2. 上海市宝山区中西医结合医院泌尿外科, 上海 201900;
3. 安徽医科大学公共卫生学院营养与食品卫生学系, 合肥 230032
2. Department of Urology, Shanghai Baoshan Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai 201900, China;
3. Department of Nutrition and Food Hygiene, School of Public Health, Anhui Medical University, Hefei 230032, Anhui, China
前列腺癌是全球高发的恶性肿瘤之一,2020年全球前列腺癌新发病例数超141万例,死亡数超37万例[1]。在我国,前列腺癌新发病例数位列恶性肿瘤第6位,占全球前列腺癌新发病例数的8.2%;死亡病例数位列第7位,占全球前列腺癌死亡病例数的13.6%[2]。我国前列腺癌发病率低于欧美发达国家,但随着人口老龄化、环境污染及生活和饮食习惯改变,前列腺癌发病率在未来仍将继续上升[3-4]。定期筛检可显著提高前列腺癌早诊率,从而通过早期治疗提高患者5年生存率[5-6]。目前我国前列腺癌筛检普及率低且筛检手段效能有限,大量前列腺癌患者在确诊时已是中晚期,从而错失最佳治疗时机,5年生存率<70%[3]。前列腺癌筛查的首要方法为前列腺特异抗原(prostate-specific antigen,PSA)检测,其他检查包括影像学检查、直肠指诊等[7-8]。影像学检查和直肠指诊对早期患者灵敏度较低,漏诊率较高,不推荐单独使用[8-9]。PSA是目前公认最有价值的前列腺癌肿瘤标志物,但其特异度较低,易受前列腺炎症、前列腺增生、尿潴留及前列腺检查等因素的影响,筛检假阳性率高[10]。因此,寻找新的高效能肿瘤标志物极其重要。
miRNA是一类长约18~22 bp的短链非编码RNA,广泛存在于真核细胞中。miRNA具有多种生物学功能,参与细胞增殖、分化、免疫反应等过程,在恶性肿瘤的发生和发展中也起着重要作用[11-13]。miRNA在肿瘤中的特异性表达及其可在血液、尿液等多种体液中检测的特性为其成为肿瘤检测标志物提供了可能[14-15],如miRNA-22、miRNA-122等被证明在原发性肝细胞癌中具有较好的诊断价值[16-17]。针对前列腺癌的研究亦显示miRNA在前列腺癌诊断中具有潜在作用,但目前尚无有效的miRNA标志物得到应用[18-19]。本研究拟通过公共数据库筛选出具有作为前列腺癌血浆检测标志物潜能的miRNA分子,再利用不同人群血浆样本验证其诊断价值。
1 材料和方法 1.1 候选miRNA的筛选利用基因表达汇编(Gene Expression Omnibus,GEO)数据库中GSE118038、GSE112264两个数据集对前列腺癌和非前列腺癌人群血浆及血清miRNA表达水平进行差异性分析,GSE118038数据集的筛选条件为|差异倍数(fold change,FC)|≥2、错误发现率(false discovery rate,FDR)<0.05,GSE112264数据集的筛选条件为|FC|≥1.5、FDR<0.05,寻找两组共有的差异表达miRNA。在GSE112264数据集中使用10 000次Bootstrap,获得两组共有miRNA出现差异表达的可重复概率。选取差异表达可重复概率达100%的miRNA,通过最近邻中心分类器曲线下面积优化模型(nearest centroid classifier for area under curve optimization,NCC-AUC)计算其在GSE112264中的模型权重,选择3个权重最高的miRNA分子进行临床验证。
1.2 临床样本收集选取2019年7月至2021年12月期间在上海市宝山区中西医结合医院就诊的前列腺癌患者45例(前列腺癌组)、慢性前列腺炎患者40例(前列腺炎组),并选取同期健康体检者42例(健康对照组)。前列腺癌纳入标准:(1)经病理活检确诊为前列腺癌,且为初发;(2)未接受前列腺癌相关治疗。排除标准:(1)近3个月内存在泌尿生殖系统感染;(2)有其他恶性肿瘤史或患有自身免疫性疾病、重要脏器功能不全;(3)前列腺癌复发或接受过抗肿瘤治疗。慢性前列腺炎纳入标准:(1)符合慢性前列腺炎诊断标准,且目前处于炎症状态;(2)影像学检查及肛门指诊未发现前列腺占位。健康体检者纳入标准:(1)影像学检查未发现前列腺增生及占位;(2)无泌尿生殖系统炎症;(3)无恶性肿瘤、自身免疫性疾病等疾病史。45例前列腺癌患者年龄为47~83岁,平均(66.58±10.05)岁;40例前列腺炎患者年龄为21~75岁,平均(41.13±12.91)岁;42例健康体检者年龄为26~78岁,平均(49.74±13.30)岁。所有纳入本研究的患者均自愿签署知情同意书,样本收集和临床信息收集符合上海市宝山区中西医结合医院伦理委员会要求。
1.3 血浆候选miRNA表达水平的检测取受试者外周静脉血3~5 mL置于EDTA抗凝管中,立即于4 ℃下以2 500×g离心20 min,分离血浆。取0.5 mL血浆,加入0.7 mL QIAzol试剂(美国Qiagen公司)裂解,严格按照miRNA提取试剂盒(miRNeasy Mini Kit,美国Qiagen公司)说明书提取血浆总miRNA,全程注意避免RNA酶污染,提取后若不立即进行反转录,则冻存于-80 ℃冰箱待测。
按照反转录试剂盒[miRcute Plus microRNA First-Strand cDNA Kit,天根生化科技(北京)有限公司]说明书配制20 μL反转录反应体系,使用Applied Biosystems PCR仪(美国ThermoFisher公司)进行反转录,获得cDNA并冻存于-20 ℃冰箱待测。按照qPCR试剂盒[miRcute Plus microRNA qPCR Kit,天根生化科技(北京)有限公司]说明书配制20 μL反应体系,使用LightCycler 480Ⅱ实时荧光定量PCR系统(瑞士Roche公司)进行qPCR检测并记录数据。反应条件:95 ℃ 15 min;94 ℃ 20 s、65 ℃ 30 s、72 ℃ 34 s,5个循环,富集低丰度miRNA,无需信号记录;94 ℃ 20 s、60 ℃ 34 s,50个循环,在60 ℃时记录荧光信号。以U6作为内参照,计算各候选miRNA的相对定量值(ΔCt值)。各基因上游引物均由生工生物工程(上海)股份有限公司合成,U6正向引物序列为5'-GC-TTCGGCAGCACATATACTAAAAT-3',miRNA-21-5p正向引物序列为5'-AAGAGCGTTAGC-TTATCAGACTG-3',miRNA-5189-5p正向引物序列为5'-AAGAAGATCTGGGCACAGGC-3',miRNA-6780a-5p正向引物序列为5'-AACGGCTTGGGAG-GGAAGA-3'。反向引物均由试剂盒提供。
1.4 统计学处理采用SPSS 21.0软件进行统计学分析,用GraphPad Prism 5.0软件绘图。计量资料不服从正态分布,以中位数(下四分位数,上四分位数)表示,组间比较采用秩和检验。采用ROC曲线评估候选miRNA在前列腺癌中的诊断价值,以约登指数最大值对应的检验变量值作为最佳临界值。采用logistic回归模型建立联合检测诊断模型。检验水准(α)为0.05。
2 结果 2.1 候选miRNA的确定从GSE118038数据集中共获得115个差异表达miRNA(|FC|≥2、FDR<0.05),从GSE112264数据集中共获得703个差异表达miRNA(|FC|≥1.5、FDR<0.05),结果见图 1。两数据集共获得26个共同差异表达miRNA,结果见表 1。对26个差异表达miRNA进行重复性验证,得到9个差异表达可重复概率为100%的miRNA。通过NCC-AUC评估,选择3个权重最高的miRNA(miRNA-21-5p、miRNA-5189-5p、miRNA-6780a-5p)作为候选miRNA进行实验验证。生物信息学分析结果显示,miRNA-21-5p在前列腺癌患者血浆及血清中低表达,miRNA-5189-5p、miRNA-6780a-5p则为高表达。

|
图 1 GSE112264和GSE118038数据集中前列腺癌和非前列腺癌人群差异表达miRNA的火山图 Fig 1 Volcanic maps of differential miRNAs between prostate cancer and non-prostate cancer groups in GSE112264 and GSE118038 datasets A: GSE112264; B: GSE118038. miRNA: MicroRNA; FC: Fold change; FDR: False discovery rate. |
|
|
表 1 GSE112264和GSE118038数据集共有的26个前列腺癌和非前列腺癌人群差异表达miRNA Tab 1 The 26 differential miRNAs between prostate cancer and non-prostate cancer groups in GSE112264 and GSE118038 datasets |
2.2 各组人群血浆候选miRNA表达水平
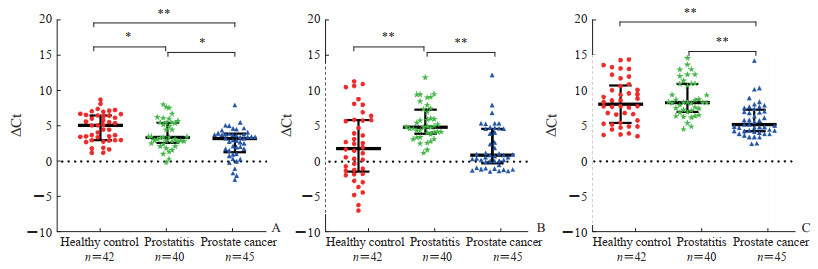
通过qPCR检测3组人群血浆中候选miRNA表达水平,结果见图 2。血浆miRNA-21-5p表达水平在3组间差异有统计学意义(P<0.05),其中前列腺癌组最高、前列腺炎组次之、健康对照组最低,这与生物信息学分析结果相反。血浆miRNA-5189-5p表达水平在前列腺炎组最低,与另外两组差异均有统计学意义(P均<0.01),但前列腺癌组与健康对照组之间差异无统计学意义(P>0.05)。血浆miRNA-6780a-5p表达水平在前列腺癌组最高,与另外两组差异均有统计学意义(P均<0.01),但前列腺炎组与健康对照组之间差异无统计学意义(P>0.05)。由此可见,血浆miRNA-21-5p和miRNA-6780a-5p具备成为前列腺癌诊断标志物的基础条件;而miRNA-5189-5p无法鉴别前列腺癌患者与健康对照者,因此不考虑将其作为前列腺癌诊断的潜在标志物。
|
图 2 各组人群候选血浆miRNA表达水平 Fig 2 Expression levels of candidate plasma miRNAs in each group A: miRNA-21-5p; B: miRNA-5189-5p; C: miRNA-6780a-5p. *P < 0.05, **P < 0.01. miRNA: MicroRNA. |
2.3 血浆miRNA-21-5p和miRNA-6780a-5p在前列腺癌中的诊断价值
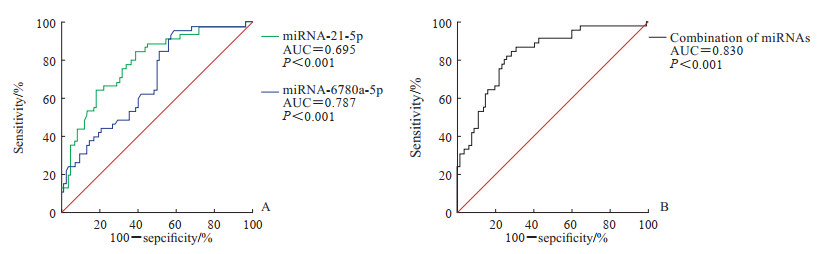
将前列腺炎组与健康对照组合并作为对照组,采用ROC曲线对血浆miRNA-21-5p和miRNA-6780a-5p的诊断效能进行验证。由图 3A可见,miRNA-21-5p单独检测诊断前列腺癌的AUC为0.695(P<0.001),当约登指数取最大值0.370时,其临界值为5.108,灵敏度为95.6%,特异度为41.5%;miRNA-6780a-5p单独检测诊断前列腺癌的AUC为0.787(P<0.001),当约登指数取最大值0.462时,其临界值为6.143,灵敏度为64.4%,特异度为81.7%。对miRNA-21-5p和miRNA-6780a-5p的ΔCt值进行logistic回归模型拟合分析,获得预测概率,模型具有统计学意义(表 2),对原始数据解释度理想。ROC曲线分析显示miRNA-21-5p与miRNA-6780a-5p联合诊断模型诊断前列腺癌的AUC为0.830(P<0.001),约登指数取最大值0.566时,灵敏度为82.2%,特异度为74.4%,具有较好的诊断价值(图 3B)。
|
图 3 miRNA-21-5p和miRNA-6780a-5p单独及联合检测诊断前列腺癌的ROC曲线 Fig 3 ROC curves of miRNA-21-5p and miRNA-6780a-5p alone and in combination for the diagnosis of prostate cancer A: miRNA-21-5p or miRNA-6780a-5p alone; B: Combination of miRNA-21-5p and miRNA-6780a-5p. miRNA: MicroRNA; ROC: Receiver operating characteristic; AUC: Area under curve. |
|
|
表 2 miRNA-21-5p和miRNA-6780a-5p诊断前列腺癌的logistic回归模型拟合分析 Tab 2 Logistic regression analysis of miRNA-21-5p and miRNA-6780a-5p for the diagnosis of prostate cancer |
3 讨论
本研究通过生物信息学分析筛选出3个候选miRNA:miRNA-21-5p、miRNA-5189-5p和miRNA-6780a-5p,随后通过设立前列腺癌、慢性前列腺炎、健康体检者3组人群样本进行实验验证,3个miRNA显示出不同的表达特征。
miRNA-21-5p源自于染色体17q23.2,位于空泡膜蛋白1编码基因内。本研究生物信息学分析结果显示miRNA-21-5p在前列腺癌患者血浆及血清中低表达,而临床标本验证结果显示其在前列腺癌患者血浆中呈高表达。多项研究证明,miRNA-21-5p在肺癌、乳腺癌、结直肠癌等多种恶性肿瘤组织或体液样本中高表达,与肿瘤的发生和发展相关[20-22]。目前,已有部分研究证明miRNA-21-5p在前列腺癌患者组织、血浆、前列腺液中高表达,具有成为肿瘤标志物的潜能[23-24]。因此,在生物信息学分析结果与实验结果矛盾的情况下,上述研究可以佐证实验结果的可靠性。有研究显示,miRNA-21调节NF-κB/核苷酸结合寡聚化结构域样受体蛋白3(NOD-like receptor protein 3,NLRP3)通路,参与炎症过程,是重要的炎症性miRNA[25-26]。本研究中血浆miRNA-21-5p表达水平在慢性前列腺炎患者中高于健康体检者,而在前列腺癌患者中高于慢性前列腺炎患者,提示miRNA-21-5p可能在健康状态-前列腺炎-前列腺癌的疾病演变中起到持续性作用。miRNA-21-5p具有成为鉴别前列腺炎、前列腺癌、健康人群诊断标志物的潜力。
miRNA-5189-5p来自于16号染色体。目前关于miRNA-5189-5p的研究较少,其在机体中的作用尚不明确。本研究结果显示,miRNA-5189-5p在慢性前列腺炎患者血浆中表达下调,而在前列腺癌患者与健康体检者间差异无统计学意义。因此,miRNA-5189-5p不具备成为前列腺癌诊断标志物的潜能,但其在炎症中的作用值得进一步探索。
miRNA-6780a-5p来自于染色体17q21.2。有研究发现,miRNA-6780a-5p参与到Runt相关转录因子3(Runt-related transcription factor 3,RUNX3)/miRNA-6780a-5p/上皮钙黏素(E-cadherin)信号通路中,促进肾癌的转移[27]。另一项研究证明miRNA-6780b-5p参与上皮-间质转化过程,促进卵巢癌转移[28]。本研究结果显示miRNA-6780a-5p在前列腺癌患者血浆中表达上调,应该是与前列腺癌高度相关的分子。但其是否与在肾细胞癌及卵巢癌中一样影响前列腺癌的复发与转移,以及是否深度关联其他泌尿及生殖系统肿瘤需进一步研究验证。
本研究结果显示miRNA-21-5p和miRNA-6780a-5p在前列腺癌患者血浆中的表达水平高于慢性前列腺炎患者和健康体检人群,具备成为前列腺癌诊断标志物的可能性。而miRNA-5189-5p表达水平在前列腺癌与健康体检人群血浆中差异无统计学意义,无法有效区分前列腺癌患者与健康人群,所以在考虑使用miRNA作为诊断前列腺癌的标志物时,miRNA-5189-5p被排除。对miRNA-21-5p、miRNA-6780a-5p诊断前列腺癌的效能进行分析,结果显示两者单独检测均具有较好的筛检能力,而两者联合诊断模型能进一步提高筛检效能,灵敏度和特异度分别达到82.2%和74.4%。考虑到PSA在前列腺癌诊断中较低的特异度,将PSA与miRNA-21-5p、miRNA-6780a-5p联合进行前列腺癌诊断可能更有利于提高检测效能。
本研究存在以下局限性:(1)由于临床多从PSA阳性人群中筛检前列腺癌,导致前列腺癌血液样本的PSA阳性率极高,样本收集存在偏倚,因此未将样本PSA纳入实验考量。(2)对照组未纳入其他可影响前列腺功能及PSA水平的特殊人群,存在一定的局限性。(3)纳入人群临床信息收集不完整,无法对临床诊断指标进行分析。(4)候选miRNA在不同肿瘤人群血浆中的表达水平是否具有差异性暂未探讨。针对以上局限性,可考虑扩大人群范围,增加样本量,全面收集临床资料,分析各miRNA与各种肿瘤分期和分型的关联,筛选出高特异度和高灵敏度的标志物。
综上所述,血浆miRNA-21-5p、miRNA-6780a-5p具有前列腺癌诊断潜能,两者联合使用可有效提高前列腺癌筛检效能。miRNA-5189-5p无法区分健康人群和前列腺癌患者,不适合作为前列腺癌肿瘤标志物,但其在慢性前列腺炎患者中呈高表达,可能在炎症反应中发挥重要作用。
| [1] |
SUNG H, FERLAY J, SIEGEL R L, LAVERSANNE M, SOERJOMATARAM I, JEMAL A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71: 209-249. DOI:10.3322/caac.21660 |
| [2] |
CAO W, CHEN H D, YU Y W, LI N, CHEN W Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020[J]. Chin Med J (Engl), 2021, 134: 783-791. DOI:10.1097/CM9.0000000000001474 |
| [3] |
李星, 曾晓勇. 中国前列腺癌流行病学研究进展[J]. 肿瘤防治研究, 2021, 48: 98-102. |
| [4] |
鲁欣, 蒋栋铭, 胡明, 杨印辉, 张丽娟, 侯建国, 等. 2004—2018年全国前列腺癌死亡率的流行特征及时间趋势[J]. 上海预防医学, 2021, 33: 899-904, 912. DOI:10.19428/j.cnki.sjpm.2021.21084 |
| [5] |
CARLSSON S V, VICKERS A J. Screening for prostate cancer[J]. Med Clin North Am, 2020, 104: 1051-1062. DOI:10.1016/j.mcna.2020.08.007 |
| [6] |
SCHRÖDER F H, HUGOSSON J, ROOBOL M J, TAMMELA T L J, ZAPPA M, NELEN V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up[J]. Lancet, 2014, 384: 2027-2035. DOI:10.1016/S0140-6736(14)60525-0 |
| [7] |
MOTTET N, VAN DEN BERGH R C N, BRIERS E, VAN DEN BROECK T, CUMBERBATCH M G, DE SANTIS M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent[J]. Eur Urol, 2021, 79: 243-262. DOI:10.1016/j.eururo.2020.09.042 |
| [8] |
赫捷, 陈万青, 李霓, 曹巍, 叶定伟, 马建辉, 等. 中国前列腺癌筛查与早诊早治指南(2022, 北京)[J]. 中国肿瘤杂志, 2022, 44: 29-53. |
| [9] |
NAJI L E, RANDHAWA H, SOHANI Z, DENNIS B, LAUTENBACH D, KAVANAGH O, et al. Digital rectal examination for prostate cancer screening in primary care: a systematic review and meta-analysis[J]. Ann Fam Med, 2018, 16: 149-154. DOI:10.1370/afm.2205 |
| [10] |
PASCHEN U, STURTZ S, FLEER D, LAMPERT U, SKOETZ N, DAHM P. Assessment of prostate-specific antigen screening: an evidence-based report by the German Institute for Quality and Efficiency in Health Care[J]. BJU Int, 2022, 129: 280-289. DOI:10.1111/bju.15444 |
| [11] |
HILL M, TRAN N. miRNA interplay: mechanisms and consequences in cancer[J/OL]. Dis Model Mech, 2021, 14: dmm047662. DOI: 10.1242/dmm.047662.
|
| [12] |
ALI SYEDA Z, LANGDEN S S S, MUNKHZUL C, LEE M, SONG S J. Regulatory mechanism of microRNA expression in cancer[J/OL]. Int J Mol Sci, 2020, 21: 1723. DOI: 10.3390/ijms21051723.
|
| [13] |
LEITÃO A L, ENGUITA F J. A structural view of miRNA biogenesis and function[J/OL]. Noncoding RNA, 2022, 8: 10. DOI: 10.3390/ncrna8010010.
|
| [14] |
VALIHRACH L, ANDROVIC P, KUBISTA M. Circulating miRNA analysis for cancer diagnostics and therapy[J/OL]. Mol Aspects Med, 2020, 72: 100825. DOI: 10.1016/j.mam.2019.10.002.
|
| [15] |
HE B X, ZHAO Z Y, CAI Q D, ZHANG Y Q, ZHANG P F, SHI S, et al. miRNA-based biomarkers, therapies, and resistance in cancer[J]. Int J Biol Sci, 2020, 16: 2628-2647. DOI:10.7150/ijbs.47203 |
| [16] |
ZHU Y M, WANG S Q, XI X C, ZHANG M F, LIU X F, TANG W N, et al. Integrative analysis of long extracellular RNAs reveals a detection panel of noncoding RNAs for liver cancer[J]. Theranostics, 2021, 11: 181-193. DOI:10.7150/thno.48206 |
| [17] |
FANG Y J, YAN D, WANG L X, ZHANG J, HE Q F. Circulating microRNAs (miR-16, miR-22, miR-122) expression and early diagnosis of hepatocellular carcinoma[J/OL]. J Clin Lab Anal, 2022, 36: e24541. DOI: 10.1002/jcla.24541.
|
| [18] |
MUNTEANU V C, MUNTEANU R A, ONACIU A, BERINDAN-NEAGOE I, PETRUT B, COMAN I. miRNA-based inspired approach in diagnosis of prostate cancer[J/OL]. Medicina (Kaunas), 2020, 56: 94. DOI: 10.3390/medicina56020094.
|
| [19] |
BHAGIRATH D, YANG T L, TABATABAI Z L, SHAHRYARI V, MAJID S, DAHIYA R, et al. Role of a novel race-related tumor suppressor microRNA located in frequently deleted chromosomal locus 8p21 in prostate cancer progression[J]. Carcinogenesis, 2019, 40: 633-642. DOI:10.1093/carcin/bgz058 |
| [20] |
TANG J M, LI X, CHENG T L, WU J. MiR-21-5p/SMAD7 axis promotes the progress of lung cancer[J]. Thorac Cancer, 2021, 12: 2307-2313. DOI:10.1111/1759-7714.14060 |
| [21] |
LIU M, MO F, SONG X H, HE Y, YUAN Y, YAN J Y, et al. Exosomal hsa-miR-21-5p is a biomarker for breast cancer diagnosis[J/OL]. PeerJ, 2021, 9: e12147. DOI: 10.7717/peerj.12147.
|
| [22] |
LI A, YANG P M. Overexpression of miR-21-5p in colorectal cancer cells promotes self-assembly of E-cadherin-dependent multicellular tumor spheroids[J/OL]. Tissue Cell, 2020, 65: 101365. DOI: 10.1016/j.tice.2020.101365.
|
| [23] |
ABRAMOVIC I, VRHOVEC B, SKARA L, VRTARIC A, NIKOLAC GABAJ N, KULIS T, et al. miR-182-5p and miR-375-3p have higher performance than PSA in discriminating prostate cancer from benign prostate hyperplasia[J/OL]. Cancers (Basel), 2021, 13: 2068. DOI: 10.3390/cancers13092068.
|
| [24] |
DANARTO R, ASTUTI I, UMBAS R, HARYANA S M. Urine miR-21-5p and miR-200c-3p as potential non-invasive biomarkers in patients with prostate cancer[J]. Turk J Urol, 2019, 46: 26-30. |
| [25] |
OLIVIERI F, PRATTICHIZZO F, GIULIANI A, MATACCHIONE G, RIPPO M R, SABBATINELLI J, et al. miR-21 and miR-146a: the microRNAs of inflammaging and age-related diseases[J/OL]. Ageing Res Rev, 2021, 70: 101374. DOI: 10.1016/j.arr.2021.101374.
|
| [26] |
FUJITA S, ITO T, MIZUTANI T, MINOGUCHI S, YAMAMICHI N, SAKURAI K, et al. miR-21 gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism[J]. J Mol Biol, 2008, 378: 492-504. DOI:10.1016/j.jmb.2008.03.015 |
| [27] |
CHEN F F, LIU X, CHENG Q, ZHU S D, BAI J, ZHENG J N. RUNX3 regulates renal cell carcinoma metastasis via targeting miR-6780a-5p/E-cadherin/EMT signaling axis[J]. Oncotarget, 2017, 8: 101042-101056. DOI:10.18632/oncotarget.13205 |
| [28] |
CAI J, GONG L Q, LI G D, GUO J, YI X Q, WANG Z H. Exosomes in ovarian cancer ascites promote epithelial-mesenchymal transition of ovarian cancer cells by delivery of miR-6780b-5p[J/OL]. Cell Death Dis, 2021, 12: 210. DOI: 10.1038/s41419-021-03490-5.
|
 2023, Vol. 44
2023, Vol. 44


