2. 上海海事大学信息工程学院计算机科学系, 上海 201306;
3. 海军军医大学(第二军医大学)第二附属医院超声诊断科, 上海 200003;
4. 同济大学附属上海市第四人民医院超声医学科, 上海 200434
2. Department of Computer Science, College of Information Engineering, Shanghai Maritime University, Shanghai 201306, China;
3. Department of Ultrasound, The Second Affiliated Hospital of Naval Medical University (Second Military Medical University), Shanghai 200003, China;
4. Department of Ultrasound, Shanghai Fourth People's Hospital Affiliated to Tongji University, Shanghai 200434, China
超声造影被誉为“第三次超声医学技术革命”,自问世以来其多用于实质性脏器血供的观察和良恶性病灶的鉴别研究[1-2],但涉及骨骼肌肉系统的报道相对较少。实验研究表明与常规彩色多普勒血流成像相比,超声造影显示骨骼肌内血流信号的灵敏度更高,可用于检测挤压伤骨骼肌内微循环变化、运动后肌肉血流灌注改变,以及评估炎症性肌病或外周动脉疾病导致的肌肉血流灌注异常[3-5]。超声造影剂六氟化硫微泡(商品名为声诺维)在低机械指数造影模式下被成功用于活体肌肉组织血流灌注的定量评估,这可为糖尿病患者肌肉微血管密度的临床评估提供客观信息[6]。本团队前期采用计算机骨骼肌纹理自动提取与定量分析技术,建立了2D灰阶超声所示骨骼肌损伤区域纹理特征的定量评估方法[7]。然而,在损伤修复过程中肌肉形态结构改变常与血流灌注变化合并存在,特别是超声造影表现为造影剂灌注分布及其相对应的微血管密度的变化规律尚未完全阐明。本研究通过建立大鼠下肢骨骼肌急性钝挫伤模型,观察钝挫伤修复过程中的肌肉组织血流灌注变化,探讨骨骼肌损伤修复过程中的超声造影特征变化,并运用计算机辅助技术定量分析造影剂平均回声强度灰阶值(Mean)与微血管平均光密度值(microvascular density,MVD)的相关性。
1 材料和方法 1.1 动物模型的建立健康成年雄性SD大鼠48只(体重为180~220 g)购自上海市计划生育科学研究所实验动物经营部[实验动物生产许可证号为SCXK(沪)2018-0006],适应性饲养1周后随机分为8组:正常对照组及损伤后1 h、12 h、24 h、48 h、7 d、14 d、28 d组,每组6只。正常对照组大鼠不予任何干预。各损伤组大鼠经2%戊巴比妥钠30 mg/kg腹腔注射麻醉后,右后肢剪毛备皮,取俯卧位,于伸膝、踝背屈90°固定于实验台。采用自行设计的重物打击装置(图 1),使800 g自制重物锤从高处以自由落体方式直接一次性撞击大鼠右后肢大腿后外侧,撞击肌肉内上方邻近耻骨,外侧紧靠股骨干,撞击肌肉面积约为1.0 cm2,撞击后相关部位皮肤完整、无出血、无骨折,此时模型即制作完成。术后将大鼠置于保温毯上保温,除损伤后1 h组外,其他损伤组大鼠禁水禁食6 h,随后恢复正常饮食,尽量保持伤口周围清洁,每天采用碘伏棉球消毒护理,直至创面皮肤恢复正常。实验过程中对动物的处置符合国家科学技术委员会发布的《实验动物管理条例》,所有动物实验均经海军军医大学(第二军医大学)第二附属医院动物伦理委员会审查批准(20ZR1457900)。

|
图 1 自制大鼠打击模型装置 Fig 1 Self-made model device for striking rats Black arrow indicates bracket for hanging heavy hammer and white arrow indicates animal fixed platform. |
1.2 超声检查流程与操作要点
采用REDWOOD超声成像仪(德国Siemens公司),探头频率为5~14 MHz。系统参数恒定:灰阶增益为13 dB,时间增益零补偿,彩色增益为2 dB,彩色血流M级,高精细动态组织谐波成像模式,聚焦深度为1.0~1.5 cm,机械指数为1.06。各损伤组大鼠在相应时间节点对损伤处骨骼肌及正常对照组相同部位进行2D灰阶超声和超声造影检查。超声检查均以股直肌长轴平面为观察平面,避开股动脉,完整显示上方的右侧耻骨,然后存储获得的超声图像以备脱机分析。超声造影采用六氟化硫微泡(4.8 mg/5 mL),注射剂量为0.025 mL/kg,经静脉快速团注(造影时保持机械指数为0.15,频率为10 MHz)后再注入生理盐水1 mL。
1.3 超声造影图像特征提取与分析由1名具有5年以上超声造影检查经验的高年资主治医师采集各组大鼠3 min超声造影视频。采用超声设备自带的造影分析软件获得时间-强度曲线,观察正常肌肉组织造影强度开始出现的时间(一般在注入造影剂后2 s)及到达峰值的时间(一般在注入造影剂后5 s)。检测完毕后回放录像,选择注入造影剂后4~6 s的图像3帧。由3名主治医师按照增强程度同时将3帧图像分为1、2、3级,最终选取增强程度为3级的图像用于计算机分析处理。
首先,在结构化纹理特征提取与独立成分分析等方法的基础上,采用信息极大化分解方法确定损伤肌肉的边缘[8]。其次,由1名有10年以上肌骨超声工作经验的主治医师和1名有10年以上计算机图像处理经验的工程师共同选定并勾画大小为60像素×60像素、形状相同的感兴趣区域(region of interest,ROI),应用Matlab R2019a软件自动计算出ROI造影剂最高能量显影时该帧图像的回声强度灰阶值,每张超声造影图像ROI的回声强度灰阶值重复测量5次,取平均值即为Mean。
1.4 肌肉组织的组织病理学检测超声检查后,各损伤组大鼠均予腹腔注射2%戊巴比妥钠350 mg/kg处死,取损伤区肌肉组织,面积约1.0 cm×0.5 cm,放入4%多聚甲醛PBS溶液中固定24 h,经石蜡包埋、5 μm厚度连续切片后进行常规H-E染色[苏木精,货号H9627,美国Sigma公司;伊红Y(水溶性),货号71014544,中国医药集团有限公司]和CD34免疫组织化学染色(CD34抗体EP373Y,货号ab81289,英国Abcam公司,稀释比例为1∶1 000)。每组连续取3张切片于光镜下观察染色阳性部位,染色呈黄色者为阳性,并在放大200倍视野下采用图像分析系统Vectra多色荧光系统(美国PerkinElmer公司)测定染色阳性区域的光密度值。各损伤组每张切片随机选择5个染色阳性视野测定CD34含量,取平均值即为MVD。
1.5 统计学处理应用SPSS22.0软件进行统计学分析。呈正态分布的计量资料以x±s表示,若方差齐多组间比较采用单因素方差分析,若方差不齐则采用近似F检验,多重比较采用Bonferroni校正法。MVD与Mean的相关性分析采用Pearson相关分析(|r|≥0.7为具有显著相关性,0.4≤|r|<0.7为相关性一般,|r|<0.4为相关性差)。采用Kendall’s W检验分析3名医师对超声造影图像评价分级的一致性。检验水准(α)为0.05。
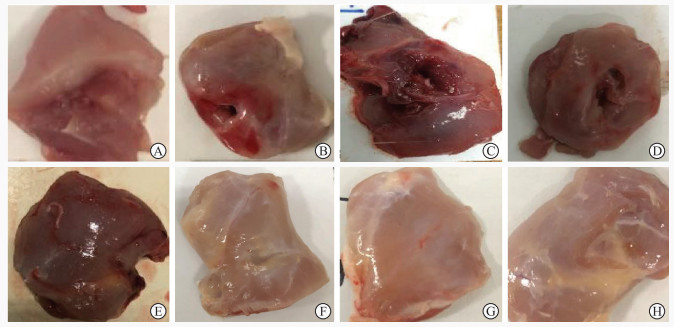
2 结果 2.1 各组大鼠骨骼肌的大体观察与正常对照组大鼠(图 2A)相比,损伤后1 h、12 h、24 h、48 h组大鼠下肢肌肉损伤处肿胀明显,可见深瘀斑及出血(图 2B~2E);损伤后7 d、14 d、28 d组大鼠下肢肌肉损伤处肿胀逐渐减轻,无明显出血,瘀斑逐渐减少甚至消失(图 2F~2H)。
|
图 2 各组大鼠股部骨骼肌的大体标本 Fig 2 Gross specimens of femoral skeletal muscle of rats in each group A: Normal control group; B: 1 h after contusion, the muscle was obviously swollen; C: 12 h after contusion, the muscle was swollen with deep ecchymosis; D: 24 h after contusion, the muscle was also swollen; E: 48 h after contusion, the muscle congestion was obvious; F: 7 d after contusion, the muscle swelling was alleviated; G: 14 d after contusion, there was no obvious bleeding; H: 28 d after contusion, the ecchymosis disappeared. |
2.2 各组大鼠骨骼肌的超声图像特征 2.2.1 2D灰阶超声图像特征
在沿肌束长轴走行的切面上,正常对照组大鼠下肢骨骼肌纹理表现为低回声的肌束与强回声的肌束膜相间排列的线状或轻度网络样分布(图 3A)。损伤后1 h、12 h、24 h、48 h组受损区骨骼肌回声增高,其内肌纹理连续性中断,网络样结构消失,呈磨玻璃样模糊不清,部分肌束间有血肿形成(图 3B~3E);随着肌纤维的修复,损伤后7 d、14 d组受损区骨骼肌纹理逐渐清晰,回声减低(图 3F、3G);损伤后28 d组受损区骨骼肌的结构形态基本恢复正常(图 3H)。

|
图 3 各组大鼠股部骨骼肌的灰阶超声图像特征 Fig 3 Characteristics of gray-scale ultrasound images of rat femoral skeletal muscle in each group A: In the normal control group, the skeletal muscle texture was arranged in a linear and network pattern with hypoechoic muscle bundle and hyperechoic muscle bundle membrane alternately; B: 1 h after contusion, the echo of the injured area was obviously increased and some muscle fibers were broken; C: 12 h after contusion, the echo of the injured area was slightly increased and there was hypoechoic area; D: 24 h after contusion, the edema was obvious and muscle texture was blurred; E: 48 h after contusion, the hypoechoic zone was reduced; F: 7 d after contusion, the echo of the injured area was slightly decreased; G: 14 d after contusion, the hypoechoic zone disappeared; H: 28 d after contusion, the texture of muscle in the injured area was clear, which was basically similar to that of the normal control group. Arrows indicate hematoma. |
2.2.2 超声造影图像特征
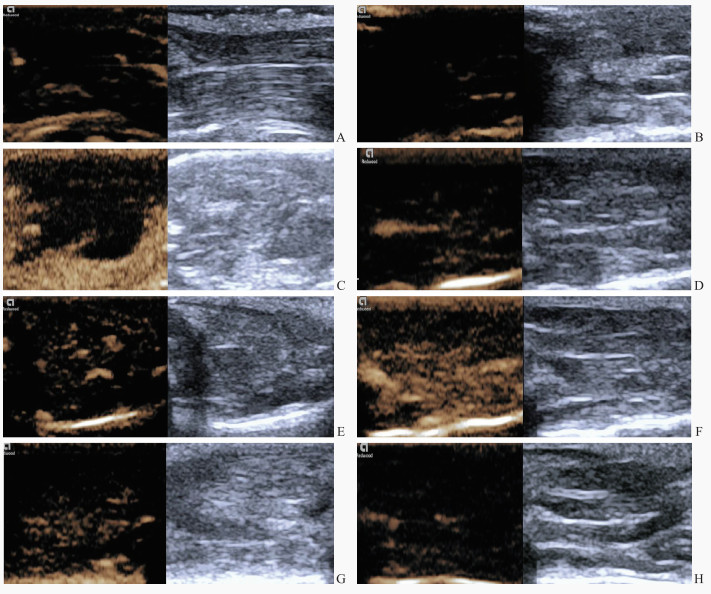
造影剂注入后,正常对照组大鼠股直肌显影均匀,供血动脉主干先于其内的微血管均匀显影(图 4A)。损伤后1 h、12 h组大鼠打击部位肌肉造影剂信号微弱,可见充盈缺损(图 4B、4C);损伤后24 h组损伤区造影剂呈星点状分布,为低增强(图 4D);损伤后48 h组损伤区造影剂呈斑片状分布,为轻度增强(图 4E);损伤后7 d组肌肉损伤区造影剂信号密集增多,呈高增强(图 4F);损伤后14 d组肌肉损伤区造影剂信号较损伤后7 d组减低(图 4G),呈轻度增强;损伤后28 d组损伤区造影剂信号与对照组相似,呈低增强(图 4H)。
|
图 4 各组大鼠股部骨骼肌的超声造影图像特征 Fig 4 Characteristics of contrast-enhanced ultrasound images of rat femoral skeletal muscle in each group A: In the normal control group, contrast agent filling could be seen in muscular vascular branches, and trace contrast agent distribution could be seen in muscle tissue; B: 1 h after contusion, there was minimal contrast agent perfusion in the muscle tissue of the injured area; C: 12 h after contusion, there was also minimal contrast agent perfusion; D: 24 h after contusion, a small amount of contrast agent perfusion could be seen in the muscle tissue of the injured area; E: 48 h after contusion, contrast agent perfusion signals characterized by multiple patchy enhancement; F: 7 d after contusion, contrast agent perfusion was obvious in muscle tissue of injured area; G: 14 d after contusion, contrast agent perfusion signals characterized by multiple patchy enhancement; H: 28 d after contusion, contrast agent perfusion had weak enhancement. |
2.3 各组大鼠骨骼肌超声造影图像的Mean分析
3位医师对正常对照组及损伤后1 h、12 h、24 h、48 h、7 d、14 d、28 d组超声造影图像的分级均有较强的一致性(Kendall’s W系数分别为0.917、0.835、0.825、0.888、0.853、0.917、0.824、0.940,P均<0.05)。损伤后1 h、12 h、24 h组损伤区Mean(分别为3.99±0.97、5.74±1.68、5.55±1.62)与正常对照组(14.99±3.65)相比均减低(P均<0.05);损伤后48 h组损伤区Mean(20.02±7.41)略高于正常对照组但差异无统计学意义(P>0.05);损伤后7 d组损伤区Mean进一步增高并达到峰值(59.98±10.13),损伤后14 d组损伤区Mean也保持在较高水平(27.11±4.67),与正常对照组相比差异均有统计学意义(P均<0.05);损伤后28 d组Mean(16.02±3.97)接近正常对照组水平(P>0.05)。
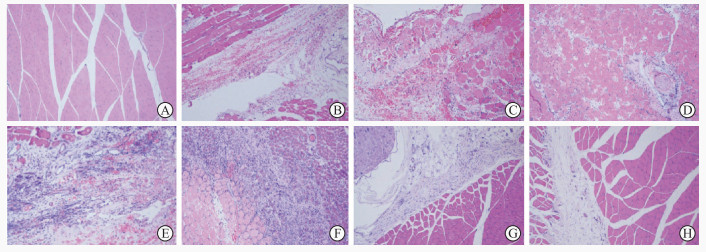
2.4 各组大鼠骨骼肌的组织病理学分析 2.4.1 H-E染色正常对照组大鼠骨骼肌肌纤维排列紧密、横纹清晰,细胞质呈粉红色(图 5A)。损伤后1 h、12 h、24 h组损伤区肌纤维排列紊乱,组织间隙出血、水肿明显,伴有中性粒细胞浸润(图 5B~5D);损伤后48 h,损伤区肌纤维变性坏死,出现肌卫星细胞,间质出血减少,多形核白细胞和中性粒细胞开始减少(图 5E);损伤后7 d,损伤区可见成纤维细胞和胶原、瘢痕形成,以及大量新生血管(图 5F);损伤后14 d,新生骨骼肌多已与损伤周边区肌纤维残存端融合,损伤区以成纤维样细胞为主,中性粒细胞大幅减少,肌卫星细胞较多(图 5G);损伤后28 d,损伤区骨骼肌的形态结构基本恢复正常,肌卫星细胞和成纤维样细胞减少,间质致密胶原沉积减少(图 5H)。
|
图 5 各组大鼠股部骨骼肌H-E染色(100×) Fig 5 H-E staining of femoral skeletal muscle of rats in each group (100×) A: In normal control group, normal myofibers staining pink arranged closely and nuclei staining blue were located in the sarcolemma; B: 1 h after contusion, the muscle fibers in the injured area were different in size and were divided and arranged disorderly, and there were a lot of hemorrhage and a small amount of inflammatory cell infiltration; C: 12 h after contusion, interstitial edema with obvious hemorrhage and increased infiltration of polymorphonuclear leukocytes and neutrophils; D: 24 h after contusion, infiltration of large numbers of nucleated leukocytes and neutrophils in the injured area; E: 48 h after contusion, muscle fiber degeneration and necrosis occurred in the injured area, and muscle satellite cells appeared; F: 7 d after contusion, neonatal skeletal muscle could be seen in the center of the injured area; G: 14 d after contusion, most newborn skeletal muscles fused with the residual ends of muscle fibers in the injured peripheral area, and a large number of dense collagen deposits can be seen in the stroma; H: 28 d after contusion, skeletal muscle structure in injured area basically returned to normal. H-E: Hematoxylin-eosin. |
2.4.2 骨骼肌损伤区的MVD分析
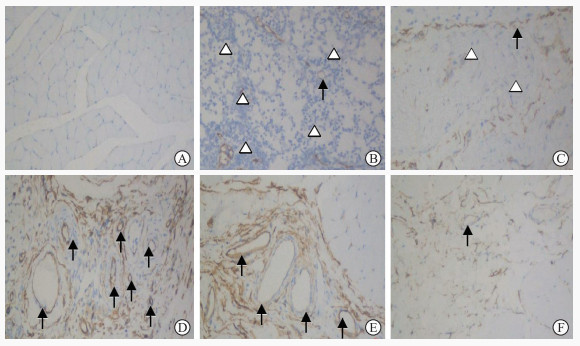
免疫组织化学染色显示大鼠骨骼肌损伤区CD34主要表达在血管内皮细胞,染色呈棕黄色(图 6)。损伤后1 h、12 h、24 h、48 h、7 d、14 d、28 d组大鼠骨骼肌损伤区MVD分别为3.08±0.42、3.63±0.41、4.73±0.63、6.30±0.85、8.93±1.06、6.38±0.94、4.19±0.44,总体差异有统计学意义(P<0.001);与损伤后1 h相比,损伤后12 h组MVD无明显变化(P>0.05),损伤后24 h至28 d各组的MVD均增高且以损伤后7 d组最高(P均<0.05)。
|
图 6 免疫组织化学染色检测各组大鼠股部骨骼肌CD34的表达(200×) Fig 6 CD34 expression in rat femoral skeletal muscle tissue in each group detected by immunohistochemical staining (200×) A: Normal control group; B: 1 h after contusion; C: 48 h after contusion; D: 7 d after contusion; E: 14 d after contusion; F: 28 d after contusion. Black arrows indicate blood vessel and white triangles indicate inflammatory cells. |
2.5 大鼠骨骼肌损伤区Mean与MVD的相关性分析
Pearson相关分析显示,大鼠骨骼肌损伤区MVD与Mean呈正相关(r=0.840,P<0.001)。
3 讨论骨骼肌损伤后其形态结构及血流灌注均有不同程度改变,借助于多种超声检测技术,不仅可直观地观察肌肉组织的变性、坏死情况及滋养血管的走行变异、血栓形成、血管闭塞等病变[9],还有助于临床医师术前准确了解骨骼肌损伤程度,从而制定科学合理的治疗方案[10-11]。采用超声造影能够通过观察造影剂的实时分布信息,动态追踪血流灌注的变化规律,从而对肌肉损伤做出微循环水平的快速诊断[12]。
既往有学者提出,超声造影定量参数与肌肉挤压伤后微循环变化有较强的相关性,可用于新西兰白兔下肢骨骼肌组织微血管血流灌注的研究[13]。与常规超声和MRI等比较,超声造影可灵敏检出急性肌肉损伤早期微循环的变化,且识别肌肉水肿等病理改变更准确[14]。然而,超声造影对急性肌肉损伤的诊断效果,一方面取决于超声医师根据其主观经验对病灶结构形态的定位及定性判读,另一方面需要依赖相关超声造影软件对病灶血流灌注特征进行定量分析[15],且操作程序较为复杂。近年来,随着计算机科学和超声医学交叉研究的深入,以深度学习为代表的人工智能迅速崛起,成为辅助超声影像定量诊断的热点技术[16],例如,基于弱监督学习的目标定位算法实现了超声造影图像中ROI目标的自动定位[17],多个超声造影视频分类算法也被验证认为可用于良恶性肿瘤的诊断与鉴别。
对于骨骼肌损伤的修复过程,笔者前期研究发现超声检查可达到与病理组织微观结构、血清标志物随时间变化一致的动态监测效果[7]。为了避免超声图像的识别因受检查医师主观经验的影响产生偏差,有研究者利用计算机辅助分析技术,从肌纤维结构分布的不同层次对肌肉2D灰阶超声图像ROI提取的纹理几何参数进行定量分析,更客观地描述了兔骨骼肌损伤修复不同时期的超声纹理特征[18]。与2D灰阶超声图像不同,超声造影图像除了可以采集肌肉不同时间节点、不同状态下反映微血流灌注的造影剂微泡信号改变,还能捕捉到肌肉结构和噪声等混叠信号[19],本研究采用一种基于结构化信息极大分解的肌肉损伤超声造影图像分割方法[8],与超声造影定性诊断相比,能更加精准地确定肌肉损伤区域的范围,然后对损伤区域内的造影剂信号强度特征进行计算机辅助提取与定量分析,有望快速实现定量描述骨骼肌损伤修复过程中超声造影所反映的肌群滋养血管束排列分布密度的演变规律。
组织损伤一般经历炎症、增生修复与组织重塑3个阶段,微循环新生血管生成是组织创伤愈合的一个重要环节。在骨骼肌损伤修复过程中,毛细血管的新生与构建调节着肌肉组织血流量有规律的升降,是创伤修复的主要病理生理基础。CD34单克隆抗体作为一种灵敏的血管内皮细胞标志物[20],可直观地反映受损肌肉组织内新生毛细血管的密度及分布情况。本研究结果显示,相较于大鼠骨骼肌损伤早期(即损伤后1 h)的MVD,损伤后12 h MVD无明显改变;至损伤后24~48 h损伤区肌肉内有少量新生毛细血管形成,MVD增高;损伤后7 d时新生毛细血管明显增多,MVD达到高峰;损伤后14~28 d MVD亦轻度增高。
超声造影剂经静脉注入后可快速分布于各级毛细血管中,组织的总体增强程度代表了组织内毛细血管总体分布情况,Doria等[21]使用第一代超声造影剂对特发性关节炎儿童的膝关节进行成像,并利用图像分析软件定量评估髌上囊内滑膜的增强程度。结果显示与健康对照组相比,疾病组超声造影前后膝关节的平均像素值均显著增高,且无症状和亚临床阶段之间膝关节的平均像素值亦存在显著差异。因此,提取并分析骨骼肌超声造影图像特征的Mean可间接反映受损肌肉内微血流灌注情况,能用于定量评估肌肉组织的损伤程度或修复情况。本研究结果亦显示,大鼠骨骼肌挫伤后,计算机辅助提取的反映损伤区回声强度的超声造影图像特征参数Mean与反映组织病理学微血管形成的参数MVD呈正相关(r=0.840,P<0.001)。可能原因如下:(1)本研究中损伤组大鼠股部肌肉受重力打击后即刻局部肌肉细胞或组织发生一系列炎症反应,出现肿胀、出血或坏死;同时,由于损伤区微血管结构破损,管腔内正常血流停滞,损伤后1~24 h组大鼠在超声造影图像上损伤区基本无造影剂灌注信号,Mean明显降低。(2)肌肉损伤修复过程中的一个重要环节是微血管再生,文献报道新西兰兔腓肠肌损伤后3 d毛细血管开始新生,7 d时新生毛细血管壁开始增厚、血流量较低,14 d后损伤区域毛细血管增生更明显,21 d时损伤区毛细血管增生达到高峰,之后毛细血管闭塞,28 d时毛细血管的数目基本恢复至损伤前水平[22]。本实验在大鼠中观察到,相较于正常对照组,损伤后1 h、24 h组损伤区造影剂灌注信号均减少,损伤后7 d损伤区造影剂灌注信号达到最高峰,损伤后14 d组损伤区灌注信号仍轻度增加,损伤后28 d组Mean达到与正常对照组较为接近的水平(P>0.05)。这一超声造影剂的分布特征与光镜下所见的损伤区微血管分布较为一致,但与前述文献报道的损伤修复时间略提前,组织重塑也较早完成,可能原因是相较于新西兰兔,大鼠的骨骼肌修复再生机制启动更快。由以上结果可见,通过计算机辅助定量分析超声造影图像中的造影剂强度变化,总体实现了对受损骨骼肌内微血管排列、分布、演变的定量评估,能够反映肌肉损伤后不同时期的微循环变化,有望成为临床超声定量诊断骨骼肌损伤修复的一种简便且快捷的方法[23-24]。
本研究存在以下局限性:(1)本研究为动物实验,重物打击所致钝挫伤的程度相对较轻,研究结果可能与真实世界的骨骼肌损伤严重程度或修复情况存在差别,今后还需要进一步针对严重肢体损伤的超声造影特征展开研究。(2)由于本实验在既往研究的基础上细化了损伤时间节点,所需实验动物数量较多,故选择了体格偏小、生存率较高的大鼠作为实验模型。而在超声造影过程中,采用个体化的静脉团注造影剂方式可能会影响大鼠骨骼肌造影剂灌注的显示效果,在后续研究中需要不断地改进实验流程,排除人为干扰。(3)当切取病理组织检测微血管密度时,或多或少涉及局部组织的炎症反应,这可能影响MVD的检测结果。因此,未来将基于骨骼肌损伤修复的病理生理研究,结合临床场景进一步深入探索人工智能辅助超声造影图像全自动定量分析工具的可行性和应用前景[25]。
| [1] |
丁炎, 周锋盛, 朱巧英, 吴鹏西. 超声造影与MRI鉴别诊断乳腺钙化性病变良恶性的对比研究[J]. 南京医科大学学报(自然科学版), 2022, 42: 103-107. |
| [2] |
李梦, 严昆, 尹珊珊, 姜彬彬, 董坤. 混合型肝癌超声造影表现与病理类型及分化程度的关系探讨[J]. 中国超声医学杂志, 2022, 38: 49-52. DOI:10.3969/j.issn.1002-0101.2022.01.016 |
| [3] |
张春东, 王欣, 唐杰, 李明明, 贺蓓, 刘凤飞, 等. 肢体肌肉挤压伤后微循环改变与超声造影表现相关性的实验研究[J]. 中华超声影像学杂志, 2021, 30: 1088-1093. DOI:10.3760/cma.j.cn131148-20210517-00334 |
| [4] |
NTOULIA A, BARNEWOLT C E, DORIA A S, HO-FUNG V M, LORENZ N, MENTZEL H J, et al. Contrast-enhanced ultrasound for musculoskeletal indications in children[J]. Pediatr Radiol, 2021, 51: 2303-2323. DOI:10.1007/s00247-021-04964-6 |
| [5] |
FISCHER C, KRIX M, WEBER M A, LOIZIDES A, GRUBER H, JUNG E M, et al. Contrast-enhanced ultrasound for musculoskeletal applications: a world federation for ultrasound in medicine and biology position paper[J]. Ultrasound Med Biol, 2020, 46: 1279-1295. DOI:10.1016/j.ultrasmedbio.2020.01.028 |
| [6] |
XU Z H, CHEN J H, HUANG F B, LV G R. Evaluation of skeletal muscle microcirculation and reserve function of the type 2 diabetes with contrast-enhanced ultrasonography[J]. Ultrasound Q, 2020, 36: 38-42. DOI:10.1097/RUQ.0000000000000459 |
| [7] |
赵佳琦, 章建全, 赵璐璐, 宋家琳, 潘倩, 盛建国, 等. 不同消融功率致兔骨骼肌急性微波热损伤修复的超声影像演变特征[J/CD]. 中华医学超声杂志(电子版), 2016, 13: 780-789.
|
| [8] |
陈东太郎, 徐琪, 曾卫明. 一种基于结构化信息极大分解的肌肉损伤超声造影图像分割方法: CN104732517A[P]. 2017-10-03.
|
| [9] |
徐振宏, 颜小斌, 李丽雅. 超声造影评估2型糖尿病骨骼肌微循环功能的研究[J]. 中国糖尿病杂志, 2021, 29: 200-204. |
| [10] |
WEBER M A, KRIX M, JAPPE U, HUTTNER H B, HARTMANN M, MEYDING-LAMADÉ U, et al. Pathologic skeletal muscle perfusion in patients with myositis: detection with quantitative contrast-enhanced US: initial results[J]. Radiology, 2006, 238: 640-649. DOI:10.1148/radiol.2382041822 |
| [11] |
KUNZ P, MICK P, GROSS S, SCHMIDMAIER G, ZEIFANG F, WEBER M A, et al. Contrast-enhanced ultrasound (CEUS) as predictor for early retear and functional outcome after supraspinatus tendon repair[J]. J Orthop Res, 2020, 38: 1150-1158. DOI:10.1002/jor.24535 |
| [12] |
JÄSCHKE M, WEBER M A, FISCHER C. [CEUS-application possibilities in the musculoskeletal system][J]. Radiologe, 2018, 58: 579-589. DOI:10.1007/s00117-018-0404-6 |
| [13] |
ZHANG C D, WANG X, TANG J. Correlation between microcirculation and contrast-enhanced ultrasonography after crush injury of limbs[J]. J Med Ultrasonics, 2018, 45: 307-313. DOI:10.1007/s10396-017-0841-2 |
| [14] |
HOTFIEL T, HOPPE M W, HEISS R, LUTTER C, TISCHER T, FORST R, et al. Quantifiable contrast-enhanced ultrasound explores the role of protection, rest, ice (cryotherapy), compression and elevation (PRICE) therapy on microvascular blood flow[J]. Ultrasound Med Biol, 2021, 47: 1269-1278. DOI:10.1016/j.ultrasmedbio.2021.01.003 |
| [15] |
毛丽娟, 曹佳颖, 杨敏捷, 李翠仙, 韩红, 董怡, 等. 三维超声造影定量分析评价肝细胞肝癌血流灌注的可行性研究[J]. 中华超声影像学杂志, 2021, 30: 932-937. DOI:10.3760/cma.j.cn131148-20210705-00456 |
| [16] |
胡妍璐, 张麒, 李超伦. 计算机辅助定量分析多发性大动脉炎颈动脉病变的超声造影图像[J]. 生物医学工程学杂志, 2017, 34: 790-796. |
| [17] |
王嘉琦. 基于时相超声造影图像的辅助诊断算法研究[D]. 成都: 电子科技大学, 2020.
|
| [18] |
赵佳琦, 章建全, 盛建国, 赵璐璐, 潘倩. 不同技术级别超声医师对离体猪横纹肌理化损伤模型超声定性诊断的局限性分析[J/CD]. 中华医学超声杂志(电子版), 2014, 11: 763-769.
|
| [19] |
WEBER M A, KRAKOWSKI-ROOSEN H, DELORME S, RENK H, KRIX M, MILLIES J, et al. Relationship of skeletal muscle perfusion measured by contrast-enhanced ultrasonography to histologic microvascular density[J]. J Ultrasound Med, 2006, 25: 583-591. DOI:10.7863/jum.2006.25.5.583 |
| [20] |
尹秋萍, 张玉英, 马钦风, 陈鲜霞, 沈淼, 张静妮. 不同类型肝泡型包虫病病灶边缘区超声造影参数与微血管密度的相关性[J]. 中国介入影像与治疗学, 2022, 19: 85-89. |
| [21] |
DORIA A S, KISS M H, LOTITO A P, MOLNAR L J, DE CASTRO C C, MEDEIROS C C, et al. Juvenile rheumatoid arthritis of the knee: evaluation with contrast-enhanced color Doppler ultrasound[J]. Pediatr Radiol, 2001, 31: 524-531. DOI:10.1007/s002470100474 |
| [22] |
江媛媛. 超声造影评价兔骨骼肌损伤修复动态变化的实验性研究[D]. 泰安: 泰山医学院, 2013.
|
| [23] |
MOLINARI F, CARESIO C, ACHARYA U R, MOOKIAH M R, MINETTO M A. Advances in quantitative muscle ultrasonography using texture analysis of ultrasound images[J]. Ultrasound Med Biol, 2015, 41: 2520-2532. DOI:10.1016/j.ultrasmedbio.2015.04.021 |
| [24] |
MARZOLA F, VAN ALFEN N, DOORDUIN J, MEIBURGER K M. Deep learning segmentation of transverse musculoskeletal ultrasound images for neuromuscular disease assessment[J/OL]. Comput Biol Med, 2021, 135: 104623. DOI: 10.1016/j.compbiomed.2021.104623.
|
| [25] |
赵佳琦, 徐琪, 章建全, 黄禾菁, 刁宗平. 骨骼肌超声诊断迈向人工智能新领域: 计算机辅助骨骼肌损伤超声定量诊断[J]. 第二军医大学学报, 2017, 38: 1217-1224. ZHAO J Q, XU Q, ZHANG J Q, HUANG H J, DIAO Z P. Ultrasound diagnosis of skeletal muscle promoted by artificial intelligence: a quantitative evaluation of injured skeletal muscle by computer-aided ultrasonographic texture analysis[J]. Acad J Sec Mil Med Univ, 2017, 38: 1217-1224. |
 2023, Vol. 44
2023, Vol. 44


