吞噬脑膜炎大肠杆菌中的作用[J]. 中国细胞生物学学报, 2020, 42: 620-628. |
| [15] |
ROUGERIE P, MISKOLCI V, COX D. Generation of membrane structures during phagocytosis and chemotaxis of macrophages: role and regulation of the actin cytoskeleton[J]. Immunol Rev, 2013, 256: 222-239. DOI:10.1111/imr.12118 |
| [16] |
SCOTT C L, T'JONCK W, MARTENS L, TODOROV H, SICHIEN D, SOEN B, et al. The transcription factor ZEB2 is required to maintain the tissue-specific identities of macrophages[J]. Immunity, 2018, 49: 312-325. DOI:10.1016/j.immuni.2018.07.004 |
| [17] |
BI R, KONG L L, XU M, LI G D, ZHANG D F, Alzheimer's Disease Neuroimaging Initiative, et al. The Arc gene confers genetic susceptibility to Alzheimer's disease in Han Chinese[J]. Mol Neurobiol, 2018, 55: 1217-1226. DOI:10.1007/s12035-017-0397-6 |
| [18] |
OKUNO H. [Synapse-to-nucleus signaling and activity-dependent gene expression in neurons: mechanisms of synaptic activity-dependent regulation of the Arc/Arg3.1 gene][J]. Seikagaku, 2010, 82: 841-846. |
| [19] |
NAGATA S. Apoptosis and clearance of apoptotic cells[J]. Ann Rev Immunol, 2018, 36: 489-517. DOI:10.1146/annurev-immunol-042617-053010 |
| [20] |
YE M S, HE Y, LIN H, YANG S X, ZHOU Y H, ZHOU L L, et al. High expression of atonal homolog 8 predicts a poor clinical outcome in patients with colorectal cancer and contributes to tumor progression[J]. Oncol Rep, 2017, 37: 2955-2963. |
| [21] |
CARREGARO F, STEFANINI A C, HENRIQUE T, TAJARA E H. Study of small proline-rich proteins (SPRRs) in health and disease: a review of the literature[J]. Arch Dermatol Res, 2013, 305: 857-866. |
| [22] |
YAO L, YAN J H, CHENG F, GAN L H, HUANG Y Q, ZHENG L, et al. Small proline-rich protein 2B facilitates gastric adenocarcinoma proliferation via MDM2-p53/p21 signaling pathway[J]. Onco Targets Ther, 2021, 14: 1453-1463. |
| [23] |
DOLCET X, LLOBET D, PALLARES J, MATIAS-GUIU X. NF-κB in development and progression of human cancer[J]. Virchows Arch, 2005, 446: 475-482. |
| [24] |
AOKI M, FUJISHITA T. Oncogenic roles of the PI3K/AKT/mTOR axis[J]. Curr Top Microbiol Immunol, 2017, 407: 153-189. |
| [25] |
GIANNONE G, GHISONI E, GENTA S, SCOTTO G, TUNINETTI V, TURINETTO M, et al. Immuno-metabolism and microenvironment in cancer: key players for immunotherapy[J/OL]. Int J Mol Sci, 2020, 21: 4414. DOI: 10.3390/ijms21124414.
|
| [26] |
SHI X, ZHU W, CHEN T, CUI W, LI X, XU S. Paraquat induces apoptosis, programmed necrosis, and immune dysfunction in CIK cells via the PTEN/PI3K/AKT axis[J]. Fish Shellfish Immunol, 2022, 130: 309-316. |
 2023, Vol. 44
2023, Vol. 44


