2. 华北理工大学附属医院心内科, 唐山 063000
2. Department of Cardiology, Affiliated Hospital of North China University of Science and Technology, Tangshan 063000, Hebei, China
主动脉夹层(aortic dissection,AD)和动脉瘤是较常见的主动脉疾病,在世界范围内具有较高的发病率[1]。AD的发病机制主要有炎症、缺氧、氧化应激和基质金属蛋白酶(matrix metalloproteinase,MMP)表达增加[2],其中血管炎症是常见的致病因素。血管炎症是一个复杂的过程,包括外渗和循环单核白细胞进入血管壁的局部活化,这一多步骤过程是局部分泌趋化因子、巨噬细胞激活细胞因子、血管黏附分子和MMP上调产生的结果[2-3]。
研究表明S100钙结合蛋白(S100 calcium binding protein,S100)A4、S100A12参与AD炎症过程[4-5]。S100蛋白家族是一个多基因钙结合家族,由S100A8/9和S100A12等20多个成员组成,其在细胞炎症和细胞生长等方面功能广泛。糖基化终末产物受体(receptor for advanced glycation end-product,RAGE)是一种多配体蛋白质,而S100是其配体之一。研究证实S100蛋白家族成员S100A11是RAGE的配体之一,在细胞生长、凋亡和炎症中发挥作用[6-7]。实验表明,S100A11/RAGE能够介导多种信号通路调控生物学过程,如通过介导p38 MAPK信号转导通路调控小鼠骨关节炎软骨细胞肥大和细胞外基质的代谢[8],介导腺苷一磷酸依赖的蛋白激酶-信号转导及转录激活因子3信号通路调控鼻上皮细胞的重构和炎症[9],介导Akt-哺乳动物雷帕霉素靶蛋白信号通路促进肝脏脂肪变性[10]。然而,近年来对S100A11在血管疾病中的作用研究较少。本研究通过动物实验探究S100A11能否诱导AD炎症,并通过细胞实验探讨S100A11诱导AD炎症的发生机制。
1 材料和方法 1.1 实验动物55只3周龄雌性SD大鼠,体重48~50 g,购自北京华阜康生物科技股份有限公司[实验动物生产许可证号为SCXK(京)2019-0008],自由采食和饮水,饲养环境为温度22~26 ℃,相对湿度50%~60%,人工光照明暗各12 h,适应性喂养3 d。
1.2 细胞及主要试剂人胚胎肾细胞HEK293T购于中国科学院上海细胞库,人主动脉平滑肌细胞(human aortic smooth muscle cell,HASMC)购自美国Invitrogen公司。戊巴比妥钠(货号P3761)、β-氨基丙腈(货号A3134-10G)、血管紧张素Ⅱ(货号A9525-10MG)购自美国Sigma公司,质粒提取试剂盒(货号D6950-02)购自美国OMEG公司,DNA凝胶回收试剂盒(货号DP209-02)、LA Taq(货号RR002A)购自武汉华联科生物技术有限公司,T4 DNA连接酶(货号2011A)购自日本TaKaRa公司,内切酶BamH Ⅰ(货号R0136V)和XhoⅠ(货号R0146V)购自美国NEB公司,Lipofectamine 2000(货号11668-027)购自美国Invitrogen公司,pIRES2-GFP空载体(货号52107)购自美国addgene公司,Opti-MEM(货号31985-062)购自美国Gibco公司,苏木精(货号PAB180015)、伊红(货号PAB180016)、膜联蛋白Ⅴ-FITC/PI凋亡检测试剂盒(货号PAB180012)、RIPA(强)组织细胞快速裂解液(货号PAB180006)、BCA蛋白浓度测定试剂盒(货号PAB180007)、HRP标记的山羊抗兔和山羊抗小鼠二抗(货号分别为PAB160011、PAB160009)购自武汉贝茵莱生物科技有限公司,TUNEL细胞凋亡检测试剂盒(货号11684817910)购自瑞士Roche公司,DMEM(货号SH30022.01B)购自美国HyClone公司,蛋白质G磁珠(货号9006S)购自美国CST公司,蛋白质Marker(货号26634)购自美国Thermo公司,PVDF膜(货号IPVH00010)、化学发光试剂(货号WBKLS0010)购自美国Millipore公司,吐温-20(货号P-1379)购自美国Amresco公司,兔抗β肌动蛋白(货号4970)购自美国CST公司,兔抗S100A11(货号ab169530)、兔抗RAGE(货号ab30381)、兔抗磷酸化p38(phosphorylated p38,p-p38)(货号ab4822)、兔抗p38(货号ab31828)、兔抗MMP2(货号ab37150)、兔抗MMP9(货号ab76003)、兔抗Bcl-2相关X蛋白(Bcl-2-associated X,Bax)(货号ab182733)、兔抗Bcl-2(货号ab59348)、兔抗增殖细胞核抗原(proliferating cell nuclear antigen,PCNA)(货号ab29)、兔抗Ki-67(货号ab16667)购自英国Abcam公司。
1.3 细胞培养细胞HEK293T和HASMC复苏后用含10% FBS和1%青霉素、链霉素的DMEM培养基置于37 ℃、5% CO2条件下培养,HEK293T细胞密度达70%~80%时即可用于转染;HASMC取4~7代细胞进行后续实验。
1.4 慢病毒S100A11-短发夹RNA(short hairpin RNA,shRNA)质粒的构建构建S100A11基因的shRNA慢病毒载体,S100A11-shRNA靶序列为5'-GGTGGCTTAGCTATAGCATGC-3'(shRNA 1)、5'-GCTTAGCTATAGCATGCCATG-3'(shRNA 2)、5'-GCTATAGCATGCCATGAGTCC-3'(shRNA 3),将干扰序列打乱并随机突变得到阴性对照序列为5'-GATCCGCAACTGAGAAGATTG-3'(NC-shRNA)。将合成的序列连入用BamHⅠ和XhoⅠ双酶切过的pSICOR载体中,然后取连接产物转入大肠杆菌DH5α,提取质粒并进行测序。将载体用慢病毒包装获得Lv-NC-shRNA和Lv-S100A11-shRNA 1/2/3并转染到HEK293T细胞中,收集病毒Lv-NC-shRNA和Lv-S100A11-shRNA 1/2/3并将最终浓度调整为1×108 TU/mL。选取15只大鼠,尾静脉分别注射300 μL重组病毒或生理盐水,72 h后用1%戊巴比妥钠(40 mg/kg)腹腔注射麻醉大鼠,取主动脉组织,采用蛋白质印迹法检测S100A11的表达。
1.5 大鼠AD模型的构建及实验分组将大鼠随机分为5组,每组8只,分别为对照组(不做任何处理)、假手术组(尾静脉注射300 μL生理盐水)、AD组(饮水中加入0.25% β-氨基丙腈连续3周构建AD模型[11])、AD+Lv-NC-shRNA组(AD模型大鼠予尾静脉注射300 μL 1×108 TU/mL Lv-NC-shRNA)和AD+Lv-S100A11-shRNA组(AD模型大鼠予尾静脉注射300 μL 1×108 TU/mL Lv-S100A11-shRNA)。
1.6 病理组织学检测(1)H-E染色:1%戊巴比妥钠(40 mg/kg)腹腔注射麻醉大鼠,取大鼠主动脉组织,用4%多聚甲醛溶液固定,石蜡包埋后切片,厚度约为4 µm,将切片置于载玻片上,展片烤片后,根据试剂说明书步骤进行苏木精-伊红染色,显微镜拍照。
(2)TUNEL染色:取主动脉组织切片,加入蛋白酶K工作液孵育8~10 min,PBS洗涤5 min,加入TUNEL反应液37 ℃避光孵育1 h,最后加入DAPI复染细胞核,封片,荧光显微镜下观察并拍照。
1.7 免疫组织化学染色主动脉组织包埋石蜡后切成4 μm厚切片,烤片,用3% H2O2孵育10 min,PBS洗涤3次。0.5% BSA封闭30 min,加入S100A11、RAGE和p-p38(稀释比例均为1∶100)一抗,4 ℃孵育过夜;加入二抗,室温孵育30 min。最后用DAPI对细胞核进行复染,光学显微镜下观察并拍照。
1.8 S100A11过表达质粒的构建与细胞实验分组设计大鼠S100A11(NM_001004095.1)引物序列5'-cgcGGATCCTGCCTACAGAGACTGAG-3'、5'-cgcGAATTCTTAGATACGCTTCTGGGA-3'(斜体字母表示限制位点,小写字母表示保护位点)。双酶切(BamHⅠ和EcoRⅠ)分别对恢复的目的基因片段和真核表达载体pIRES2-GFP质粒进行酶切。利用T4 DNA连接酶将纯化的大鼠S100A11基因靶片段定向连接到pIRES2-GFP载体上。37 ℃振荡培养过夜,在大肠杆菌DH5α中扩增后,按质粒提取试剂盒说明书步骤从菌液中提取目标质粒。重组载体命名为pIRES2-GFP-S100A11。将HASMC分为3组:对照组(未进行任何干预)、EV组(转染pIRES2-GFP空载体)和OV-S100A11组(转染pIRES2-GFP-S100A11)。EV组、OV-S100A11组细胞分别用Lipofectamine 2000将pIRES2-GFP空载体和pIRES2-GFP-S100A11瞬时转染到HASMC细胞中,培养48 h后采用荧光显微镜观察和蛋白质印迹法检测转染效率,然后进行后续实验。
1.9 流式细胞术检测HASMC凋亡各组取5万~10万重悬的细胞于1000×g 4 ℃离心5 min,弃上清后加入1 mL预冷PBS,振荡使细胞悬浮后再次于1 000×g 4 ℃离心5 min,弃上清;将细胞重悬于200 μL结合缓冲液,加入10 μL膜联蛋白Ⅴ-FITC和10 μL PI,混匀后4 ℃避光孵育30 min;加入300 μL结合缓冲液,随即进行流式细胞仪检测,使用贝克曼FC500流式细胞仪自带软件CXP进行分析。
1.10 FPS ZM1或SB203580处理用200 nmol/L的RAGE抑制剂FPS ZM1或10 μmol/L的p38磷酸化抑制剂SB203580处理转染pIRES2-GFP-S100A11的HASMC 1 h,收集细胞进行后续实验。
1.11 蛋白质印迹法检测蛋白表达水平细胞用PBS洗涤后加入裂解液,4 ℃充分裂解细胞,转移至EP管中,95 ℃以上加热10 min,12 000×g离心10 min,取上清进行蛋白定量。采用SDS-PAGE分离蛋白,然后通过湿转法转至PVDF膜上,用5 %脱脂奶粉溶液4 ℃封闭过夜。加入一抗(S100A11、MMP2、Bax一抗稀释比例均为1∶2 000,RAGE、p-p38、p38、Bcl-2、PCNA、Ki-67、GAPDH一抗稀释比例均为1∶1 000,MMP9一抗稀释比例为1∶5 000)于室温孵育1 h,洗膜;加入二抗(稀释比例为1∶10 000)室温孵育1 h,洗膜后加入化学发光试剂,采用全自动化学发光分析仪检测。
1.12 统计学处理使用GraphPad Prism 8.0软件进行数据分析。计量资料以x±s表示,多组间比较采用单因素方差分析,组间两两比较采用最小显著性差异法。检验水准(α)为0.05。
2 结果 2.1 Lv-S100A11-shRNA降低了HEK293T细胞中S100A11的表达见图 1,与对照组(0.73±0.01)相比,转染Lv-NC-shRNA后HEK293T细胞中S100A11的表达水平(0.75±0.01)无明显变化(P>0.05);与Lv-NC-shRNA组相比,转染Lv-S100A11-shRNA 1/2/3后S100A11的表达水平(0.50±0.01、0.30±0.01、0.34±0.00)均降低(P均<0.01),其中Lv-S100A11-shRNA 2组S100A11的表达水平最低,因此后续实验选用Lv-S100A11-shRNA2。

|
图 1 Lv-S100A11-shRNA转染HEK293T细胞后S100A11蛋白的表达 Fig 1 Expression of S100A11 protein after Lv-S100A11-shRNAs transfection into HEK293T cells Lv: Lentiviral vector; S100A11: S100 calcium binding protein A11; shRNA: Short hairpin RNA; NC: Negative control. |
2.2 AD模型鉴定
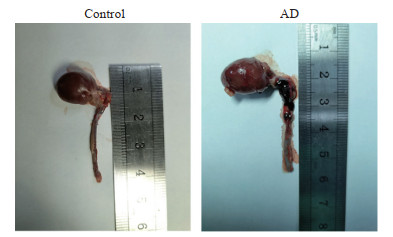
由图 2可见,对照组大鼠主动脉血管正常;AD组大鼠主动脉血管出现明显血肿,表明AD模型构建成功。
|
图 2 大鼠AD模型鉴定 Fig 2 Identification of AD model rats AD: Aortic dissection. |
2.3 Lv-S100A11-shRNA改善大鼠主动脉病变
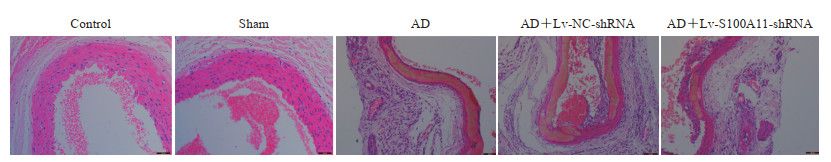
H-E染色结果(图 3)显示,对照组和假手术组大鼠主动脉血管正常,而AD组主动脉血管撕裂形成充满血液的夹层;与AD组相比,AD+Lv-NC-shRNA组大鼠主动脉病变无明显变化,而AD+Lv-S100A11-shRNA组大鼠主动脉病变改善。
|
图 3 H-E染色观察大鼠主动脉的组织学变化(200×) Fig 3 Histological changes of rat aorta showed by H-E staining (200×) H-E: Hematoxylin-eosin; AD: Aortic dissection; Lv: Lentiviral vector; NC: Negative control; shRNA: Short hairpin RNA; S100A11: S100 calcium binding protein A11. |
2.4 Lv-S100A11-shRNA减少大鼠主动脉组织细胞凋亡
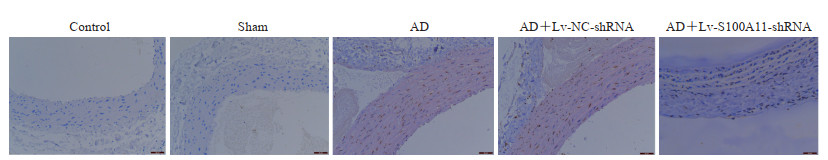
TUNEL染色结果(图 4)显示,对照组和假手术组主动脉组织中细胞凋亡均较少;与假手术组相比,AD组主动脉组织中细胞凋亡增多;与AD组相比,AD+Lv-NC-shRNA组主动脉组织中细胞凋亡无明显变化,而AD+Lv-S100A11-shRNA组细胞凋亡减少。
|
图 4 TUNEL染色检测大鼠主动脉组织中细胞凋亡情况(200×) Fig 4 TUNEL staining detecting cell apoptosis level in aortic tissue of rats (200×) TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling; AD: Aortic dissection; Lv: Lentiviral vector; NC: Negative control; shRNA: Short hairpin RNA; S100A11: S100 calcium binding protein A11. |
2.5 Lv-S100A11-shRNA降低大鼠主动脉组织中S100A11、RAGE和p-p38的表达
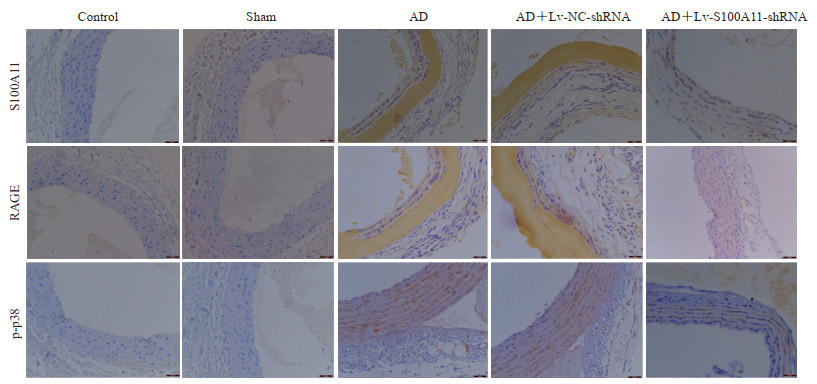
免疫组织化学染色法检测结果(图 5)显示,与对照组相比,假手术组大鼠主动脉组织中S100A11、RAGE、p-p38的表达无明显变化;与假手术组相比,AD组大鼠主动脉组织中S100A11、RAGE、p-p38表达均升高;与AD组相比,AD+Lv-NC-shRNA组大鼠主动脉组织中S100A11、RAGE、p-p38表达无明显变化,而AD+Lv-S100A11-shRNA组大鼠主动脉组织中S100A11、RAGE、p-p38表达均降低,表明RAGE和p-p38可能参与S100A11引起的AD形成。
|
图 5 免疫组织化学染色检测大鼠主动脉组织中S100A11、RAGE和p-p38蛋白的表达(200×) Fig 5 Expression of S100A11, RAGE and p-p38 proteins in aorta of rats detected by immunohistochemical staining (200×) S100A11: S100 calcium binding protein A11; RAGE: Receptor for advanced glycation end-product; p-p38: Phosphorylated p38; AD: Aortic dissection; Lv: Lentiviral vector; NC: Negative control; shRNA: Short hairpin RNA. |
2.6 Lv-S100A11-shRNA对大鼠主动脉组织中MMP2、MMP9、Bax、Bcl-2、PCNA、Ki-67蛋白表达的影响
蛋白质印迹法检测结果(图 6、表 1)显示,与对照组相比,假手术组大鼠主动脉组织中MMP2、MMP9、Bax、Bcl-2、PCNA和Ki-67的表达均无显著变化(P均>0.05);与假手术组相比,AD组大鼠主动脉组织中MMP2、MMP9和Bax表达均升高(P均<0.01),Bcl-2、PCNA和Ki-67表达均降低(P均<0.01);与AD组相比,AD+Lv-NC-shRNA组MMP2、MMP9、Bax、Bcl-2、PCNA和Ki-67的表达均无显著变化(P均>0.05),而AD+Lv-S100A11-shRNA组MMP2、MMP9、Bax表达均降低(P均<0.01),Bcl-2、PCNA、Ki-67表达均升高(P均<0.01)。

|
图 6 蛋白质印迹法检测大鼠主动脉组织中MMP2、MMP9、Bax、Bcl-2、PCNA、Ki-67蛋白的表达 Fig 6 Expression of MMP2, MMP9, Bax, Bcl-2, PCNA and Ki-67 proteins in aorta of rats detected by Western blotting MMP: Matrix metalloproteinase; Bax: Bcl-2-associated X; Bcl-2: B-cell lymphoma-2; PCNA: Proliferating cell nuclear antigen; Ki-67: Cell proliferation associated antigen; AD: Aortic dissection; Lv: Lentiviral vector; NC: Negative control; shRNA: Short hairpin RNA; S100A11: S100 calcium binding protein A11. |
|
|
表 1 各组大鼠主动脉组织中MMP2、MMP9、Bax、Bcl-2、PCNA、Ki-67蛋白的表达水平 Tab 1 Protein expression levels of MMP2, MMP9, Bax, Bcl-2, PCNA and Ki-67 in aortic tissue of rats in each group |
2.7 S100A11过表达质粒的转染效率
荧光显微镜观察结果(图 7A)显示,EV组和OV-S100A11组HASMC均表达GFP,且转染pIRES2-GFP-S100A11促进了S100A11的表达。蛋白质印迹法检测结果(图 7B)显示,OV-S100A11组HASMC中S100A11蛋白的表达高于对照组和EV组(0.72±0.01 vs 0.34±0.01、0.35±0.00,P均<0.01),表明pIRES2-GFP-S100A11转染成功。

|
图 7 HASMC中S100A11过表达质粒的转染效率 Fig 7 Transfection efficiency of S100A11 overexpression plasmid in HASMCs A: Immunofluorescence detection results of HASMCs transfected with OV-S100A11 (100×); B: Western blotting detecting the expression of S100A11. Control group: Untreated cells; EV group: Transfected empty vector cells; OV-S100A11 group: Transfected overexpression of S100A11 vector cells. HASMC: Human aortic smooth muscle cell; S100A11: S100 calcium binding protein A11. |
2.8 S100A11过表达促进HASMC凋亡
流式细胞术检测结果(图 8)显示,与对照组[(6.09±0.56)%]相比,EV组HASMC凋亡率[(6.37±0.54)%]无显著变化(P>0.05),而OV-S100A11组HASMC凋亡率[(18.60±1.07)%]明显升高(P<0.01)。
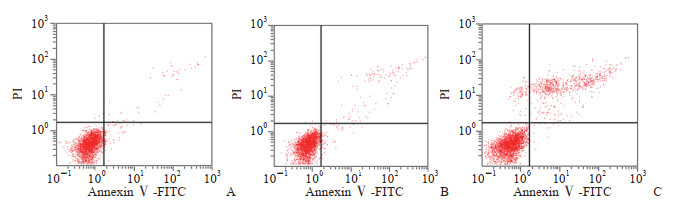
|
图 8 流式细胞术检测过表达S100A11对HASMC细胞凋亡的影响 Fig 8 Effect of S100A11 overexpression on apoptosis of HASMCs detected by flow cytometry A: Control group, untreated cells; B: EV group, transfected empty vector cells; C: OV-S100A11 group, transfected overexpression of S100A11 vector cells. S100A11: S100 calcium binding protein A11; HASMC: Human aortic smooth muscle cell; PI: Propidium iodide; FITC: Fluorescein isothiocyanate. |
2.9 S100A11过表达对HASMC中RAGE/p38 MAPK通路的影响
蛋白质印迹法检测结果(图 9、表 2)显示,与对照组相比,OV-S100A11组HASMC中RAGE、p-p38、MMP2、MMP9、Bax的表达均升高(P均<0.01),Bcl-2、PCNA、Ki-67的表达均下降(P均<0.01);与OV-S100A11组相比,OV-S100A11+FPS ZM1组S100A11、RAGE、p-p38、MMP2、MMP9、Bax的表达均下降(P均<0.01),Bcl-2、PCNA和Ki-67的表达均升高(P均<0.01),说明S100A11通过RAGE影响AD的发生;与OV-S100A11组相比,OV-S100A11+SB203580组p-p38、MMP2、MMP9和Bax的表达均降低(P均<0.01),Bcl-2、PCNA和Ki-67的表达均升高(P均<0.01),而S100A11和RAGE的表达无明显变化(P均>0.05),表明S100A11/RAGE对AD的作用通过p38 MAPK介导。
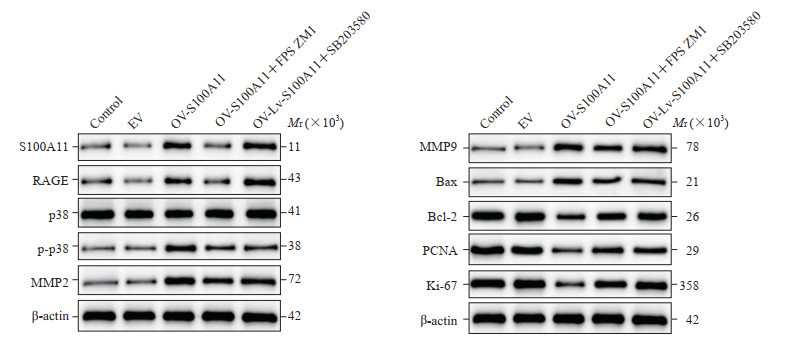
|
图 9 蛋白质印迹法检测HASMC中S100A11、RAGE、p38、p-p38、MMP2、MMP9、Bax、Bcl-2、PCNA和Ki-67蛋白质的表达 Fig 9 Protein expression of S100A11, RAGE, p38, p-p38, MMP2, MMP9, Bax, Bcl-2, PCNA and Ki-67 in HASMCs detected by Western blotting Control group: Untreated cells; EV group: Transfected empty vector cells; OV-S100A11 group: Transfected overexpression of S100A11 vector cells; OV-S100A11+FPS ZM1group: OV-S100A11 cells treated with RAGE inhibitor FPS ZM1; OV-Lv-S100A11+SB203580 group: OV-S100A11 cells treated with p38 phosphorylation inhibitor SB203580. HASMC: Human aortic smooth muscle cell; S100A11: S100 calcium binding protein A11; RAGE: Receptor for advanced glycation endproducts; p-p38: Phosphorylated p38; MMP: Matrix metalloproteinase; Bax: Bcl-2-associated X; Bcl-2: B-cell lymphoma-2; PCNA: Proliferating cell nuclear antigen; Ki-67: Cell proliferation associated antigen. |
|
|
表 2 各组HASMC中S100A11、RAGE、p-p38、p38、MMP2、MMP9、Bax、Bcl-2、PCNA和Ki-67蛋白的表达水平 Tab 2 Protein expression levels of S100A11, RAGE, p-p38, p38, MMP2, MMP9, Bax, Bcl-2, PCNA and Ki-67 in HASMCs of each group |
3 讨论
S100A11是大型钙结合蛋白家族S100的成员,被认为在胞吞、胞吐、酶活性调节[12]、细胞生长[13]、凋亡[14]和炎症[15]等过程中发挥特定的生物学作用。S100A11在不同组织中表达水平存在差异[16],且主要集中在细胞质[17-18]。S100A11与许多肿瘤的发生有关,在一些恶性肿瘤中被确定为肿瘤抑制因子,但在有些恶性肿瘤中其也被确定为促癌因子[19]。研究发现,S100A11在生长调节中也发挥矛盾作用[17, 20],在人角质形成细胞中通过RAGE依赖信号转导发挥作用[17]。
本研究建立了β-氨基丙腈诱导的AD大鼠模型,探究S100A11在AD中的作用与相关机制。研究结果显示,在AD模型中β-氨基丙腈能够促进主动脉细胞的凋亡,促进主动脉组织中S100A11、RAGE和p-p38的表达,上调主动脉组织中MMP2、MMP9和Bax的表达,下调主动脉组织中Bcl-2、PCNA和Ki-67的表达。进一步用Lv-S100A11-shRNA降低AD大鼠中S100A11的表达,观察到下调S100A11表达可以抑制RAGE和p-p38的表达、减轻主动脉组织细胞凋亡和AD的形成,提示S100A11的表达与AD的形成有密切的关系,S100A11或可通过调控RAGE和p-p38参与AD形成。
p38 MAPK是MAPK家族中的一个成员,包括p38α、p38β、p38γ和p38δ,其中p38α是最常见的一种蛋白质[21]。据报道,p38 MAPK通路在转录和翻译水平上是促炎细胞因子生物合成的关键调控因子,这使得该通路的不同成分成为治疗自身免疫病和炎症性疾病的潜在靶点[22]。研究表明,S100A11是RAGE的配体,RAGE能够与S100A11相互作用并在众多疾病中发挥作用[10]。为了探究S100A11的下游分子,本研究构建了S100A11过表达载体转染HASMC。研究结果显示过表达S100A11能促进HASMC的凋亡,上调RAGE和p-p38的表达,并能调节迁移、凋亡、增殖相关蛋白质的表达水平。为了进一步确认RAGE及p38的作用,本实验将RAGE抑制剂FPS ZM1与OV-S100A11转染的HASMC共孵育,抑制受体结合,结果显示,FPS ZM1组S100A11、RAGE和p-p38的表达均下调,且能够逆转过表达S100A11引起的迁移、凋亡、增殖相关蛋白质表达水平的改变,提示S100A11通过与RAGE结合发挥作用。最后又用p38磷酸化抑制剂SB203580处理过表达S100A11的HASMC,抑制p38的磷酸化,结果显示,SB203580能降低p-p38的表达且迁移、凋亡、增殖相关蛋白质的表达趋势与FPS ZM1干预效果一致,表明RAGE和p38 MAPK是S100A11的下游分子,S100A11可能通过其受体RAGE激活p38 MAPK信号通路参与AD形成。
综上所述,体内和体外实验表明,S100A11通过RAGE受体和p38 MAPK效应因子对AD的发生起促进作用,提示S100A11可能是AD的诊断指标之一。
| [1] |
NIENABER C A, CLOUGH R E, SAKALIHASAN N, SUZUKI T, GIBBS R, MUSSA F, et al. Aortic dissection[J/OL]. Nat Rev Dis Primers, 2016, 2: 16053. DOI: 10.1038/nrdp.2016.53.
|
| [2] |
WU D, SHEN Y H, RUSSELL L, COSELLI J S, LEMAIRE S A. Molecular mechanisms of thoracic aortic dissection[J]. J Surg Res, 2013, 184: 907-924. DOI:10.1016/j.jss.2013.06.007 |
| [3] |
CIFANI N, PROIETTA M, TRITAPEPE L, DI GIOIA C, FERRI L, TAURINO M, et al. Stanford-A acute aortic dissection, inflammation, and metalloproteinases: a review[J]. Ann Med, 2015, 47: 441-446. DOI:10.3109/07853890.2015.1073346 |
| [4] |
DAUGHERTY A, RATERI D L, LU H. S100A12 links to thoracic aortic aneurysms[J]. Circ Res, 2010, 106: 13-15. DOI:10.1161/CIRCRESAHA.109.210757 |
| [5] |
JIANG W L, WANG Z W, HU Z P, WU H B, ZHANG M, HU X P, et al. Highly expressed S100A12 in aortic wall of patients with DeBakey type Ⅰ aortic dissection could be a promising marker to predict perioperative complications[J]. Ann Vasc Surg, 2014, 28: 1556-1562. DOI:10.1016/j.avsg.2014.03.020 |
| [6] |
赵本正, 陈俊宇, 徐萍, 陈莹莹, 邱俊, 郑晶莹, 等. S100A11, 一种多生物学功能的Ca2+结合蛋白[J]. 中国实验诊断学, 2018, 22: 1655-1658. |
| [7] |
HUANG Y K, CHOU R H, YU C. Tranilast blocks the interaction between the protein S100A11 and receptor for advanced glycation end products (RAGE) V domain and inhibits cell proliferation[J]. J Biol Chem, 2016, 291: 14300-14310. DOI:10.1074/jbc.M116.722215 |
| [8] |
赵晓, 黄飞麒, 姚乃婕, 陈扬声. S100A11-RAGE通过P38MAPK信号转导通路调控小鼠骨关节炎软骨细胞肥大和细胞外基质代谢[J]. 中国现代医学杂志, 2016, 26: 6-11. |
| [9] |
LIU C C, DU H J, WANG Y J, GONG N Y, QI W W, ZHOU X M, et al. S100A11 regulates nasal epithelial cell remodeling and inflammation in CRSwNPs via the RAGE-mediated AMPK-STAT3 pathway[J]. Mol Immunol, 2021, 140: 35-46. DOI:10.1016/j.molimm.2021.09.014 |
| [10] |
TENG F, JIANG J J, ZHANG J H, YUAN Y W, LI K L, ZHOU B, et al. The S100 calcium-binding protein A11 promotes hepatic steatosis through RAGE-mediated AKT-mTOR signaling[J/OL]. Metabolism, 2021, 117: 154725. DOI: 10.1016/j.metabol.2021.154725.
|
| [11] |
LI J S, LI H Y, WANG L, ZHANG L, JING Z P. Comparison of β-aminopropionitrile-induced aortic dissection model in rats by different administration and dosage[J]. Vascular, 2013, 21: 287-292. DOI:10.1177/1708538113478741 |
| [12] |
ZHAO X Q, NAKA M, MUNEYUKI M, TANAKA T. Ca2+-dependent inhibition of actin-activated myosin ATPase activity by S100C (S100A11), a novel member of the S100 protein family[J]. Biochem Biophys Res Commun, 2000, 267: 77-79. DOI:10.1006/bbrc.1999.1918 |
| [13] |
SAKAGUCHI M, MIYAZAKI M, INOUE Y, TSUJI T, KOUCHI H, TANAKA T, et al. Relationship between contact inhibition and intranuclear S100C of normal human fibroblasts[J]. J Cell Biol, 2000, 149: 1193-1206. DOI:10.1083/jcb.149.6.1193 |
| [14] |
MAKINO E, SAKAGUCHI M, IWATSUKI K, HUH N H. Introduction of an N-terminal peptide of S100C/A11 into human cells induces apoptotic cell death[J]. J Mol Med (Berl), 2004, 82: 612-620. |
| [15] |
CECIL D L, JOHNSON K, REDISKE J, LOTZ M, SCHMIDT A M, TERKELTAUB R. Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products[J]. J Immunol, 2005, 175: 8296-8302. DOI:10.4049/jimmunol.175.12.8296 |
| [16] |
INADA H, NAKA M, TANAKA T, DAVEY G E, HEIZMANN C W. Human S100A11 exhibits differential steady-state RNA levels in various tissues and a distinct subcellular localization[J]. Biochem Biophys Res Commun, 1999, 263: 135-138. DOI:10.1006/bbrc.1999.1319 |
| [17] |
SAKAGUCHI M, SONEGAWA H, MURATA H, KITAZOE M, FUTAMI J, KATAOKA K, et al. S100A11, an dual mediator for growth regulation of human keratinocytes[J]. Mol Biol Cell, 2008, 19: 78-85. DOI:10.1091/mbc.e07-07-0682 |
| [18] |
BROOME A M, ECKERT R L. Microtubule-dependent redistribution of a cytoplasmic cornified envelope precursor[J]. J Invest Dermatol, 2004, 122: 29-38. DOI:10.1046/j.0022-202X.2003.22105.x |
| [19] |
SALAMA I, MALONE P S, MIHAIMEED F, JONES J L. A review of the S100 proteins in cancer[J]. Eur J Surg Oncol, 2008, 34: 357-364. DOI:10.1016/j.ejso.2007.04.009 |
| [20] |
SAKAGUCHI M, MIYAZAKI M, TAKAISHI M, SAKAGUCHI Y, MAKINO E, KATAOKA N, et al. S100C/A11 is a key mediator of Ca2+-induced growth inhibition of human epidermal keratinocytes[J]. J Cell Biol, 2003, 163: 825-835. DOI:10.1083/jcb.200304017 |
| [21] |
RODRÍGUEZ-CARBALLO E, GÁMEZ B, VENTURA F. p38 MAPK signaling in osteoblast differentiation[J/OL]. Front Cell Dev Biol, 2016, 4: 40. DOI: 10.3389/fcell.2016.00040.
|
| [22] |
CUENDA A, ROUSSEAU S. p38 MAP-kinases pathway regulation, function and role in human diseases[J]. Biochim Biophys Acta, 2007, 1773: 1358-1375. DOI:10.1016/j.bbamcr.2007.03.010 |
 2023, Vol. 44
2023, Vol. 44


