肾结石是一种易复发性疾病,发病的结石成分主要是钙结石,以草酸钙最为常见[1]。研究表明,草酸钙结石的重要触发事件在于磷酸钙晶体在肾髓质间质组织中形成,蚀穿肾乳头上皮形成典型的Randall斑,草酸钙晶体可能沉积于该病灶顶部,附着于肾乳头形成结石[2]。肾结石的外科治疗通常只处理位于集合系统内的结石[3],理论上肾髓质内的钙斑可能继续生长并促进残留结石或晶体在肾乳头上皮处黏附和聚集[4-6]。目前尚不明确影响晶体在肾小管上皮细胞黏附聚集的靶点及药物。
研究表明,肾小管细胞损伤是促进草酸钙晶体在细胞表面黏附沉积的重要因素[7],损伤引起的细胞膜结构及蛋白表达变化会促进草酸钙晶体在肾小管上皮细胞上的黏附沉积[8]。晶体沉积诱导的细胞毒性涉及受体相互作用的丝氨酸/苏氨酸蛋白激酶(receptor-interacting serine/threonine-protein kinase,RIPK)3-混合谱系激酶结构域样蛋白(mixed lineage kinase domain-like,MLKL)介导的坏死性凋亡[9]。坏死性凋亡是程序性死亡模式的一种,其信号通路可通过RIPK3等进行调节,有望通过调节肾组织细胞坏死性凋亡、减轻细胞损伤来干预肾脏的草酸钙晶体沉积[10]。然而Mulay等[11]通过构建坏死性凋亡相关的肿瘤坏死因子受体(tumour necrosis factor receptor,Tnfr)1基因敲除和Tnfr1/Tnfr2基因双敲除小鼠的肾脏晶体沉积模型,发现其肾损伤指标低于对照组,但其肾脏晶体沉积并无差异。
尿液中草酸钙微晶体的聚集是导致晶体快速增大和结石形成的关键因素[12-13]。从化学角度考虑,首先是尿液中的尿石盐成核,然后这些<10 nm的晶核生长或聚集到病理性尺寸范围(约几十微米),这些微米级的晶体与受损伤的肾上皮细胞黏附后进一步长大并最终形成结石(几毫米到几十毫米)。调控草酸钙微晶的物理和化学性质,减少其在尿液中的聚集可能是预防肾结石形成的方法之一[14-15]。聚乙二醇(polyethylene glycol,PEG)是一种无毒的高分子聚合物,具有良好的水溶性、润滑性、保湿性、分散性、粘接性等[16],其具备的分散剂的特性可以使固体颗粒保持悬浮稳定、不沉淀,使整个悬浮液体系的物理和化学性质一致[17],因此以PEG为代表的聚合物对草酸钙晶体生长聚集和结石形成的干预作用值得研究。
本研究拟通过观察RIPK3抑制剂GSK-872以及分子量为4 000的PEG(PEG-4000)对小鼠肾小管上皮细胞TCMK-1表面一水草酸钙(calcium oxalate monohydrate,COM)晶体沉积的影响,并检测其细胞增殖活性、细胞内活性氧(reactive oxygen species,ROS)水平、坏死性凋亡相关分子表达及细胞表面晶体黏附量的变化,初步探究影响COM晶体在TCMK-1黏附聚集的靶点及药物,为复发性肾结石的预防和治疗提供新思路。
1 材料和方法 1.1 细胞与试剂小鼠肾小管上皮细胞系TCMK-1购自美国菌种保藏中心;FBS购自美国Hyclone公司;COM晶体购自上海阿达玛斯试剂有限公司;DMEM高糖培养基购自上海泰坦科技股份有限公司;GSK-872购自美国葛兰素史克公司;兔源磷酸化混合系列激酶结构域样蛋白(phospho-mixed lineage kinase domain-like,p-MLKL)单克隆抗体购自北京博奥森生物技术有限公司;CCK-8试剂盒、ROS检测试剂盒、增强型二辛可酸(bicinchoninic acid,BCA)蛋白定量试剂盒、兔源RIPK1多克隆抗体、兔源RIPK3多克隆抗体、兔源GAPDH单克隆抗体均购自上海碧云天生物技术有限公司。
1.2 细胞培养TCMK-1细胞用含10% FBS和1%青霉素/链霉素的高糖DMEM完全培养基于5% CO2、37 ℃培养箱中培养。每2~3 d换液1次,培养至细胞融合度为80% 时用含0.25% EDTA的胰酶消化、传代。
1.3 镜下观测实验一:将细胞接种于6 cm培养皿,常规培养24 h后吸弃原培养基并用PBS洗涤,设置A1、A2、A3、A4、B1、B2、B3、B4、C1、C2、C3、C4组,A1~A4组加入含10% FBS的DMEM培养基,B1~B4组加入含20 μmol/L GSK-872和10% FBS的DMEM培养基,C1~C4组加入含10% FBS的DMEM培养基,放入培养箱孵育30 min,吸弃培养基并用PBS洗涤;A1~A4组、B1~B4组分别加入4 mL含质量浓度200、400、600、800 μg/mL COM的DMEM培养基,C1~C4组加入等体积DMEM培养基,在摇床上80 r/min振荡15 min。放入培养箱37 ℃孵育12 h后弃去晶体,用不含钙、镁的PBS洗涤细胞3次,再加入PBS铺底,在倒置相差显微镜下观察TCMK-1细胞表面的晶体黏附情况。
实验二:将细胞接种于6 cm培养皿,常规培养24 h后吸弃原培养基并用PBS洗涤备用。配制500 g/L的PEG-4000水溶液备用。设置a、b、c组,a组培养皿中加入4 mL含10% FBS的DMEM培养基,加入COM至800 μg/mL,摇床上80 r/min振荡15 min。b组培养皿中加入4 mL含10% FBS的DMEM培养基,加入400 μL PEG-4000溶液充分振荡混匀,再加入COM至800 μg/mL,摇床上80 r/min振荡15 min。c组培养皿中加入4 mL含10% FBS的DMEM培养基,加入COM至800 μg/mL,摇床上80 r/min振荡15 min,再向培养基中加入400 μL PEG-4000溶液,充分混匀。培养箱孵育12 h后弃去晶体,用不含钙、镁的PBS洗涤细胞3次,再加入PBS铺底,在倒置相差显微镜下观察TCMK-1细胞表面的晶体黏附情况。
1.4 CCK-8法测定细胞增殖活性实验一:按照每孔100 μL培养基、2×103个细胞进行96孔细胞培养板铺板,设置A、B、C组,每组重复7孔,放入细胞培养箱培养8 h。吸弃培养基并用PBS洗涤。A组每孔加入含10% FBS的DMEM培养基,B组每孔加入含20 μmol/L GSK-872和10% FBS的DMEM培养基,C组每孔加入含10% FBS的DMEM培养基。放入培养箱孵育30 min后,吸弃各孔培养基并用PBS洗涤。A、B组每孔加入100 μL含400 μg/mL COM的DMEM培养基,C组每孔加入等体积的DMEM培养基。将培养板振荡混匀后放入培养箱孵育12 h。吸弃各孔培养基,于每孔加入10 μL CCK-8检测工作液,孵育2 h后用酶标仪测定450 nm波长处光密度(D450)值。将上述CCK-8方法中COM浓度换为800 μg/mL且其余步骤不变再次进行实验。
实验二:按照每孔100 μL培养基、2×103个细胞进行96孔细胞培养板铺板,设置a、b、c组,每组重复7孔,放入细胞培养箱培养8 h。吸弃培养基并用PBS洗涤。a组每孔加入100 μL含10% FBS和800 μg/mL COM的DMEM培养基,振荡混匀。b组每孔加入100 μL含10% FBS的DMEM培养基,并加入10 μL PEG-4000溶液振荡混匀,再加入COM至800 μg/mL,振荡混匀。c组每孔加入100 μL含10% FBS和800 μg/mL COM的DMEM培养基,振荡混匀,再向培养基中加入10 μL PEG-4000溶液振荡混匀。各组放入培养箱孵育12 h。吸弃各孔培养基,于每孔加入10 μL CCK-8检测工作液,孵育2 h后用酶标仪测定D450值。
1.5 2’, 7’- 二氯二氢荧光素二乙酸酯(2’, 7’-dichlorodihydrofluorescein diacetate,DCFH-DA)探针法检测细胞ROS实验一:按照每孔100 μL培养基、2×103个细胞进行96孔黑底细胞培养板铺板,设置A、B、C组,每组重复7孔,放入细胞培养箱培养8 h。吸弃培养基并用PBS洗涤。A组每孔加入含10% FBS的DMEM培养基,B组每孔加入含20 μmol/L GSK-872和10% FBS的DMEM培养基,C组每孔加入含10% FBS的DMEM培养基。放入培养箱孵育30 min后,吸弃培养基并用PBS洗涤。A、B组每孔加入100 μL含400 μg/mL COM的DMEM培养基,C组每孔加入等体积的DMEM培养基,将培养板振荡混匀后放入培养箱孵育12 h。吸弃各孔培养基,于每孔加入50 μL含10 mmol/LDCFH-DA的无血清培养基,放入细胞培养箱内孵育20 min,取出后用无血清细胞培养基洗涤细胞3次,以488 nm为激发波长、525 nm为发射波长用荧光酶标仪检测。将上述DCFH-DA探针法中COM浓度换为800 μg/mL且其余步骤不变再次进行实验。
实验二:按照每孔100 μL培养基、2×103个细胞进行96孔黑底细胞培养板铺板,设置a、b、c组,每组重复7孔,放入细胞培养箱培养8 h。吸弃培养基并用PBS洗涤。a组每孔加入100 μL含10% FBS和800 μg/mL COM的DMEM培养基,震荡混匀。b组每孔加入100 μL含10% FBS的DMEM培养基,先加入10 μL PEG-4000溶液振荡混匀,再加入COM至800 μg/mL,振荡混匀。c组每孔加入100 μL含10% FBS和800 μg/mL COM的DMEM培养基,振荡混匀,再加入10 μL PEG-4000溶液,振荡混匀。将培养板放入培养箱孵育12 h。吸弃各孔培养基,于每孔加入50 μL含10 mmol/LDCFH-DA的无血清培养基,放入细胞培养箱内孵育20 min,取出后用无血清细胞培养基洗涤细胞3次,以488 nm为激发波长、525 nm为发射波长用荧光酶标仪检测。
1.6 蛋白质印迹法检测坏死性凋亡特异性分子RIPK1、RIPK3、p-MLKL的表达取1.3节实验一中镜下观测后的细胞,用RIPA裂解液充分裂解后提取总蛋白质,BCA法测定蛋白质浓度。按照每孔30 μg的上样量进行SDS-PAGE,转膜,采用5% 牛血清白蛋白(bovine serum albumin,BSA)室温封闭2 h后,加入一抗于4 ℃孵育过夜。次日加入HRP标记的二抗于室温孵育1 h,然后进行ECL反应,使用化学发光成像仪曝光显影。
1.7 COM晶体黏附量的测定取1.3节实验一和实验二中镜下观测后的细胞,将培养皿放置在小烧杯中,加入5 mL浓HNO3和1.0 mL HClO4,在电磁炉上消化至溶液透明,继续加热至溶液即将蒸干时关闭电源,利用余热将溶液烘干,自然冷却后加入3 mL超纯水,混合均匀,同时设空白组(即5 mL浓HNO3与1.0 mL HClO4的混合溶液),利用电感耦合等离子体发射光谱法(inductively coupled plasma emission spectroscopy,ICP)准确测定钙离子的浓度,并换算成COM晶体的黏附量。
1.8 统计学处理应用GraphPad Prism 9软件进行统计学分析。计量资料以x±s表示,多组间比较采用单因素方差分析,多重比较采用Tukey法。检验水准(α)为0.05。
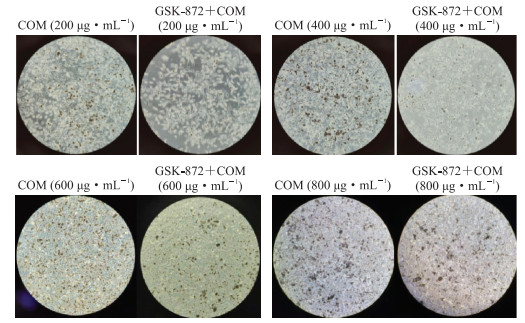
2 结果 2.1 镜下观察TCMK-1细胞表面的COM晶体黏附情况倒置相差显微镜下可见,在200、400、600、800 μg/mL COM处理后,各组TCMK-1细胞表面晶体黏附随晶体浓度增加而明显增多(图 1)。COM浓度为200、400 μg/mL时,GSK872预处理组镜下细胞表面晶体黏附明显少于单纯COM处理组;当COM浓度为800 μg/mL时,GSK872预处理组多数晶体聚集形成不定型沉淀并滞留于细胞表面,与单纯COM处理组无明显差异。
|
图 1 倒置相差显微镜下观测GSK-872预处理对不同浓度COM作用的TCMK-1细胞表面晶体黏附的影响(100×) Fig 1 Effect of GSK-872 pretreatment on TCMK-1 cell-crystal adhesion treated with different concentrations of COM observed under phase inversed microscope (100×) GSK-872: A potent and specific inhibitor of receptor-interacting serine/threonine-protein kinase 3; COM: Calcium oxalate monohydrate; TCMK-1: Transformed C3H mouse kidney 1. |
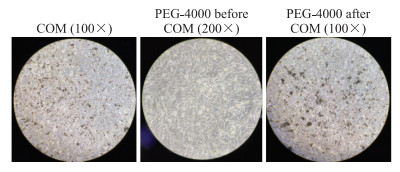
与浓度为800 μg/mL COM处理组相比,预加入PEG-4000使COM处理的TCMK-1细胞表面晶体黏附明显减轻,而加入COM后再加入PEG-4000处理的TCMK-1细胞表面晶体黏附无明显改变(图 2)。
|
图 2 倒置相差显微镜下观测COM干预前后加入PEG-4000对TCMK-1细胞表面晶体黏附的影响 Fig 2 Effect of adding PEG-4000 on TCMK-1 cells before and after COM treatment observed under phase inverted microscope COM: Calcium oxalate monohydrate; TCMK-1: Transformed C3H mouse kidney 1; PEG: Polyethylene glycol. |
2.2 GSK-872及PEG-4000对COM处理的TCMK-1细胞增殖的影响
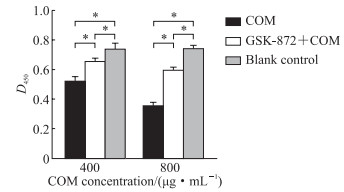
CCK-8检测结果(图 3)显示,与空白对照组相比,400、800 μg/mL COM处理12 h时TCMK-1细胞的增殖活性均受到抑制,而使用GSK-872预处理组相比对应浓度的COM处理组TCMK-1细胞的增殖活性均增强(P均<0.05)。
|
图 3 CCK-8法检测GSK-872预处理对不同浓度COM作用的TCMK-1细胞增殖活性的影响 Fig 3 Effect of GSK-872 pretreatment on proliferation activity of TCMK-1 cells treated with different concentrations of COM detected by CCK-8 *P < 0.05. n=7, x±s. CCK-8: Cell counting kit 8; GSK-872: A potent and specific inhibitor of receptor-interacting serine/ threonine-protein kinase 3; COM: Calcium oxalate monohydrate; TCMK-1: Transformed C3H mouse kidney 1. |
CCK-8检测结果显示,预加入PEG-4000组细胞增殖活性(光密度值0.659±0.029)较COM处理组(0.365±0.011)增高(P<0.05),而加入COM后再加入PEG-4000处理组细胞增殖活性(光密度值0.342±0.012)与COM处理组(0.365±0.011)相比差异无统计学意义(P>0.05)。
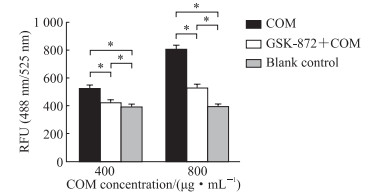
2.3 GSK-872及PEG-4000对COM处理的TCMK-1细胞ROS的影响DCFH-HA探针法检测结果显示,与空白对照组相比,400、800 μg/mL COM处理细胞12 h时TCMK-1细胞的ROS水平均升高;与COM处理组相比,预加入GSK-872降低了COM诱导细胞产生的ROS水平(P均<0.05,图 4)。
|
图 4 DCFH-DA探针法检测GSK-872预处理对不同浓度COM作用的TCMK-1细胞ROS水平的影响 Fig 4 Effect of GSK-872 pretreatment on ROS levels of TCMK-1 cells treated with different concentrations of COM detected by the DCFH-DA probe method *P < 0.05. n=7, x±s. DCFH-DA: 2', 7'-dichlorodihydrofluorescein diacetate; GSK-872: A potent and specific inhibitor of receptor-interacting serine/threonine-protein kinase 3; COM: Calcium oxalate monohydrate; TCMK-1: Transformed C3H mouse kidney 1; ROS: Reactive oxygen species; RFU: Relative fluorescence unit. |
与COM处理组TCMK-1细胞ROS水平(808.1±20.5)相比,预加入PEG-4000使COM处理的TCMK-1细胞ROS水平(438.9±11.9)降低(P<0.05),而加入COM后再加入PEG-4000处理的TCMK-1细胞ROS水平(823.7±14.3)差异无统计学意义(P>0.05)。
2.4 GSK-872对COM处理的TCMK-1细胞坏死性凋亡相关蛋白表达的影响蛋白质印迹法检测结果(图 5)显示,与空白对照组比较,800 μg/mLCOM处理12 h时细胞坏死性凋亡相关蛋白RIPK1、RIPK3、p-MLKL表达增加;与COM处理组比较,GSK-872预处理组的细胞RIPK1表达差异无统计学意义(0.678±0.020 vs 0.698±0.015,P>0.05),RIPK3(0.155±0.021 vs 0.641±0.029)及p-MLKL(0.451±0.103 vs 1.162±0.137)表达均减少(P均<0.05)。

|
图 5 GSK-872预处理对800 μg/mL COM处理的细胞坏死性凋亡相关蛋白表达的影响 Fig 5 Effect of GSK-872 pretreatment on the expression of necroptosis-related proteins in cells treated with 800 μg/mL COM 1: COM group; 2: GSK-872+COM group; 3: Blank control group. GSK-872: A potent and specific inhibitor of RIPK3; COM: Calcium oxalate monohydrate; RIPK: Receptorinteracting serine/threonine-protein kinase; p-MLKL: Phosphomixed lineage kinase domain-like; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. |
2.5 GSK-872及PEG-4000对COM处理的TCMK-1细胞表面晶体黏附量的影响
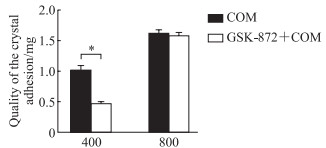
结果如图 6所示,COM浓度为400 μg/mL时,与COM处理组相比,GSK-872预处理组晶体黏附量减少(P<0.05);COM浓度为800 μg/mL时,两组晶体黏附量差异无统计学意义(P>0.05)。
|
图 6 ICP检测GSK-872预处理对不同浓度COM作用的TCMK-1细胞表面晶体黏附量的影响 Fig 6 Effect of GSK-872 pretreatment on TCMK-1 cellcrystal adhesion treated with different concentrations of COM detected by ICP *P < 0.05. n=7, x±s. ICP: Inductively coupled plasma emission spectroscopy; GSK-872: A potent and specific inhibitor of receptor-interacting serine/threonine-protein kinase 3; COM: Calcium oxalate monohydrate; TCMK-1: Transformed C3H mouse kidney 1. |
COM浓度为800 μg/mL时,与COM处理组[(1.643±0.032)mg]相比,预加入PEG-4000使COM处理的TCMK-1细胞的晶体黏附量[(0.405±0.040)mg]减少(P<0.05),而加入COM后再加入PEG-4000处理的TCMK-1细胞的晶体黏附量[(1.678±0.026)mg]差异无统计学意义P>0.05。
3 讨论有研究认为晶体的细胞毒性涉及RIPK3-MLKL介导的坏死性凋亡,作为调控坏死性凋亡的重要靶点,抑制RIPK3可防止细胞晶体性损伤[9]。
尿液中草酸钙微晶体的过饱和析出和聚集是导致晶体快速增大和结石形成的重要因素,减少其在尿液中的聚集可能是预防肾结石形成的方法之一。PEG因具有高结构灵活性、生物相容性、两亲性、高水化能力等物理、化学特性以及公认的安全性,成为载入《中华人民共和国药典》及美国FDA批准使用的多用途聚合物[18]。分子量100~700的PEG在室温下为液态,分子量1 000~2 000的PEG为软固态,分子量>2 000的PEG为硬结晶固态或玻璃态,熔点约为63 ℃[19]。但这并不妨碍PEG的生物安全性,它在水中具有高溶解度[20],且在所有pH值下都保持电中性,在生物环境中保持惰性,蛋白质吸附差,细胞活化和黏附性低,细胞摄取差,炎症程度可忽略不计[21]。短期和长期毒性研究、致突变性试验、大鼠致畸试验和人体临床试验均表明PEG通过口服和非口服途径是安全的[22]。通过腹腔注射、皮下注射、肌内注射及静脉注射进入小鼠血液中的分子量为6 000的PEG(PEG-6000)几乎在10 h内被完全清除,且PEG-6000在体内清除速度明显较更大分子量的PEG快[23]。人体每日可接受各种分子量的PEG摄入量高达10 mg/kg体重(来自WHO关于食品添加剂的评价)[24]。PEG的罕见不良反应包括静脉和口服给药后过敏或过敏性休克[25]。美国FDA发布的非活性成分数据库(Inactive Ingredient Database)列出了PEG在口服、局部和静脉制剂中的用途,还描述了该特定组合物中使用的PEG的最大浓度。另外,PEG-4000及其复方制剂作为轻泻剂在临床中被长期广泛应用,进一步佐证了它的化学稳定性和生物安全性。同时具备分散剂特性的PEG-4000可能在水溶液中通过其分散稳定作用抑制草酸钙微晶体的聚集和生长,并使其保持悬浮稳定[26]。本研究中PEG-4000水溶液稀释后作用于细胞及COM的实际质量分数约为5%,略小于复方聚乙二醇散中6%的使用浓度建议。
本研究结果显示,与400、800 μg/mL COM处理组相比,预加入RIPK3特异性抑制剂GSK-872可减轻400、800 μg/mL COM处理下TCMK-1细胞损伤。预加入GSK-872可以使400 μg/mL COM处理的细胞表面晶体黏附量明显减少,但不能减少800 μg/mL COM处理的细胞表面晶体黏附量。PEG-4000相关的实验显示,与800 μg/mL COM处理组相比,预加入PEG-4000能够减轻COM处理组TCMK-1的细胞损伤,并降低细胞表面晶体黏附量。在加入800 μg/mL COM之后再加入PEG-4000时,细胞表面晶体黏附及细胞损伤相比COM处理组没有明显差异。
实验结果说明RIPK3特异性抑制剂GSK-872对于坏死性凋亡通路的调控作用明显,并验证了坏死性凋亡是草酸钙晶体刺激下主要的细胞损伤方式之一。实验发现在较低浓度晶体处理下抑制细胞坏死性凋亡能够降低细胞表面对晶体的黏附作用,但不能完全消除细胞-晶体黏附作用。这可能与细胞损伤的分子机制多样性有关,仅通过少数位点的抑制很难消除细胞损伤以及细胞膜对晶体的黏附作用,GSK-872选择性抑制了细胞坏死性凋亡,同时也会启动细胞凋亡等细胞死亡模式。
实验通过检测细胞增殖能力、ROS水平、坏死性凋亡相关蛋白表达及细胞表面晶体黏附量表明,COM浓度达到800 μg/mL时,预使用GSK-872仍旧能够减轻晶体性细胞损伤、减少ROS产生的水平,但是细胞表面晶体黏附总量没有明显差异。可能的解释为,当晶体密度达到一定程度时晶体颗粒间依靠静电作用等相互吸引并聚集形成不定型沉淀,这些滞留下来的不定型沉淀吸引了大量微晶颗粒并且占据的空间远大于单晶颗粒,成为影响晶体黏附量的主要因素。
预加入PEG-4000能够使晶体在悬液体系中保持悬浮稳定并降低细胞的晶体性损伤,细胞表面晶体黏附聚集明显减少。另外在加入COM之后再加入PEG-4000,细胞表面晶体黏附相比COM处理组没有减少,细胞损伤无明显差异,说明PEG-4000在晶体与细胞接触作用之前起到分散作用,而没有逆转已经形成的晶体-细胞黏附的作用。
以 PEG-4000 为代表的聚合物或生物材料可能 对肾结石的防治研究有所帮助,其影响细胞表面晶 体黏附聚集的作用机制有待进一步研究。
| [1] |
ASOUDEH F, TALEBI S, JAYEDI A, MARX W, NAJAFI M T, MOHAMMADI H. Associations of total protein or animal protein intake and animal protein sources with risk of kidney stones: a systematic review and dose-response meta-analysis[J]. Adv Nutr, 2022, 13: 821-832. DOI:10.1093/advances/nmac013 |
| [2] |
WINFREE S, WEILER C, BLEDSOE S B, GARDNER T, SOMMER A J, EVAN A P, et al. Multimodal imaging reveals a unique autofluorescence signature of Randall's plaque[J]. Urolithiasis, 2021, 49: 123-135. DOI:10.1007/s00240-020-01216-4 |
| [3] |
LILDAL S K, ANDREASSEN K H, BAARD J, BREHMER M, BULTITUDE M, ERIKSSON Y, et al. Consultation on kidney stones, Copenhagen 2019:aspects of intracorporeal lithotripsy in flexible ureterorenoscopy[J]. World J Urol, 2021, 39: 1673-1682. DOI:10.1007/s00345-020-03481-9 |
| [4] |
CHANTHICK C, THONGBOONKERD V. Hyaluronic acid promotes calcium oxalate crystal growth, crystal-cell adhesion, and crystal invasion through extracellular matrix[J/OL]. Toxicol In Vitro, 2022, 80: 105320.
10.1016/j.tiv.2022.105320.
|
| [5] |
NOONIN C, PEERAPEN P, YOODEE S, KAPINCHARANON C, KANLAYA R, THONGBOONKERD V. Systematic analysis of modulating activities of native human urinary Tamm-Horsfall protein on calcium oxalate crystallization, growth, aggregation, crystal-cell adhesion and invasion through extracellular matrix[J/OL]. Chem Biol Interact, 2022, 357: 109879.
10.1016/j.cbi.2022.109879.
|
| [6] |
STREEPER N M, GALIDA M, BOLTZ S, LI S, NAKADA S Y, RAFFIN E P, et al. Is stone-free status after surgical intervention for kidney stones associated with better health-related quality of life?-A multicenter study from the North American stone quality of life consortium[J]. Urology, 2021, 148: 77-82. DOI:10.1016/j.urology.2020.09.058 |
| [7] |
YE Q L, WANG D M, WANG X, ZHANG Z Q, TIAN Q X, FENG S Y, et al. Sirt1 inhibits kidney stones formation by attenuating calcium oxalate-induced cell injury[J/OL]. Chem Biol Interact, 2021, 347: 109605.
10.1016/j.cbi.2021.109605.
|
| [8] |
LI X R, CHEN S Y, FENG D M, FU Y Q, WU H, LU J Z, et al. Calcium-sensing receptor promotes calcium oxalate crystal adhesion and renal injury in Wistar rats by promoting ROS production and subsequent regulation of PS ectropion, OPN, KIM-1, and ERK expression[J]. Ren Fail, 2021, 43: 465-476. DOI:10.1080/0886022X.2021.1881554 |
| [9] |
MULAY S R, DESAI J, KUMAR S V, EBERHARD J N, THOMASOVA D, ROMOLI S, et al. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis[J/OL]. Nat Commun, 2016, 7: 10274.
10.1038/ncomms10274.
|
| [10] |
MENG Y X, HORNE C R, SAMSON A L, DAGLEY L F, YOUNG S N, SANDOW J J, et al. Human RIPK3 C-lobe phosphorylation is essential for necroptotic signaling[J/OL]. Cell Death Dis, 2022, 13: 565.
10.1038/s41419-022-05009-y.
|
| [11] |
MULAY S R, EBERHARD J N, DESAI J, MARSCHNER J A, KUMAR S V R, WEIDENBUSCH M, et al. Hyperoxaluria requires TNF receptors to initiate crystal adhesion and kidney stone disease[J]. J Am Soc Nephrol, 2017, 28: 761-768. DOI:10.1681/ASN.2016040486 |
| [12] |
SUN X Y, OUYANG J M, GAN Q Z, LIU A J. Renal epithelial cell injury induced by calcium oxalate monohydrate depends on their structural features: size, surface, and crystalline structure[J]. J Biomed Nanotechnol, 2016, 12: 2001-2014. DOI:10.1166/jbn.2016.2289 |
| [13] |
HEWAGAMA S P, HEWAWASAM R P. Antiurolithiatic potential of three Sri Lankan medicinal plants by the inhibition of nucleation, growth, and aggregation of calcium oxalate crystals in vitro[J/OL]. Sci World J, 2022, 2022: 8657249.
10.1155/2022/8657249.
|
| [14] |
HE J Y, DENG S P, OUYANG J M. Morphology, particle size distribution, aggregation, and crystal phase of nanocrystallites in the urine of healthy persons and lithogenic patients[J]. IEEE Trans Nanobioscience, 2010, 9: 156-163. DOI:10.1109/TNB.2010.2045510 |
| [15] |
AKHTAR K, HAQ I U. Chemical modulation of crystalline state of calcium oxalate with nickel ions[J]. Clin Chim Acta, 2013, 418: 12-16. DOI:10.1016/j.cca.2012.12.027 |
| [16] |
KOLATE A, BARADIA D, PATIL S, VHORA I, KORE G, MISRA A. PEG—a versatile conjugating ligand for drugs and drug delivery systems[J]. J Control Release, 2014, 192: 67-81. DOI:10.1016/j.jconrel.2014.06.046 |
| [17] |
ELISTRATOVA A A, GUBAREV A S, LEZOV A A, VLASOV P S, SOLOMATINA A I, LIAO Y C, et al. Amphiphilic diblock copolymers bearing poly(ethylene glycol) block: hydrodynamic properties in organic solvents and water micellar dispersions, effect of hydrophobic block chemistry on dispersion stability and cytotoxicity[J/OL]. Polymers (Basel), 2022, 14: 4361.
10.3390/polym14204361.
|
| [18] |
HARRIS J M, CHESS R B. Effect of pegylation on pharmaceuticals[J]. Nat Rev Drug Discov, 2003, 2: 214-221. DOI:10.1038/nrd1033 |
| [19] |
THOMAS A, MÜLLER S S, FREY H. Beyond poly(ethylene glycol): linear polyglycerol as a multifunctional polyether for biomedical and pharmaceutical applications[J]. Biomacromolecules, 2014, 15: 1935-1954. DOI:10.1021/bm5002608 |
| [20] |
PASUT G, VERONESE F M. State of the art in PEGylation: the great versatility achieved after forty years of research[J]. J Control Release, 2012, 161: 461-472. DOI:10.1016/j.jconrel.2011.10.037 |
| [21] |
IBRAHIM M, RAMADAN E, ELSADEK N E, EMAM S E, SHIMIZU T, ANDO H, et al. Polyethylene glycol (PEG): the nature, immunogenicity, and role in the hypersensitivity of PEGylated products[J]. J Control Release, 2022, 351: 215-230. DOI:10.1016/j.jconrel.2022.09.031 |
| [22] |
WEBSTER R, DIDIER E, HARRIS P, SIEGEL N, STADLER J, TILBURY L, et al. PEGylated proteins: evaluation of their safety in the absence of definitive metabolism studies[J]. Drug Metab Dispos, 2007, 35: 9-16. DOI:10.1124/dmd.106.012419 |
| [23] |
YAMAOKA T, TABATA Y, IKADA Y. Fate of water-soluble polymers administered via different routes[J]. J Pharm Sci, 1995, 84: 349-354. DOI:10.1002/jps.2600840316 |
| [24] |
D'SOUZA A A, SHEGOKAR R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications[J]. Expert Opin Drug Deliv, 2016, 13: 1257-1275. DOI:10.1080/17425247.2016.1182485 |
| [25] |
BIGINI P, GOBBI M, BONATI M, CLAVENNA A, ZUCCHETTI M, GARATTINI S, et al. The role and impact of polyethylene glycol on anaphylactic reactions to COVID-19 nano-vaccines[J]. Nat Nanotechnol, 2021, 16: 1169-1171. DOI:10.1038/s41565-021-01001-3 |
| [26] |
PHAM LE KHANH H, NEMES D, RUSZNYÁK Á, UJHELYI Z, FEHÉR P, FENYVESI F, et al. Comparative investigation of cellular effects of polyethylene glycol (PEG) derivatives[J/OL]. Polymers, 2022, 14: 279.
10.3390/polym14020279.
|
 2023, Vol. 44
2023, Vol. 44


