前床突脑膜瘤(anterior clinoidal meningioma,ACM)通常归类于鞍旁脑膜瘤或蝶骨嵴脑膜瘤,起源部位很难确定,有时会与起源于鞍结节、鞍膈、视神经管的脑膜瘤相混淆[1]。ACM通常位于前床突及蝶骨嵴内侧1/3,在所有蝶骨嵴脑膜瘤中占34%~43.9%[2]。由于肿瘤倾向于向前床突及蝶骨嵴外侧延伸,巨大ACM会侵犯颈内动脉及其分支、海绵窦等重要血管结构,同时肿瘤与视神经、动眼神经的紧密关系更增加了手术难度[3-5]。尽管颅底显微外科技术进展迅速,但如何完整、安全地切除ACM对于神经外科仍然是一个巨大的挑战。海军军医大学(第二军医大学)第二附属医院神经外科于2010年7月至2020年7月采用显微手术治疗了ACM患者64例,肿瘤全切率高且患者神经功能恢复较好。本研究回顾性分析了64例患者的临床资料,对ACM显微手术策略及患者术后早期视力恢复情况进行评价。
1 资料和方法 1.1 研究对象回顾性分析2010年7月至2020年7月于海军军医大学(第二军医大学)第二附属医院神经外科住院并行显微手术治疗的64例ACM患者的临床资料。纳入标准:患者出院诊断为ACM;排除标准:术前存在动眼神经功能障碍或合并其他颅脑疾病。64例患者中,男19例(29.7%)、女45例(70.3%),年龄为27~73岁,平均年龄(52.3±12.2)岁。临床表现:头晕30例,头痛26例,单侧视力下降19例,双侧视力下降10例;癫痫综合征6例。根据Al-Mefty分类标准[6],Ⅰ型21例、Ⅱ型39例、Ⅲ型4例。根据肿瘤直径[7],大型ACM(直径>4.0 cm)32例(50.0%)、中型ACM(直径2.0~4.0 cm)27例(42.2%)、小型ACM(直径<2.0 cm)5例(7.8%)。46例(71.9%)肿瘤累及重要神经、血管结构。
1.2 影像学资料64例患者术前均行头颅计算机断层扫描血管造影(computed tomography angiography,CTA)或磁共振血管成像(magnetic resonance angiography,MRA)、头颅MRI平扫+增强检查。27例患者行颅脑数字减影血管造影(digital subtraction angiography,DSA)检查以评估肿瘤中涉及血管的通畅程度及狭窄程度,并确定血管在肿瘤中的位置。36例ACM位于左侧前床突区域,28例位于右侧前床突区域。所有患者术后当天即刻复查头颅CT平扫,术后72 h内复查头颅MRI平扫+增强评估肿瘤切除情况,术后3个月复查头颅MRI平扫+增强评估肿瘤有无复发或进展。
1.3 手术方法64例患者均为全身麻醉下显微手术治疗,49例采用标准翼点入路(扩大翼点入路),15例采用眶颧入路。根据术前头颅MRI平扫+增强及CTA检查(图 1)显示的肿瘤位置、大小及是否侵犯神经、血管等特点,决定是否离断眶顶壁、眶外侧壁及颧弓。患者取仰卧位,以蝶骨嵴为中心铣开骨瓣,沿蝶骨嵴外侧向内分离并磨平蝶骨嵴,根据肿瘤基底附着情况决定是否磨除前床突。以蝶骨嵴为中心弧形剪开硬膜,切开侧裂蛛网膜并释放脑脊液,依次开放侧裂池、颈动脉池及视交叉池。显露肿瘤后,评估肿瘤与颈内动脉及其分支、动眼神经、视神经的位置关系,沿蛛网膜界面分离被肿瘤所包裹的颈内动脉及其分支,在距离视神经和动眼神经的安全范围内电凝肿瘤基底部阻断血供,分块切除肿瘤。
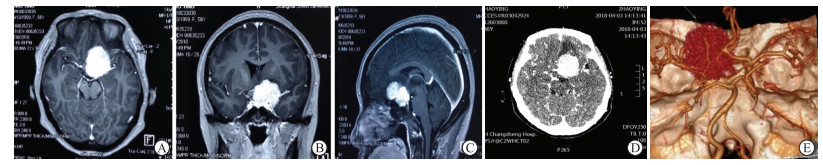
|
图 1 ACM患者术前影像学检查 Fig 1 Preoperative imaging examination of an ACM patient A: Transverse MRI image; B: Coronal MRI image; C: Sagittal MRI image; D, E: CTA examination showed the tumor encircling the anterior cerebral artery, middle cerebral artery, and the clinoid segment of the left internal carotid artery. ACM: Anterior clinoidal meningioma; MRI: Magnetic resonance imaging; CTA: Computed tomography angiography. |
1.4 疗效评估及随访
采用Simpson分级[8]评估肿瘤的切除程度,Simpson Ⅰ~Ⅱ级切除为肿瘤全切除。在评估肿瘤质地时,将术中可直接通过吸引器吸除的肿瘤定义为质地软,反之则为质地硬。统计患者术后动眼神经损伤、脑脊液漏、脑梗死等并发症情况。通过门诊或电话进行随访,平均随访时间为(24.3±13.2)个月,随访内容包括头颅MRI平扫+增强检查,视力、视野、眼底检查,以及动眼神经功能检查。
1.5 统计学处理应用SPSS 21.0软件进行数据分析。服从或近似服从正态分布的计量资料以x±s表示,计数资料以例数和百分数表示。
2 结果 2.1 手术切除情况64例ACM患者中,52例(81.2%)为Simpson Ⅰ~Ⅱ级切除,8例(12.5%)为Simpson Ⅲ级切除,4例(6.2%)为Simpson Ⅳ级切除;46例累及重要神经、血管结构的患者中,19例(41.3%,19/46)为Simpson Ⅰ~Ⅱ级切除。32例大型ACM中,25例为Simpson Ⅰ~Ⅱ级切除,5例为Simpson Ⅲ级切除,2例为Simpson Ⅳ级切除;32例中、小型ACM中,27例为Simpson Ⅰ~Ⅱ级切除,5例为Simpson Ⅲ~Ⅳ级切除。见表 1、图 2。
|
|
表 1 ACM直径与切除程度Simpson分级的关系 Tab 1 Relationship between the diameter of ACMs and surgical resection Simpson classification |
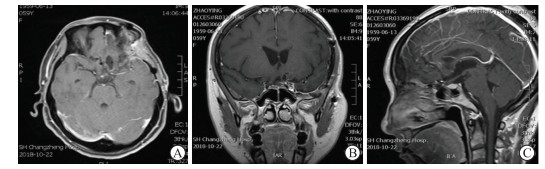
|
图 2 ACM患者术后72 h影像学检查 Fig 2 Imaging examination of an ACM patient at 72 h postoperation MRI examination showed that the ACM patient had a SimpsonⅠ resection. A: Transverse MRI image; B: Coronal MRI image; C: Sagittal MRI image. ACM: Anterior clinoidal meningioma; MRI: Magnetic resonance imaging. |
根据Al-Mefty分类的Ⅰ型ACM中,21例肿瘤质地均较硬,13例(61.9%,13/21)为Simpson Ⅰ~Ⅱ级切除,8例(38.1%,8/21)为Simpson Ⅲ~Ⅳ级切除;Ⅱ型ACM中,33例肿瘤质地较软、6例肿瘤质地较硬,35例(89.7%,35/39)为Simpson Ⅰ~Ⅱ级切除,4例(10.3%,4/39)为Simpson Ⅲ~Ⅳ级切除;Ⅲ型ACM中,4例肿瘤质地均较软,4例(100.0%,4/4)均为Simpson Ⅰ~Ⅱ级切除。当肿瘤质地较软时,Simpson Ⅰ~Ⅱ级切除率较高。见表 2。
|
|
表 2 Al-Mefty分类、ACM质地与切除程度Simpson分级的关系 Tab 2 Relationship between Al-Mefty classification, texture of ACMs and surgical resection Simpson classification |
2.2 术后患者症状改善及视力恢复情况
手术后1周内,56例术前头痛、头晕的患者中,40例(71.4%,40/56)术后头痛、头晕症状好转,其余16例(28.6%,16/56)头痛、头晕症状较前相仿,但在随后的长期随访中均得到改善;29例术前存在视力下降的患者中,12例(41.4%,12/29)术后视力较术前改善,其余17例(58.6%,17/29)视力均保持在术前水平,无患者术后出现视力下降情况。6例术前存在癫痫综合征的患者术后均无癫痫发作。
2.3 手术并发症64例患者中,共6例(9.4%,6/64)患者术后出现动眼神经损伤表现(上睑下垂、瞳孔对光反射迟钝),2例(3.1%,2/64)术后出现脑脊液漏并行脑脊液漏修补术,1例(1.6%,1/64)患者因术中脑血管损伤导致术后大面积脑梗死而死亡。见表 3。
|
|
表 3 ACM患者术后并发症统计 Tab 3 Postoperative complications in ACM patients |
3 讨论
ACM在所有中颅底脑膜瘤中占比较低,在手术切除前较难确定其起源。Cui等[9]和Pamir等[10]将脑膜瘤中心位于前床突周围者及前床突发生骨化的脑膜瘤归类为ACM。文献报道ACM的手术入路多为传统入路,其中以翼点入路(扩大翼点入路)最为多见[11-14]。眶颧入路对于大型ACM具有一定的优势,其可增加肿瘤的显露范围,但该入路创伤较大,术后脑脊液漏的发生率较高[15-16]。既往文献报道ACM总切除率(Simpson Ⅰ~Ⅱ级切除)为59%~91%[17-18],本组64例患者均根据其个体化情况选择显微手术策略,在保证患者神经功能的前提下最大程度地切除肿瘤,总切除率达81.2%(52/64,Simpson Ⅰ~Ⅱ级切除),部分术前存在视力下降的患者在术后1周内视力得到好转,且术后并发症较少。
Romani等[19]报道了经外侧眶上入路行ACM切除术,结果证明该入路的安全性和可靠性较高。Kim等[13]回顾性分析了59例ACM切除术资料,经外侧眶上入路及眶颧入路占78%,扩大翼点入路占15.3%,经额下入路占6.8%;总切除率为64.4%,总复发率为18.6%(新发病灶和复发病灶);23.7%的患者发生术后并发症,最常见的术后并发症为颅神经损伤,其次是术后脑出血或脑梗死、脑积水和颅内感染等。本组的64例患者中,共9例(14.1%,9/64)术后出现动眼神经损伤、脑脊液漏、脑梗死等相关并发症,其中1例(1.6%,1/64)患者因术中脑血管损伤导致术后大面积脑梗死而死亡。46例累及重要神经、血管结构的患者中,19例(41.3%,19/46)为Simpson Ⅰ~Ⅱ级切除。29例术前视力下降的患者中,12例(41.4%,12/29)术后1周内视力改善;无患者视力水平较术前下降。
李洋等[20]提出利用双向分离法可最大程度切除肿瘤组织并分离、保护颈内动脉及其穿支。Giammattei等[21]认为经硬膜内入路可以早期减轻肿瘤对周围脑组织的压迫,并通过释放脑脊液来减轻颅内压,经硬膜外入路可行硬膜外视神经管减压术及前床突切除术,将硬膜内入路与硬膜外入路相结合可以更好地保护视神经等结构。笔者认为翼点入路(扩大翼点入路)或眶颧入路在肿瘤切除、重要神经及血管保护方面具有其独特的优势:(1)当肿瘤向前侵犯眼眶时,暴露范围较其他入路广,并可经硬膜外切断肿瘤供血动脉,在磨除部分蝶骨嵴后可离断肿瘤基底部后切除肿瘤;(2)术者能自硬膜下和硬膜外充分处理肿瘤供血动脉,在保持术野清晰的同时减少术中出血;(3)在剥离与肿瘤相粘连的神经、血管时可直视神经、血管并减少副损伤,在磨除前床突时可减少视神经及颈内动脉损伤的发生率。
巨大ACM切除术的重点在于如何安全定位穿行于肿瘤组织内的重要神经、血管结构及避免术中对脑组织的副损伤[18]。笔者经验如下:(1)避免损伤颈内动脉和视神经最好的方法是在相对正常的解剖区域定位并解剖分离颈内动脉及视神经管,且最小限度地牵拉肿瘤组织;(2)经翼点入路(扩大翼点入路)或眶颧入路开颅后,在额底硬脑膜外脑压板置入之前,可在额底硬脑膜开几个小切口逐步释放脑脊液,在降低颅内压的同时可减少对脑组织的副损伤;(3)当肿瘤质地较软时,可在神经导航定位下于肿瘤硬脑膜基底部置入显微吸引器吸除部分肿瘤组织,从而逐步释放硬膜内压力;(4)从视神经硬脑膜袖套和镰状韧带开始打开肿瘤基底部硬脑膜,再行视神经管减压术并缓慢释放基底蛛网膜池脑脊液,有助于早期切断肿瘤供血动脉并使肿瘤与颅底分离。
随着内镜技术的发展,目前关于鼻内镜下颅底肿瘤切除术的报道逐渐增多。Bardeesi等[22]报道了1例鼻内镜下鞍上入路ACM切除术,但该入路仅适用于特定病例,当肿瘤与视神经、颈内动脉及其分支粘连紧密时则切除困难。有学者报道了鼻内镜下鞍上脑膜瘤切除术,该入路有利于切除延伸至前床突外侧及视神经侧方的肿瘤,这对鼻内镜下切除ACM同样具有参考意义[23-24]。鼻内镜入路虽然脑脊液漏发生率高,但视野宽广并可全景观察肿瘤,不打开硬脑膜即可早期行视神经减压术,对于改善视力具有重要作用[25-26]。Soni等[27]认为鼻内镜入路无法处理关键的神经、血管结构,而经颅入路可精细处理海绵窦、视神经及颈内动脉内侧等结构。鼻内镜入路在切除特定位置的肿瘤及保护视力方面具有独特的优势,将鼻内镜入路和经颅入路相结合对于切除体积巨大、侵袭范围广的ACM具有重要意义。
本研究探讨了ACM的显微手术治疗及早期视力恢复情况,ACM的手术目标是在完全切除肿瘤的同时改善神经功能,避免神经、血管的副损伤。本研究中部分视力下降的患者在术后早期视力得到改善,肿瘤质地较软的患者完全切除率高(Simpson Ⅰ~Ⅱ级切除)并且神经功能恢复较好,肿瘤质地较硬的患者完全切除困难并可导致严重的神经功能并发症。但本研究样本量小,随访时间短,仍需要增加样本量、延长随访时间来进一步研究ACM患者的显微手术疗效及远期视力恢复情况。
| [1] |
PAMIR M N, ÖZDUMAN K. Clinoidal meningiomas[J]. Handb Clin Neurol, 2020, 170: 25-35. |
| [2] |
LEE J H, SADE B, PARK B J. A surgical technique
for the removal of clinoidal meningiomas[C].
Neurosurgery, 2006, 59: ONS108-ONS114.
10.1227/01.NEU.0000220023.09021.03.
|
| [3] |
SUGHRUE M, KANE A R, RUTKOWSKI M J,
BERGER M S, MCDERMOTT M W. Meningiomas
of the anterior clinoid process: is it wise to drill out
the optic canal?[C]. Cureus, 2015, 7: e321.
10.7759/cureus.321.
|
| [4] |
BASSIOUNI H, ASGARI S, SANDALCIOGLU I E, SEIFERT V, STOLKE D, MARQUARDT G. Anterior
clinoidal meningiomas: functional outcome after
microsurgical resection in a consecutive series of 106
patients. Clinical article[J]. J Neurosurg, 2009, 111: 1078-1090. DOI:10.3171/2009.3.17685 |
| [5] |
XU T, YAN Y, EVINS A I, GONG Z Y, JIANG L, SUN H Y,
et al. Anterior clinoidal meningiomas: meningeal anatomical
considerations and surgical implications[C]. Front
Oncol, 2020, 10: 634.
10.3389/fonc.2020.00634.
|
| [6] |
AL-MEFTY O. Clinoidal meningiomas[J]. J Neurosurg, 1990, 73: 840-849. DOI:10.3171/jns.1990.73.6.0840 |
| [7] |
GOEL A, GUPTA S, DESAI K. New grading system to predict resectability of anterior clinoid meningiomas[J]. Neurol Med Chir (Tokyo), 2000, 40: 610-616. DOI:10.2176/nmc.40.610 |
| [8] |
NAKASU S, FUKAMI T, JITO J, NOZAKI K. Recurrence and regrowth of benign meningiomas[J]. Brain Tumor Pathol, 2009, 26: 69-72. DOI:10.1007/s10014-009-0251-2 |
| [9] |
CUI H, WANG Y, YIN Y H, FEI Z M, LUO Q Z, JIANG J Y. Surgical management of anterior clinoidal meningiomas: a 26-case report[J]. Surg Neurol, 2007, 68(Suppl 2): S6-S10. |
| [10] |
PAMIR M N, BELIRGEN M, ÖZDUMAN K, KILI T, OZEK M. Anterior clinoidal meningiomas: analysis of 43 consecutive surgically treated cases[J]. Acta Neurochir (Wien), 2008, 150: 625-636. DOI:10.1007/s00701-008-1594-x |
| [11] |
BEHARI S, GIRI P J, SHUKLA D, JAIN V K, BANERJI D. Surgical strategies for giant medial sphenoid wing meningiomas: a new scoring system for predicting extent of resection[J]. Acta Neurochir (Wien), 2008, 150: 865-877. DOI:10.1007/s00701-008-0006-6 |
| [12] |
RUSSELL S M, BENJAMIN V. Medial sphenoid ridge meningiomas: classification, microsurgical anatomy, operative nuances, and long-term surgical outcome in 35 consecutive patients[J]. Neurosurgery, 2008, 62: 1169-1181. |
| [13] |
KIM J H, JANG W Y, JUNG T Y, KIM I Y, LEE K H,
KANG W D, et al. Predictive factors for surgical
outcome in anterior clinoidal meningiomas: analysis
of 59 consecutive surgically treated cases[C] .
Medicine (Baltimore), 2017, 96: e6594.
10.1097/MD.0000000000006594.
|
| [14] |
ABDEL-AZIZ K M, FROELICH S C, DAGNEW E, JEAN W, BRENEMAN J C, ZUCCARELLO M, et al. Large sphenoid wing meningiomas involving the cavernous sinus: conservative surgical strategies for better functional outcomes[J]. Neurosurgery, 2004, 54: 1375-1383. DOI:10.1227/01.NEU.0000125542.00834.6D |
| [15] |
王慧博, 陆嘉诚, 陈正新, 李海林, 骆慧, 汤其凯, 等. 前床突脑膜瘤显微外科手术策略与疗效分析[J]. 临床神经外科杂志, 2021, 18: 513-517. DOI:10.3969/j.issn.1672-7770.2021.05.007 |
| [16] |
王旭辉, 贺绪智, 任明亮, 梁鸿, 许明伟, 李兵, 等. 中颅底至颞下窝、翼腭窝沟通性肿瘤的手术治疗[J]. 临床神经外科杂志, 2020, 17: 259-263. DOI:10.3969/j.issn.1672-7770.2020.03.004 |
| [17] |
ATTIA M, UMANSKY F, PALDOR I, DOTAN S, SHOSHAN Y, SPEKTOR S. Giant anterior clinoidal meningiomas: surgical technique and outcomes[J]. J Neurosurg, 2012, 117: 654-665. DOI:10.3171/2012.7.JNS111675 |
| [18] |
CZERNICKI T, KUNERT P, NOWAK A, MARCHEL A. Results of surgical treatment of anterior clinoidal meningiomas-our experiences[J]. Neurol Neurochir Pol, 2015, 49: 29-35. DOI:10.1016/j.pjnns.2015.01.003 |
| [19] |
ROMANI R, LAAKSO A, KANGASNIEMI M, LEHECKA M, HERNESNIEMI J. Lateral supraorbital approach applied to anterior clinoidal meningiomas: experience with 73 consecutive patients[J]. Neurosurgery, 2011, 68: 1632-1647. DOI:10.1227/NEU.0b013e318214a840 |
| [20] |
李洋, 袁贤瑞, 谢源阳, 刘定阳, 袁健, 苏君, 等. 前床突脑膜瘤的显微手术治疗及疗效影响因素分析[J]. 中华神经外科杂志, 2019, 35: 474-479. DOI:10.3760/cma.j.issn.1001-2346.2019.05.009 |
| [21] |
GIAMMATTEI L, STARNONI D, LEVIVIER M,
MESSERER M, DANIEL R T. Surgery for clinoidal
meningiomas: case series and meta-analysis of outcomes
and complications[C]. World Neurosurg, 2019, 129:
e700-e717.
10.1016/j.wneu.2019.05.253.
|
| [22] |
BARDEESI A M, ALSALEH S, AJLAN A M.
Endoscopic transnasal suprasellar approach for anterior
clinoidal meningioma: a case report and review of the
literature[C]. Surg Neurol Int, 2017, 8: 194.
10.4103/sni.sni_147_17.
|
| [23] |
OTTENHAUSEN M, BANU M A, PLACANTONAKIS D G, TSIOURIS A J, KHAN O H, ANAND V K, et al. Endoscopic endonasal resection of suprasellar meningiomas: the importance of case selection and experience in determining extent of resection, visual improvement, and complications[J]. World Neurosurg, 2014, 82(3/4): 442-449. |
| [24] |
KOUTOUROUSIOU M, FERNANDEZ-MIRANDA J C, STEFKO S T, WANG E W, SNYDERMAN C H, GARDNER P A. Endoscopic endonasal surgery for suprasellar meningiomas: experience with 75 patients[J]. J Neurosurg, 2014, 120: 1326-1339. DOI:10.3171/2014.2.JNS13767 |
| [25] |
CLARK A J, JAHANGIRI A, GARCIA R M, GEORGE J R, SUGHRUE M E, MCDERMOTT M W, et al. Endoscopic surgery for tuberculum sellae meningiomas: a systematic review and meta-analysis[J]. Neurosurg Rev, 2013, 36: 349-359. DOI:10.1007/s10143-013-0458-x |
| [26] |
CHOWDHURY F H, HAQUE M R, GOEL A H, KAWSAR K A. Endoscopic endonasal extended transsphenoidal removal of tuberculum sellae meningioma (TSM): an experience of six cases[J]. Br J Neurosurg, 2012, 26: 692-699. DOI:10.3109/02688697.2012.673648 |
| [27] |
SONI R S, PATEL S K, HUSAIN Q, DAHODWALA M Q, ELOY J A, LIU J K. From above or below: the controversy and historical evolution of tuberculum sellae meningioma resection from open to endoscopic skull base approaches[J]. J Clin Neurosci, 2014, 21: 559-568. DOI:10.1016/j.jocn.2013.03.043 |
 2023, Vol. 44
2023, Vol. 44


