2. 中山大学中山眼科中心海南眼科医院(海南省眼科医院)眼底病科, 海南省眼科学重点实验室, 海口 571300
2. Department of Fundus Diseases, Hainan Eye Hospital, Zhongshan Ophthalmic Center, Key Laboratory of Ophthalmology of Hainan Province, Sun Yat-sen University, Haikou 571300, Hainan, China
视网膜病变是糖尿病常见的并发症之一,发生率约为30%[1]。糖尿病视网膜病变(diabetic retinopathy,DR)的主要病理变化包括视网膜微血管病变和视神经病变[2],早期防治要点是预防微血管病变[2]。研究发现视网膜神经节细胞(retinal ganglion cell,RGC)的凋亡早于微血管病变,因此抗凋亡可能是早期防治DR的措施[3]。轴索导向分子信号素蛋白3A(semaphorin 3A,SEMA3A)不仅是神经轴突生长的负调控蛋白,还通过影响细胞增殖、迁移和凋亡参与多种病理生理过程,与支气管肺发育不良[4]、缺血性脑卒中[5]、多发性硬化[6]和癌症[7]等的发生与发展有关。SEMA3A与DR的关系既往少有报道,有研究发现SEMA3A的抑制剂BI-X可以改善氧诱导视网膜病变小鼠的视网膜血运重建,提示其可能成为糖尿病黄斑缺血的治疗靶点[8]。Notch1信号通路可调控RGC的凋亡[9]。本研究探讨了SEMA3A在大鼠RGC中的表达变化及其对RGC凋亡和Notch1信号通路的影响,旨在寻找DR潜在的治疗靶点。
1 材料和方法 1.1 动物、试剂与仪器40只SPF级成年雄性SD大鼠(6~8周龄)购于上海斯莱克实验动物有限责任公司[动物生产许可证号为SCXK(沪)2018-0013],自由进食和水,饲养环境温度为22~24 ℃、相对湿度为40%~60%、明暗周期12 h。
链脲佐菌素(streptozotocin,STZ)购于上海懋康生物科技有限公司,SEMA3A-siRNA慢病毒由上海吉玛制药技术有限公司提供,Notch1通路抑制剂(3, 5-二氟苯乙酰基)-L-丙氨酰基-L-2-苯基甘氨酸叔丁酯(DAPT)购于长沙鼎国生物技术有限公司,免疫荧光染色试剂盒、H-E染色试剂盒、TUNEL染色试剂盒均购于上海歌凡生物科技有限公司,IL-6、TNF-α ELISA检测试剂盒购于武汉博士德生物科技有限公司。兔抗鼠SEMA3A、小鼠抗大鼠POU结构域转录因子3a(POU domain transcription factor 3a,Brn3a;一种RGC标志物)、羊抗兔Alexa 488、羊抗鼠Alexa 595、鼠抗兔Notch1、鼠抗兔caspase 3抗体均购于美国Sigma公司。
CKX53型荧光显微镜(日本奥林巴斯公司),DYCZ-40K型电泳仪(北京六一生物科技有限公司),HBS-1096C型酶标仪(南京德铁实验设备有限公司)。
1.2 模型制备与分组将40只大鼠随机分为对照组、模型组、si-SEMA3A组、si-SEMA3A+DAPT组,每组10只。参考文献[10]建立大鼠DR模型:大鼠腹腔注射55 mg/kg STZ,72 h后尾静脉采血检测血糖浓度,血糖浓度>16.7 mmol/L视为造模成功[11]。对照组大鼠正常喂养;模型组大鼠建立DR模型,si-SEMA3A组大鼠在造模完成后双眼玻璃体内注射SEMA3A-siRNA慢病毒(病毒滴度1×109 IU/mL)10 μL,si-SEMA3A+DAPT组大鼠在造模完成后双眼玻璃体内注射SEMA3A-siRNA慢病毒(病毒滴度1×109 IU/mL)10 μL和10 μmol/L DAPT 10 μL。12周后进行各项指标检测。
1.3 双标记免疫荧光染色检测大鼠视网膜SEMA3A蛋白表达定位建模12周后安乐处死大鼠,用40 g/L多聚甲醛溶液灌注,取出眼球并进行冰冻切片(厚度为10 μm)。将切片用PBS洗涤3次,随后用3 g/L的Triton X-100孵育30 min;再次用PBS洗涤3次,随后用5 g/L的山羊血清在温室孵育30 min;加入兔抗鼠SEMA3A抗体(稀释200倍)+小鼠抗大鼠Brn3a抗体(稀释200倍),4 ℃过夜,次日滴加二抗(羊抗兔Alexa 488抗体、羊抗鼠Alexa 594抗体),室温孵育60 min;最后封片,在显微镜下观察、拍照。
1.4 H-E染色检测大鼠RGC密度将眼球切片用PBS洗涤3次,滴加苏木精染液,2 min后冲洗,随后用95%乙醇脱水,加伊红染液,2 min后冲洗,脱水、透明后封片,在显微镜下观察。用ImageJ软件计算大鼠RGC密度。
1.5 TUNEL染色检测大鼠RGC凋亡情况在眼球切片中加入蛋白酶K工作液,室温孵育30 min;加入TUNEL反应液,温室孵育60 min;加入50 μL转化剂,温室孵育30 min。随后加入DAB显色,苏木精复染,脱水,二甲苯透明。最后封片,显微镜下观察。
1.6 ELISA检测大鼠视网膜炎症因子IL-6和TNF-α表达水平操作严格按照试剂盒说明书进行。
1.7 蛋白质印迹法检测大鼠视网膜SEMA3A、Notch1及caspase 3蛋白表达水平用含有放射免疫沉淀法裂解缓冲液的裂解液提取大鼠视网膜组织中的总蛋白,用BCA试剂盒检测蛋白浓度,通过10% SDS-PAGE分离蛋白,用半干转移法将蛋白转至PVDF膜上。随后用5%脱脂奶粉封闭1 h,加入SEMA3A抗体(稀释200倍)、Notch1抗体(稀释500倍)、caspase 3抗体(稀释1 000倍)、GAPDH抗体(稀释1 000倍),4 ℃过夜孵育。次日除去一抗,缓冲液洗涤3次后加入二抗。用ECL发光仪成像,用ImageJ软件分析灰度值。
1.8 统计学处理应用SPSS 22.0软件进行统计学分析。计量资料以x±s表示,多组间比较采用单因素方差分析,两两比较采用最小显著性差异法(least significant difference,LSD)-t检验。检验水准(α)为0.05。
2 结果 2.1 SEMA3A蛋白在大鼠RGC中的表达SEMA3A和Brn3a双标记免疫荧光染色确定SEMA3A蛋白在大鼠RGC中特异性表达(图 1),说明SEMA3A对视网膜的损伤作用可能是通过RGC实现的。

|
图 1 双标记免疫荧光染色检测大鼠RGC中SEMA3A和Brn3a共存 Fig 1 Detection of coexistence of SEMA3A and Brn3a in rat RGCs using double-labeled immunofluorescence staining A: Brn3a expression (red, Alexa 594 fluorescence labeled, arrow indicated); B: SEMA3A expression (green, Alexa 488 fluorescence labeled, arrow indicated); C: Coexistence of Brn3a and SEMA3A (merged, arrow indicated). RGC: Retinal ganglion cell; SEMA3A: Semaphorin 3A; Brn3a: POU domain transcription factor 3a. |
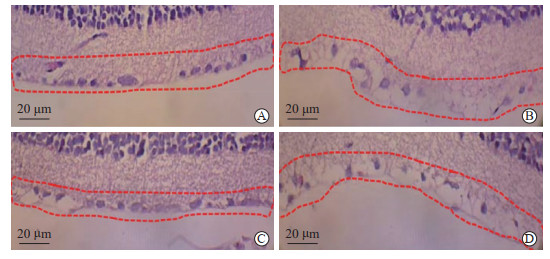
2.2 各组大鼠RGC密度比较
与对照组比较[(209.33±19.87)个/mm2],模型组大鼠RGC密度[(141.26±9.01)个/mm2]较低(P<0.05);si-SEMA3A组大鼠RGC密度[(182.94±16.22)个/mm2]大于模型组和si-SEMA3A+DAPT组[(158.09±12.25)个/mm2](P均<0.05)。见图 2。
|
图 2 H-E染色检测各组大鼠RGC密度 Fig 2 H-E staining for detecting RGC density of rats in each group A: Control group; B: Model group; C: si-SEMA3A group; D: si-SEMA3A+DAPT group. Red dashed boxes show RGCs. H-E: Hematoxylin-eosin; RGC: Retinal ganglion cell; SEMA3A: Semaphorin 3A; DAPT: N-(N-((3, 5-difluorophenyl)acetyl)-L-alanyl-L-2-phenyl)glycine-1, 1-dimethylethyl ester. |
2.3 各组大鼠RGC凋亡率比较
对照组大鼠视网膜组织中未检测到TUNEL阳性RGC;模型组大鼠视网膜组织中可见较多TUNEL阳性RGC,RGC凋亡率为(49.55±7.82)%;与模型组比较,si-SEMA3A组大鼠RGC凋亡率[(18.90±3.41)%]较低(P<0.05),而si-SEMA3A+DAPT组大鼠RGC凋亡率[(34.65±4.03)%]高于si-SEMA3A组(P<0.05)。见图 3。

|
图 3 TUNEL染色检测各组大鼠RGC凋亡情况 Fig 3 TUNEL staining for detecting RGC apoptosis of rats in each group A: Control group; B: Model group; C: si-SEMA3A group; D: si-SEMA3A+DAPT group. Black arrows indicate apoptotic RGCs. TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling; RGC: Retinal ganglion cell; SEMA3A: Semaphorin 3A; DAPT: N-(N-((3, 5-difluorophenyl)acetyl)-L-alanyl-L-2-phenyl)glycine-1, 1-dimethylethyl ester. |
2.4 各组大鼠视网膜组织中IL-6和TNF-α表达水平比较
模型组大鼠视网膜组织中IL-6和TNF-α的表达水平高于对照组和si-SEMA3A组(P均<0.05),si-SEMA3A+DAPT组大鼠视网膜组织中IL-6和TNF-α的表达水平高于si-SEMA3A组(P<0.05)。见图 4。

|
图 4 ELISA法检测各组大鼠视网膜组织中IL-6和TNF-α的表达水平 Fig 4 Expression of IL-6 and TNF-α in rat retinal tissue of each group detected by ELISA *P < 0.05 vs model group; △P < 0.05 vs si-SEMA3A group. n=10, x±s. ELISA: Enzyme-linked immunosorbent assay; IL-6: Interleukin 6; TNF-α: Tumor necrosis factor α; SEMA3A: Semaphorin 3A; DAPT: N-(N-((3, 5-difluorophenyl)acetyl)-L-alanyl-L-2-phenyl)glycine-1, 1-dimethylethyl ester. |
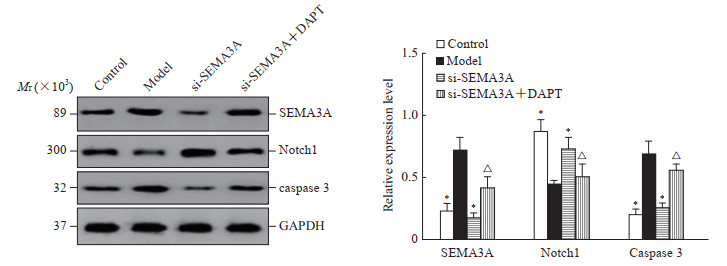
2.5 各组大鼠视网膜组织中SEMA3A、Notch1及caspase
3蛋白表达水平比较与对照组比较,模型组大鼠视网膜组织中SEMA3A和caspase 3的表达水平较高,Notch1表达水平较低(P均<0.05);与模型组比较,si-SEMA3A组大鼠视网膜组织中SEMA3A和caspase 3表达水平较低,Notch1表达水平较高(P均<0.05);而si-SEMA3A+DAPT组大鼠视网膜组织中SEMA3A、caspase 3表达水平高于si-SEMA3A组,Notch1表达水平低于si-SEMA3A组(P均<0.05)。见图 5。
|
图 5 蛋白质印迹法检测各组大鼠视网膜组织中SEMA3A、Notch1、caspase 3蛋白的表达水平 Fig 5 Expression levels of SEMA3A, Notch1, and caspase 3 in rat retinal tissue of each group detected by Western blotting *P < 0.05 vs model group; △P < 0.05 vs si-SEMA3A group. n=10, x±s. SEMA3A: Semaphorin 3A; caspase 3: Cysteine aspartic acid specific protease 3; DAPT: N-(N-((3, 5-difluorophenyl)acetyl)-L-alanyl-L-2-phenyl)glycine-1, 1-dimethylethyl ester; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. |
3 讨论
视网膜的神经元或神经胶质对长期高血糖的影响特别敏感,RGC损伤是DR的发病机制[12]。本研究发现,SEMA3A在糖尿病大鼠视网膜组织中表达水平升高,抑制SEMA3A表达可能通过Notch1信号通路改善糖尿病大鼠RGC凋亡。
SEMA3A是分泌型糖蛋白,可以由上皮细胞、内皮细胞、神经元等分泌[13]。研究表明,SEMA3A在长期高糖引起的微血管病变(包括糖尿病肾病[14]和糖尿病神经病变[15])的发展中起着至关重要的作用,其机制可能与SEMA3A调控突触可塑性、免疫炎症、细胞凋亡和细胞增殖等有关[13, 16]。SEMA3A从个体发育到成年在视网膜中持续表达[17]。视神经损伤后小胶质细胞被激活,促炎M1样小胶质细胞的数量随着视网膜SEMA3A的增加而增加,同时抗炎M2样小胶质细胞的数量减少,而RGC凋亡增加,提示SEMA3A参与小胶质细胞极化M1/M2样动态反应并促进RGC凋亡[13]。
在本研究中,SEMA3A表达于大鼠RGC内,在糖尿病状态下视网膜组织SEMA3A表达水平升高、RGC凋亡率增加且密度降低;沉默SEMA3A基因后RGC凋亡率降低,提示SEMA3A在糖尿病大鼠中介导了RGC的凋亡。ELISA检测结果发现,糖尿病大鼠视网膜组织中IL-6和TNF-α表达水平升高,而沉默SEMA3A基因后这2种炎症因子表达水平降低,说明SEMA3A对于炎症因子的分泌可能有一定的影响。这一发现扩展了目前对SEMA3A和RGC之间相互作用的理解,证明SEMA3A不仅介导RGC凋亡,还与免疫炎症损伤有关。
Notch1信号通路可以调节神经元生长,其表达水平降低与神经元损伤及凋亡有关,而激活Notch1信号通路有助于周围神经元再生[18]。研究发现,Notch1信号通路参与糖尿病RGC凋亡[19]。另外,Notch1信号通路还通过介导免疫炎症损伤而影响细胞凋亡[20]。Notch1信号通路参与的细胞凋亡依赖于caspase蛋白家族[21]。本研究发现,糖尿病模型大鼠视网膜组织中Notch1蛋白表达水平降低,而caspase 3蛋白表达水平升高;沉默SEMA3A基因可下调SEMA3A及caspase 3蛋白表达水平,而Notch1通路抑制剂DAPT可逆转上述变化,说明SEMA3A对RGC凋亡和炎症损伤的影响可能是通过Notch1细胞凋亡信号通路实现的。
综上所述,本研究发现下调SEMA3A可抑制糖尿病模型大鼠的RGC凋亡,其作用机制可能是通过Notch1信号通路实现的。依据本研究结果,我们认为SEMA3A可能成为DR的治疗靶点,但是未来仍需要开展更丰富和全面的细胞及动物研究,进一步探寻SEMA3A的生物学机制,最重要的是开展临床试验检测SEMA3A在外周血和玻璃体中的表达水平并探讨其临床实际意义。
| [1] |
SPRINZAK D, BLACKLOW S C. Biophysics of Notch signaling[J]. Annu Rev Biophys, 2021, 50: 157-189. DOI:10.1146/annurev-biophys-101920-082204 |
| [2] |
WANG W, LO A C Y. Diabetic retinopathy: pathophysiology and treatments[J]. Int J Mol Sci, 2018, 19(6): 1816. DOI:10.3390/ijms19061816 |
| [3] |
QIU A W, HUANG D R, LI B, et al. IL-17A injury to retinal ganglion cells is mediated by retinal Müller cells in diabetic retinopathy[J]. Cell Death Dis, 2021, 12(11): 1057. DOI:10.1038/s41419-021-04350-y |
| [4] |
LIANG Z, ZHANG X, LIU Y, et al. SEMA3A protects against hyperoxia-induced lung injury in a bronchopulmonary dysplasia model of newborn rat by inhibiting ERK pathway[J]. Allergol Immunopathol (Madr), 2021, 49(6): 8-15. DOI:10.15586/aei.v49i6.478 |
| [5] |
YANG L, WANG L, WANG J, et al. Long non-coding RNA Gm11974 aggravates oxygen-glucose deprivation-induced injury via miR-122-5p/SEMA3A axis in ischaemic stroke[J]. Metab Brain Dis, 2021, 36(7): 2059-2069. DOI:10.1007/s11011-021-00792-7 |
| [6] |
EIZA N, GARTY M, STAUN-RAM E, et al. The possible involvement of sema3A and sema4A in the pathogenesis of multiple sclerosis[J]. Clin Immunol, 2022, 238: 109017. DOI:10.1016/j.clim.2022.109017 |
| [7] |
FUJⅡ S, FUJIMOTO T, HASEGAWA K, et al. The Semaphorin 3A-AKT axis-mediated cell proliferation in salivary gland morphogenesis and adenoid cystic carcinoma pathogenesis[J]. Pathol Res Pract, 2022, 236: 153991. DOI:10.1016/j.prp.2022.153991 |
| [8] |
ZIPPEL N, KENNY C H, WU H, et al. Sema3A antibody BI-X prevents cell permeability and cytoskeletal collapse in HRMECs and increases tip cell density in mouse oxygen-induced retinopathy[J]. Transl Vis Sci Technol, 2022, 11(6): 17. DOI:10.1167/tvst.11.6.17 |
| [9] |
杨晓, 张胜利, 赵俊宏, 等. miR-495通过靶向Notch1信号调控高糖诱导的视神经节细胞凋亡[J]. 临床和实验医学杂志, 2019, 18(14): 1486-1489. DOI:10.3969/j.issn.1671-4695.2019.14.009 |
| [10] |
朱晓燕, 刘勤, 白惠玲, 等. 2型糖尿病视网膜病变大鼠模型建立[J]. 实验动物科学, 2022, 39(1): 28-33. DOI:10.3969/j.issn.1006-6179.2022.01.006 |
| [11] |
PARK Y G, LEE J Y, KIM C, et al. Early microglial changes associated with diabetic retinopathy in rats with streptozotocin-induced diabetes[J]. J Diabetes Res, 2021, 2021: 4920937. DOI:10.1155/2021/4920937 |
| [12] |
HU Y, GRODZKI L M, BARTSCH S, et al. Cell-based neuroprotection of retinal ganglion cells in animal models of optic neuropathies[J]. Biology (Basel), 2021, 10(11): 1181. DOI:10.3390/biology10111181 |
| [13] |
LIU Y J, CHEN X, ZHANG J Q, et al. Semaphorin3A increases M1-like microglia and retinal ganglion cell apoptosis after optic nerve injury[J]. Cell Biosci, 2021, 11(1): 97. DOI:10.1186/s13578-021-00603-7 |
| [14] |
FEI B, ZHOU H, HE Z, et al. KCNQ1OT1 inhibition alleviates high glucose-induced podocyte injury by adsorbing miR-23b-3p and regulating Sema3A[J]. Clin Exp Nephrol, 2022, 26(5): 385-397. DOI:10.1007/s10157-021-02173-x |
| [15] |
POP-BUSUI R, ANG L, HOLMES C, et al. Inflammation as a therapeutic target for diabetic neuropathies[J]. Curr Diab Rep, 2016, 16(3): 29. DOI:10.1007/s11892-016-0727-5 |
| [16] |
VADASZ Z, TOUBI E. Semaphorin3A: a potential therapeutic tool in immune-mediated diseases[J]. Eur J Rheumatol, 2018, 5(1): 58-61. DOI:10.5152/eurjrheum.2017.17076 |
| [17] |
GUO Z, LI Y, CHEN M, et al. Semaphorin3A regulates mitochondrial apoptosis in RAW264.7 cells in vitro[J]. Tissue Cell, 2022, 75: 101711. DOI:10.1016/j.tice.2021.101711 |
| [18] |
XU M, BI X Y, XUE X R, et al. Activation of the M3AChR and Notch1/HSF1 signaling pathway by choline alleviates angiotensin Ⅱ-induced cardiomyocyte apoptosis[J]. Oxid Med Cell Longev, 2021, 2021: 9979706. DOI:10.1155/2021/9979706 |
| [19] |
LI H, ZHU Z, LIU J, et al. MicroRNA-137 regulates hypoxia-induced retinal ganglion cell apoptosis through Notch1[J]. Int J Mol Med, 2018, 41(3): 1774-1782. DOI:10.3892/ijmm.2017.3319 |
| [20] |
LUO J, LI L, HU D, et al. LINC00612/miR-31-5p/Notch1 axis regulates apoptosis, inflammation, and oxidative stress in human pulmonary microvascular endothelial cells induced by cigarette smoke extract[J]. Int J Chron Obstruct Pulmon Dis, 2020, 15: 2049-2060. DOI:10.2147/COPD.S255696 |
| [21] |
ZENG Y, YIN B, WANG X, et al. Effects of the Notch1 signaling pathway on human lung cancer A549 cells[J]. Exp Lung Res, 2017, 43(4/5): 208-216. DOI:10.1080/01902148.2017.1341008 |
 2023, Vol. 44
2023, Vol. 44


