2. 电子科技大学医学院, 成都 610054;
3. 中国科学院四川转化医学研究医院, 成都 610000;
4. 德阳市第二人民医院胸外科, 德阳 618000;
5. 四川省人民医院金牛医院胸外科, 成都 610000
2. School of Medicine, University of Electronic Science and Technology of China, Chengdu 610054, Sichuan, China;
3. Chinese Academy of Sciences Sichuan Translational Medicine Research Hospital, Chengdu 610000, Sichuan, China;
4. Department of Thoracic Surgery, The Second People's Hospital of Deyang, Deyang 618000, Sichuan, China;
5. Department of Thoracic Surgery, Sichuan Provincial People's Hospital Jinniu Hospital, Chengdu 610000, Sichuan, China
最新的全球癌症统计数据显示,肺癌依然是致死率最高的恶性肿瘤;在所有病理类型肺癌中,非小细胞肺癌(non-small cell lung cancer,NSCLC)占80%~85%[1-2]。尽管随着治疗手段的改进和治疗策略的优化(如新辅助治疗的扩大应用和免疫检查点抑制剂等新药的应用),近年来NSCLC的转归得到了较大改善[3-5],然而总体上NSCLC的预后依旧不尽如人意[6]。为了更好地指导NSCLC的治疗,越来越多的学者尝试探索有效的NSCLC预后标志物[7-8]。真核生物胞质伴侣素6A(chaperonin containing TCP1 subunit 6A,CCT6A)是一个致癌基因,可通过调控细胞周期、细胞增殖、细胞侵袭、细胞干性和血管生成等多种方式促进恶性肿瘤的进展和耐药[9-12]。有研究发现CCT6A可通过激活TGF-β通路增强NSCLC细胞的转移能力[10, 13],并且在肺腺癌患者中CCT6A过表达与较差的无复发生存期和总生存期相关[13]。本研究旨在进一步评估CCT6A在NSCLC癌组织中的异常表达,并分析其与NSCLC患者临床病理特征和预后的关联。
1 资料和方法 1.1 研究对象回顾性收集2016年1月至2020年12月于四川省人民医院接受手术切除治疗的160例NSCLC患者的临床病历资料和随访信息。纳入标准:(1)经病理诊断为NSCLC;(2)年龄≥18岁;(3)美国东部肿瘤协作组体力状态(Eastern Cooperative Oncology Group performance status,ECOG PS)评分为0~1分;(4)有可获及的、可用于本研究的组织标本;(5)有可获及的、可用于本研究的临床病理特征数据。排除标准:(1)合并其他恶性实体肿瘤;(2)合并恶性血液疾病。本研究获得四川省人民医院伦理委员会审批。
1.2 数据收集收集患者的年龄、性别、吸烟史、饮酒史、高血压病史、高脂血症史、糖尿病史、EOCG PS评分等临床资料,肿瘤病理类型、分化程度、大小、淋巴结转移情况、TNM分期等病理资料,癌胚抗原、糖链抗原125等实验室检查指标数据,以及接受新辅助化疗和辅助化疗的情况。收集患者的随访信息(最终随访日期为2021年12月31日),根据随访信息计算无病生存期和总生存期。
1.3 CCT6A表达水平检测收集患者的癌组织样本和癌旁组织样本,采用免疫组织化学(immunohistochemistry,IHC)染色检测CCT6A表达水平。以CCT6A抗体(稀释比例为1∶200,美国Invitrogen公司)作为一抗,山羊抗兔IgG(H+L)(稀释比例为1∶60,美国Invitrogen公司)作为二抗,参考既往报道的方法[14]进行IHC染色。染色后在光学显微镜下观察,根据染色的强度和密度,采用半定量评分方法对CCT6A表达水平进行评估。IHC评分为染色强度评分(0~3分)和染色密度评分(0~4分)的乘积,IHC评分≤3分认为CCT6A表达水平低,IHC评分>3分认为CCT6A表达水平高[15]。
1.4 统计学处理分别应用SPSS 26.0和GraphPad Prism 7.02软件进行数据分析和绘图。服从正态分布且方差齐的计量资料以x±s表示,组间差异的比较采用独立样本t检验或单因素方差分析;不服从正态分布或方差不齐的计量资料以中位数(下四分位数,上四分位数)表示。计数资料以例数和百分数表示,组间差异的比较采用Pearson χ2检验。绘制ROC曲线评估CCT6A表达水平对NSCLC癌组织和癌旁组织的区分效能。生存分析采用Kaplan-Meier曲线进行展示,组间差异的比较采用log-rank检验。通过向前逐步的多因素Cox回归分析确定无病生存期和总生存期的独立预测因素。检验水准(α)为0.05。
2 结果 2.1 NSCLC患者的临床病历资料共160例NSCLC患者纳入本研究。160例患者的年龄为(59.4±11.7)岁,男133例(83.1%)、女27例(16.9%);95例(59.4%)有吸烟史,51例(31.9%)有饮酒史,55例(34.4%)有高血压病史,60例(37.5%)有高脂血症史,26例(16.2%)有糖尿病史;125例(78.1%)患者EOCG PS评分为0分,35例(21.9%)EOCG PS评分为1分;78例(48.8%)为肺腺癌,66例(41.2%)为肺鳞状细胞癌,16例(10.0%)为其他病理亚型;19例(11.9%)肿瘤分化程度为高分化,84例(52.5%)为中分化,57例(35.6%)为低分化;74例(46.2%)肿瘤直径≤5 cm,86例(53.8%)肿瘤直径>5 cm;69例(43.1%)有淋巴结转移;30例(18.8%)TNM分期为Ⅰ期,60例(37.5%)为Ⅱ期,70例(43.8%)为Ⅲ期;癌胚抗原为5.5(2.6,32.7)ng/mL,糖链抗原125为42.2(13.9,108.4)U/mL;68例(42.5%)接受了新辅助化疗,其中35例(21.9%)化疗方案为长春瑞滨+顺铂(vinorelbine and cisplatin,NP),17例(10.6%)化疗方案为多西他赛+顺铂(docetaxel and cisplatin,DP),11例(6.9%)化疗方案为吉西他滨+顺铂(gemcitabine and cisplatin,GP),5例(3.1%)化疗方案为紫杉醇+顺铂(paclitaxel and cisplatin,TP);123例(76.9%)接受了辅助化疗,其中70例(43.8%)化疗方案为NP,21例(13.1%)化疗方案为DP,17例(10.6%)化疗方案为GP,15例(9.4%)化疗方案为TP。
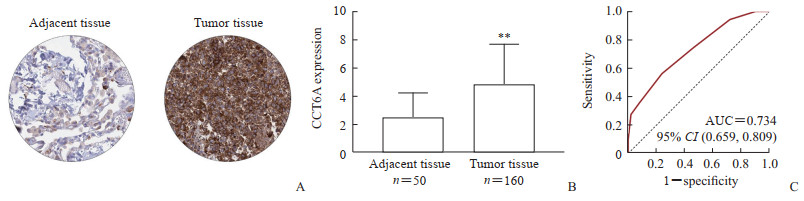
2.2 CCT6A在NSCLC患者癌组织中的表达160例NSCLC患者的癌组织均可获及,其中有50例患者的癌旁组织样本可获及,采用IHC染色检测CCT6A表达水平。结果显示,CCT6A主要表达于细胞质和细胞膜(图 1A),半定量评估结果表明CCT6A在NSCLC癌组织中的表达水平高于癌旁组织[(4.8±2.9)分vs (2.5±1.7)分,P<0.01,图 1B],ROC曲线分析表明CCT6A表达水平可以较好地区分NSCLC癌组织和癌旁组织(AUC为0.734,图 1C)。
|
图 1 NSCLC患者癌组织和癌旁组织中CCT6A的表达 Fig 1 Expression of CCT6A in cancer and adjacent tissues of NSCLC patients A: Immunohistochemical staining image (200×); B: Semi quantitative levels of CCT6A in cancer and adjacent tissues (**P < 0.01 vs adjacent tissue. x±s); C: ROC curve analysis showing that the expression level of CCT6A can effectively distinguish NSCLC cancer tissue and adjacent tissue. NSCLC: Non-small cell lung cancer; CCT6A: Chaperonin containing TCP1 subunit 6A; ROC: Receiver operating characteristic; AUC: Area under curve; CI: Confidence interval. |
2.3 NSCLC患者癌组织CCT6A表达水平与临床病理特征的关系
由表 1可见,NSCLC患者癌组织CCT6A表达水平与高的ECOG PS评分(P=0.038)、差的肿瘤分化(P=0.014)及淋巴结转移(P=0.018)有关,但与年龄、性别、吸烟史、饮酒史、高血压病史、高脂血症史、糖尿病史、肿瘤大小、肿瘤病理类型、TNM分期等临床病理特征无关(P均>0.05)。
|
|
表 1 不同临床病理特征NSCLC患者癌组织中CCT6A的表达水平 Tab 1 Expression level of CCT6A in cancer tissue of NSCLC patients with different clinicopathological characteristics |
2.4 NSCLC患者癌组织CCT6A表达水平与预后的关系
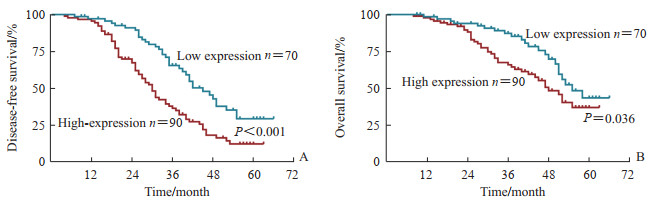
将NSCLC患者分为CCT6A高表达组(n=90)和CCT6A低表达组(n=70)。CCT6A高表达组TNM分期Ⅰ、Ⅱ、Ⅲ期分别有13、33、44例,41例接受新辅助化疗(NP、DP、GP、TP方案分别为24、8、4、5例),72例接受辅助化疗(NP、DP、GP、TP方案分别为44、10、8、10例);CCT6A低表达组TNM分期Ⅰ、Ⅱ、Ⅲ期分别有17、27、26例,27例接受新辅助化疗(NP、DP、GP、TP方案分别为11、9、7、0例),51例接受辅助化疗(NP、DP、GP、TP方案分别为26、11、9、5例)。两组患者TNM分期、接受新辅助化疗的患者比例及化疗方案、接受辅助化疗的患者比例及化疗方案差异均无统计学意义(P均>0.05)。生存分析结果表明,癌组织CCT6A高表达组患者的无病生存期(P<0.001)和总生存期(P=0.036)均短于CCT6A低表达组(图 2)。
|
图 2 癌组织CCT6A高表达组与低表达组NSCLC患者的Kaplan-Meier生存分析 Fig 2 Kaplan-Meier survival analysis of NSCLC patients with high and low expression of CCT6A in cancer tissues A: Disease-free survival; B: Overall survival. CCT6A: Chaperonin containing TCP1 subunit 6A; NSCLC: Non-small cell lung cancer. |
2.5 NSCLC患者预后的独立预测因素
多因素Cox回归分析结果显示,癌组织CCT6A高表达是NSCLC患者无病生存期差的独立预测因素(HR=2.062,95% CI 1.356~3.138,P=0.001),但不是总生存期的独立预测因素;此外,高脂血症(HR=1.794,95% CI 1.186~2.712,P=0.006)和高TNM分期(HR=1.365,95% CI 1.006~1.853,P=0.046)是NSCLC患者无病生存期差的独立预测因素,高脂血症(HR=2.295,95% CI 1.363~3.865,P=0.002)、肿瘤分化差(HR=1.830,95% CI 1.219~2.748,P=0.004)和高TNM分期(HR=2.078,95% CI 1.354~3.190,P=0.001)是NSCLC患者总生存期差的独立预测因素。
3 讨论既往研究发现,CCT6A在多种恶性肿瘤中高表达[11-12, 16-18]。例如,Ma等[16]的研究表明宫颈癌组织中CCT6A高表达,且对癌组织和正常组织有较好的鉴别能力。Peng等[17]研究报道乳头状甲状腺癌中CCT6A高表达,并且与其他常见致癌因子如细胞分裂周期20和细胞周期素B1高度相关。目前有关NSCLC患者癌组织中CCT6A表达情况及其临床意义的报道较少。本研究结果显示,CCT6A在NSCLC患者癌组织中的表达水平高于癌旁组织,表明CCT6A的异常表达可能与NSCLC的患病风险有关。
CCT6A可能通过多种渠道促进肿瘤的进展,与多种肿瘤的疾病特征相关。He等[19]研究报道胃癌患者癌组织中CCT6A的表达水平与肿瘤大小、T分期、N分期及TNM分期均呈正相关。Hu等[20]研究发现星形胶质瘤患者癌组织中CCT6A的表达水平与WHO级别及异柠檬酸脱氢酶突变缺失有关。此外,Ma等[16]的研究显示宫颈癌患者癌组织中CCT6A的表达水平与肿瘤大小、淋巴结转移及国际妇产科联合会分期有一定关联。与上述报道相符,本研究结果显示NSCLC患者癌组织中CCT6A的表达水平与肿瘤分化、淋巴结转移和ECOG PS评分有关,原因可能为:(1)CCT6A可以促进肿瘤细胞干性,因此与不良肿瘤分化相关[21];(2)CCT6A可以增强肿瘤细胞的迁移和侵袭能力,从而促进淋巴结转移[12];(3)CCT6A可以调控肿瘤细胞周期、细胞增殖、细胞侵袭、细胞干性和血管生成等促进肿瘤进展[9-12],导致患者病情更严重、体力活动状态更差,因此与ECOG PS评分有关。
多项研究报道CCT6A有作为多种肿瘤预后标志物的潜能[16-19]。Ma等[16]发现癌组织中CCT6A的高表达与宫颈癌患者无病生存期差有关,与总生存期差也有一定的关联。Peng等[17]研究表明癌组织中CCT6A高表达不仅与乳头状甲状腺癌患者较差的无病生存期和总生存期有关,还是其不良预后的独立预测因素。还有研究发现癌组织中CCT6A高表达与胃癌患者无病生存期差有关,且是其独立预测因素,但与总生存期缺乏关联[19]。本研究也发现癌组织中CCT6A高表达与NSCLC患者不良预后有关,可能原因为:(1)CCT6A可以促进肿瘤细胞干性和侵袭能力,从而诱导术后NSCLC的复发和进展[12, 21];(2)CCT6A可以促进肿瘤血管生成和肿瘤细胞干性,从而降低NSCLC对于新辅助治疗或辅助治疗的响应效果[10, 21];(3)CCT6A与NSCLC肿瘤分化差、淋巴结转移和ECOG PS评分高有关,从而间接影响患者预后。此外,本研究多因素Cox回归分析结果显示CCT6A的高表达并不能独立预测NSCLC患者总生存期,这可能是因为影响总生存期的因素众多,如患者随后接受的治疗、患者自身对于治疗的依从性等,因此CCT6A在预测总生存期中的作用受到其他因素的影响,或CCT6A通过影响其他因素从而与总生存期有一定关联,但并非是总生存期的独立预测因素。
本研究尚存在一些不足:(1)本研究仅纳入了可手术切除治疗的NSCLC患者,因此CCT6A在不可手术切除治疗NSCLC患者中的临床价值需进一步探索;(2)本研究仅采用IHC染色方法检测CCT6A在NSCLC患者中的表达,未来研究可以进一步采用其他方法进行验证;(3)本研究纳入患者样本量规模中等,将来有必要进一步扩大样本量进行深入研究和验证;(4)由于部分患者癌旁组织未保存,本研究中仅获得50例癌旁组织,癌组织和癌旁组织数量上的不匹配可能降低统计效能。
综上所述,CCT6A在NSCLC癌组织中高表达,其高表达与NSCLC患者肿瘤分化差、淋巴结转移、ECOG PS评分高有关,提示CCT6A有作为监测NSCLC病情进展标志物的潜能;CCT6A表达水平高是NSCLC患者无病生存期差的独立预测因子,提示其可预测复发风险。
| [1] |
SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI:10.3322/caac.21660 |
| [2] |
ALEXANDER M, KIM S Y, CHENG H. Update 2020:management of non-small cell lung cancer[J]. Lung, 2020, 198(6): 897-907. DOI:10.1007/s00408-020-00407-5 |
| [3] |
SAW S P L, ONG B H, CHUA K L M, et al. Revisiting neoadjuvant therapy in non-small-cell lung cancer[J]. Lancet Oncol, 2021, 22(11): e501-e516. DOI:10.1016/S1470-2045(21)00383-1 |
| [4] |
SOH J, HAMADA A, FUJINO T, et al. Perioperative therapy for non-small cell lung cancer with immune checkpoint inhibitors[J]. Cancers (Basel), 2021, 13(16): 4035. DOI:10.3390/cancers13164035 |
| [5] |
ETTINGER D S, WOOD D E, AISNER D L, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021[J]. J Natl Compr Canc Netw, 2021, 19(3): 254-266. DOI:10.6004/jnccn.2021.0013 |
| [6] |
SCHEGOLEVA A A, KHOZYAINOVA A A, FEDOROV A A, et al. Prognosis of different types of non-small cell lung cancer progression: current state and perspectives[J]. Cell Physiol Biochem, 2021, 55(S2): 29-48. DOI:10.33594/000000340 |
| [7] |
ŠUTIĆ M, VUKIĆ A, BARANAŠIĆ J, et al. Diagnostic, predictive, and prognostic biomarkers in non-small cell lung cancer (NSCLC) management[J]. J Pers Med, 2021, 11(11): 1102. DOI:10.3390/jpm11111102 |
| [8] |
VANDERLAAN P A, ROY-CHOWDHURI S. Current and future trends in non-small cell lung cancer biomarker testing: the American experience[J]. Cancer Cytopathol, 2020, 128(9): 629-636. DOI:10.1002/cncy.22313 |
| [9] |
王廷峰, 冯波, 马君俊, 等. 伴侣素CCT6A在结肠直肠癌中的表达及其意义[J]. 外科理论与实践, 2009, 14(3): 335-338. |
| [10] |
YING Z, TIAN H, LI Y, et al. CCT6A suppresses SMAD2 and promotes prometastatic TGF-β signaling[J]. J Clin Invest, 2017, 127(5): 1725-1740. DOI:10.1172/JCI90439 |
| [11] |
ZENG G, WANG J, HUANG Y, et al. Overexpressing CCT6A contributes to cancer cell growth by affecting the G1-to-S phase transition and predicts A negative prognosis in hepatocellular carcinoma[J]. Onco Targets Ther, 2019, 12: 10427-10439. DOI:10.2147/OTT.S229231 |
| [12] |
YANG X, TONG Y, YE W, et al. HOXB2 increases the proliferation and invasiveness of colon cancer cells through the upregulation of CCT6A[J]. Mol Med Rep, 2022, 25(5): 174. DOI:10.3892/mmr.2022.12690 |
| [13] |
WANG H, WANG X, XU L, et al. CCT6A and CHCHD2 are coamplified with EGFR and associated with the unfavorable clinical outcomes of lung adenocarcinoma[J]. Dis Markers, 2022, 2022: 1560199. DOI:10.1155/2022/1560199 |
| [14] |
ZHANG T, SHI W, TIAN K, et al. Chaperonin containing t-complex polypeptide 1 subunit 6A correlates with lymph node metastasis, abnormal carcinoembryonic antigen and poor survival profiles in non-small cell lung carcinoma[J]. World J Surg Oncol, 2020, 18(1): 156. DOI:10.1186/s12957-020-01911-x |
| [15] |
FU H, JIN C, ZHU Q, et al. Dysregulated expressions of PTEN, NF-κB, WWP2, p53 and c-Myc in different subtypes of B cell lymphoma and reactive follicular hyperplasia[J]. Am J Transl Res, 2019, 11(2): 1092-1101. |
| [16] |
MA J, YANG L, FENG H, et al. CCT6A may act as a potential biomarker reflecting tumor size, lymphatic metastasis, FIGO stage, and prognosis in cervical cancer patients[J]. J Clin Lab Anal, 2021, 35(8): e23793. DOI:10.1002/jcla.23793 |
| [17] |
PENG W, LI W, ZHANG X, et al. The intercorrelation among CCT6A, CDC20, CCNB1, and PLK1 expressions and their clinical value in papillary thyroid carcinoma prognostication[J]. J Clin Lab Anal, 2022, 36(9): e24609. DOI:10.1002/jcla.24609 |
| [18] |
CAI Y, WU D, ZHAN L. CCT6A expression in hepatocellular carcinoma and its correlation with clinical characteristics, liver function indexes, tumor markers and prognosis[J]. Clin Res Hepatol Gastroenterol, 2022, 46(3): 101796. DOI:10.1016/j.clinre.2021.101796 |
| [19] |
HE T, YU D, WANG Z, et al. Chaperonin-containing tailless complex polypeptide 1 subunit 6A links with aggravating tumor features and disease-free survival in surgical gastric cancer patients: a long-term follow-up study[J]. Clin Res Hepatol Gastroenterol, 2022, 46(7): 101913. DOI:10.1016/j.clinre.2022.101913 |
| [20] |
HU Y, FU P, ZHAO H, et al. Chaperonin-containing tailless complex polypeptide 1 subunit 6A correlates with increased World Health Organization grade, less isocitrate dehydrogenase mutation, and deteriorative survival of astrocytoma patients[J]. J Clin Lab Anal, 2021, 35(9): e23917. DOI:10.1002/jcla.23917 |
| [21] |
PENG X, CHEN G, LV B, et al. MicroRNA-148a/152 cluster restrains tumor stem cell phenotype of colon cancer via modulating CCT6A[J]. Anticancer Drugs, 2021, 33(1): e601-e621. DOI:10.1097/CAD.0000000000001198 |
 2023, Vol. 44
2023, Vol. 44


