2. 重庆医科大学附属第二医院肝胆外科,重庆 400010;
3. 成都市双流区妇幼保健院新生儿科,成都 610200
2. Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China;
3. Department of Neonatology, Chengdu Shuangliu District Maternal and Child Health Hospital, Chengdu 610200, Sichuan, China
脓毒症是指宿主对感染产生失控反应并出现危及生命的器官功能障碍的临床综合征,是ICU常见的死亡原因,每年患病人数高达1 900万[1]。脓毒症可导致各种类型的器官损伤,特别是肝、脑和心脏损伤,而脓毒症后肝功能障碍是多器官功能障碍和脓毒症致死的独立危险因素[2]。有证据表明,肝功能损伤作为脓毒症的并发症,可直接导致脓毒症患者病情进展甚至死亡[3]。然而针对脓毒症后肝功能损伤目前仍没有较好的治疗策略。
表没食子儿茶素没食子酸酯(epigallocatechin gallate,EGCG)在儿茶素中占比最高(50%~80%),有抗氧化、抗突变和抗感染等特性[4]。研究发现,EGCG能有效抑制内毒素诱导的血清高迁移率族蛋白B1(high mobility group protein B1,HMGB1)的释放和表达,提高败血症模型大鼠的存活率;还可通过抑制外源性HMGB1在巨噬细胞表面的黏附降低HMGB1及其标志物IL-6的聚集,阻断HMGB1介导的炎症反应[5-6]。
HMGB1是一种高度保守且广泛表达的非组蛋白染色质结合蛋白,可与特定染色质DNA结合并参与DNA重组、基因转录调控、细胞分裂和分化,是近年发现的一种重要的脓毒症炎症因子。已有研究证明多种细胞死亡方式如细胞焦亡等参与了宿主的免疫调节[7]。细胞焦亡主要由核苷酸结合寡聚化结构域样受体蛋白3(nucleotide binding oligomerization domain-like receptor protein 3,NLRP3)炎症小体介导,该炎症小体在固有免疫细胞识别抗原后被激活[8],以炎症小体激活和炎症级联为特征,被认为是减少器官和组织损伤的主要防御机制,然而细胞焦亡过度活化会导致炎症失控、细胞死亡和多器官损伤。研究表明在脓毒症中,细胞HMGB1增加可促进细胞焦亡和凋亡,介导器官损伤[9]。EGCG参与脓毒症后急性肝损伤的细胞途径及脓毒症后急性肝损伤的发病机制目前尚不明了。本研究旨在探究EGCG对脓毒症后急性肝损伤是否具有保护作用,以及EGCG的保护作用是否与减弱HMGB1/Toll样受体4(Toll like receptor 4,TLR4)/NF-κB/NLRP3途径降低肝细胞焦亡有关。
1 材料和方法 1.1 动物、细胞与试剂8~10周龄雄性C57BL/6J小鼠88只购自湖南斯莱克景达实验动物有限公司[实验动物生产许可证号为SCXK(湘)2019-0004],饲养于重庆医科大学实验动物中心SPF级动物房[实验动物使用许可证号为SYXK(渝)2018-0003],自由饮食、饮水,实验前先在SPF级动物房适应性饲养小鼠1周,实验全过程遵守《实验动物管理条例》。
人正常肝细胞系L02细胞由重庆医科大学病毒性肝炎研究所提供。
EGCG(纯度≥98%,货号IE0150)购自北京索莱宝科技有限公司,脂多糖(纯度≤100%,货号L4391)购自美国Sigma-Aldrich公司,HMGB1(纯度>95%,货号C357)购自苏州近岸蛋白质科技股份有限公司,Opti-MEMTMⅠ减血清培养基购自美国ThermoFisher Scientific公司,FBS购自澳大利亚Life公司,青霉素-链霉素复合溶液购自武汉赛维尔生物科技有限公司,DMEM购自重庆医科大学病毒性肝炎研究所,caspase 1一抗购自中国台湾Arigo公司,NF-κB p65一抗购自美国Cell Signaling Technology公司,消皮素D(gasdermin D,GSDMD)、caspase 11、IL-18、IL-1β、HMGB1和TLR4一抗均购自英国Abcam公司,NLRP3、β肌动蛋白、组蛋白H3一抗和山羊抗兔二抗均购自武汉博士德生物工程有限公司,免疫组织化学染色酶标二抗购自北京中杉金桥生物技术有限公司,BCA蛋白定量试剂盒、RIPA裂解液和4%多聚甲醛溶液均购自上海碧云天生物技术有限公司,细胞核蛋白质提取试剂盒购自江苏凯基生物技术股份有限公司,TNF-α和IL-6 ELISA检测试剂盒均购自北京四正柏生物科技有限公司,HMGB1 ELISA检测试剂盒购自生工生物工程(上海)股份有限公司。
1.2 动物模型的建立与分组小鼠随机分为盲肠结扎穿刺术(cecal ligation and puncture,CLP)组、CLP+EGCG低剂量(4 mg/kg)组、CLP+EGCG高剂量(8 mg/kg)组和假手术组。CLP手术过程:小鼠禁食、不禁水12 h后,在无菌条件下予腹腔注射异氟烷(2%~3%)麻醉。备皮、碘伏消毒,沿腹白线中段剪开一长约0.8 cm的纵行切口,分离皮肤和肌层,探查腹腔并分离盲肠(注意盲肠主要位于小鼠左下腹,避免伤及盲肠及血管)。使用镊子轻轻挤压肠内容物使盲肠远端充盈,使用3-0手术丝线结扎盲肠远端,然后使用21 G针头在结扎处远端避开血管穿刺盲肠1次,造成肠瘘,小心挤出约1 mm的粪便,最后将盲肠回纳,关腹。以假手术组作为对照,假手术组小鼠不予盲肠远端结扎与穿刺,其他手术操作与CLP相同。CLP组与假手术组小鼠术后予37 ℃无菌生理盐水(5 mL/100 g)皮下注射1次,CLP+EGCG低剂量组和CLP+EGCG高剂量组小鼠术后分别予37 ℃含80和160 mg/L EGCG的生理盐水(5 mL/100 g)皮下注射1次,随后使小鼠俯卧于保温垫上复苏。待苏醒后放回SPF级动物房[室温(22±2)℃,相对湿度40%~70%,12 h昼夜交替]继续饲养,饮食、饮水同术前。观察并记录小鼠术后24 h一般情况,然后经腹腔注射异氟烷(2%~3%)麻醉后经心脏采血并处死小鼠,收集全血、分离肝组织进行实验。
1.3 细胞培养与处理L02细胞用含有10% FBS和1%抗生素(青霉素和链霉素)的DMEM,在37 ℃、5% CO2的培养箱中培养。取对数生长期细胞用0.25%胰酶消化后重悬,调整细胞密度并以2.0×106个/孔接种于6孔板,待细胞贴壁后更换培养基为Opti-MEMTMⅠ减血清培养基,继续培养4 h后分为4组:脂多糖组(用400 ng/mL脂多糖刺激细胞24 h)、脂多糖+HMGB1组(用400 ng/mL脂多糖刺激细胞10 h后,再加入100 ng/mL HMGB1刺激14 h)、脂多糖+EGCG组(用100 μg/mL EGCG预处理细胞2 h,然后用400 ng/mL脂多糖刺激24 h)、对照组(加入无菌PBS后培养24 h),每组设8个复孔。
1.4 小鼠血常规与肝功能检测术后24 h使用EDTA抗凝管收集小鼠全血,常温下1 h内送往重庆医科大学宠物医院进行血常规检测。使用一次性凝结真空管收集小鼠血液后,先室温静置30 min,再经4 ℃ 1 000×g离心10 min收集血清,送往重庆医科大学附属第二医院,采用全自动生化分析仪检测小鼠血清丙氨酸转氨酶(alanine aminotransferase,ALT)和天冬氨酸转氨酶(aspartate aminotransferase,AST)水平。
1.5 ELISA检测小鼠血清与L02细胞上清液中炎症因子HMGB1、IL-6和TNF-α水平术后24 h,使用一次性凝结真空管收集小鼠全血,室温下静置30 min后,4 ℃ 1 000×g离心10 min收集血清。收集各组L02细胞培养上清液。严格按照ELISA检测试剂盒说明书进行操作,检测小鼠血清与细胞培养上清液中HMGB1、IL-6和TNF-α水平。
1.6 H-E染色观察小鼠肝组织学变化术后24 h,分离小鼠肝组织,使用PBS洗去血液,用滤纸擦干多余水分,剪下一块肝组织放入4%多聚甲醛溶液中固定24 h,然后经脱水、石蜡包埋处理后制作石蜡切片。肝组织石蜡切片经脱蜡、水化处理后进行H-E染色,于显微镜下观察肝组织学变化。其余肝组织放入液氮中保存备用。
1.7 蛋白质印迹法检测小鼠肝组织与L02细胞中HMGB1、TLR4、NF-κB p65、NLRP3和焦亡相关蛋白(GSDMD、caspase 1、caspase 11、IL-1β、IL-18)的表达每10 mg肝组织加入约100 μL RIPA裂解液进行匀浆,然后置于冰上充分裂解并收集总蛋白质。各组细胞弃培养基后用PBS洗涤,加入RIPA裂解液收集细胞总蛋白质。根据细胞核蛋白质提取试剂盒说明书提取细胞核蛋白质,使用BCA蛋白定量试剂盒测定蛋白质浓度。取适量蛋白质进行SDS-PAGE,电转移至PVDF膜,使用5%牛血清白蛋白溶液封闭;分别加入HMGB1一抗(稀释比例为1∶10 000)及TLR4、NF-κB p65、NLRP3、GSDMD、caspase 1、caspase 11、IL-18、IL-1β一抗(稀释比例均为1∶1 000)4 ℃孵育过夜,用TBST洗去膜表面未结合的抗体;加入山羊抗兔二抗(稀释比例为1∶10 000)室温孵育2 h,用TBST洗膜;均匀滴加ECL显影剂,采用化学发光凝胶成像仪(美国Bio-Rad公司)显影。
1.8 免疫组织化学染色检测小鼠肝组织中HMGB1与GSDMD表达与定位小鼠肝组织石蜡切片经脱蜡、水化,采用微波修复法进行抗原修复并冷却至室温后,使用PBS洗片。擦干组织周围多余液体,滴加适量的内源性过氧化物酶阻断剂室温孵育15 min,用PBS洗片;滴加适量山羊血清工作液室温封闭10 min,倾去血清,勿洗;滴加适量HMGB1、GSDMD一抗(稀释比例均为1∶50)4 ℃孵育过夜,用PBS洗片;滴加适量的生物素标记山羊抗兔IgG聚合物室温孵育15 min,用PBS洗片;滴加适量HRP标记链霉卵白素工作液室温孵育15 min后洗片;滴加适量DAB显色液(现配)室温显色3 min,用自来水冲洗;最后滴加适量苏木精染色液室温染色约30 s。经冲洗、返蓝、脱水、透明处理后,使用中性树脂封片,于显微镜下观察HMGB1与GSDMD的表达和定位情况。
1.9 统计学处理48只小鼠(每组12只)用于分析4组小鼠术后生存率,然后根据各组小鼠生存情况扩充实验小鼠数量,并为保证各项实验样本量充足及检验指标分析结果的均一性,最终确定各组统计样本量n=6,因此CLP组小鼠40只、CLP+EGCG低剂量组20只、CLP+EGCG高剂量组16只、假手术组12只。应用GraphPad Prism 8.2.1软件进行数据处理。计量资料以x±s表示,多组间比较采用单因素方差分析,多重比较采用Bonferroni校正的t检验。检验水准(α)为0.05。
2 结果 2.1 各组小鼠术后一般情况术后24 h,CLP组小鼠死亡8只,CLP+EGCG低剂量组死亡5只,CLP+EGCG高剂量组死亡3只,假手术组小鼠全部存活。CLP组小鼠术后精神萎靡,喜聚集,背部毛发脏乱,眼睑部出现白色脓性分泌物,大便溏稀;死亡小鼠腹部膨隆,解剖后可见腹腔充满黄色渗出液,肠道粘连,盲肠远端呈白色坏死。CLP+EGCG低剂量组与CLP+EGCG高剂量组存活小鼠毛发脏乱,精神尚可,大便附着于肛周。假手术组小鼠术后苏醒即基本恢复正常状态。
2.2 各组小鼠术后血常规与肝功能术后24 h血常规和肝功能检测结果显示,CLP组白细胞计数、淋巴细胞计数、中性粒细胞计数、单核细胞计数均低于假手术组(P均<0.05),血清ALT、AST水平均高于假手术组(P均<0.01),提示脓毒症后急性肝损伤模型小鼠构建成功。CLP+EGCG低剂量组、CLP+EGCG高剂量组小鼠白细胞计数、淋巴细胞计数、中性粒细胞计数、单核细胞计数等血常规指标与CLP组相比差异均无统计学意义(P均>0.05),但血清ALT、AST水平均低于CLP组(P均<0.01),且CLP+EGCG高剂量组血清ALT、AST水平低于CLP+EGCG低剂量组(P均<0.05),表明EGCG能减轻小鼠脓毒症后急性肝损伤。见表 1。
|
|
表 1 各组小鼠术后24 h血常规与肝功能指标 Tab 1 Routine blood test and liver function of mice 24 h after operation in each group |
2.3 各组小鼠血清炎症因子HMGB1、TNF-α和IL-6表达
术后24 h ELISA检测结果显示,CLP组、CLP+EGCG低剂量组及CLP+EGCG高剂量组小鼠血清中HMGB1表达量分别为(889.77±60.25)、(686.82±74.53)、(479.30±50.43)pg/mL,TNF-α表达量分别为(647.75±55.68)、(377.80±42.25)、(166.74±19.88)pg/mL,IL-6表达量分别为(1 706.37±95.81)、(1 175.61±116.12)、(791.55±85.01)pg/mL,均高于假手术组[HMGB1、TNF-α、IL-6表达量分别为(46.26±4.34)pg/mL、(45.90±0.38)pg/mL、(13.12±1.66)pg/mL],差异均有统计学意义(P均<0.01)。CLP+EGCG低剂量组和CLP+EGCG高剂量组血清HMGB1、TNF-α和IL-6的表达量均低于CLP组(P均<0.05),且CLP+EGCG高剂量组血清中HMGB1、TNF-α和IL-6的表达量均较CLP+EGCG低剂量组更低(P均<0.05)。以上结果提示EGCG对脓毒症后急性肝损伤模型小鼠有一定的抗炎作用。
2.4 各组小鼠肝组织学变化术后24 h肝组织H-E染色(图 1)显示,假手术组小鼠肝细胞形态、结构正常,中央静脉结构完整,肝小叶结构清楚,无异常表现;CLP组小鼠肝组织病理切片可见肝细胞肿胀,呈现多发灶状坏死,部分区域可见中央静脉周围多个局灶坏死融合成片,考虑脓毒症引起的炎性坏死;CLP+EGCG低剂量组及CLP+EGCG高剂量组小鼠肝组织损伤较CLP组明显减轻。

|
图 1 各组小鼠肝组织苏木精-伊红染色结果 Fig 1 Hematoxylin-eosin staining results of mouse liver tissues in each group The intervention concentrations of low-and high-dose EGCG were 4 and 8 mg/kg, respectively. CLP: Cecal ligation and puncture; EGCG: Epigallocatechin gallate. |
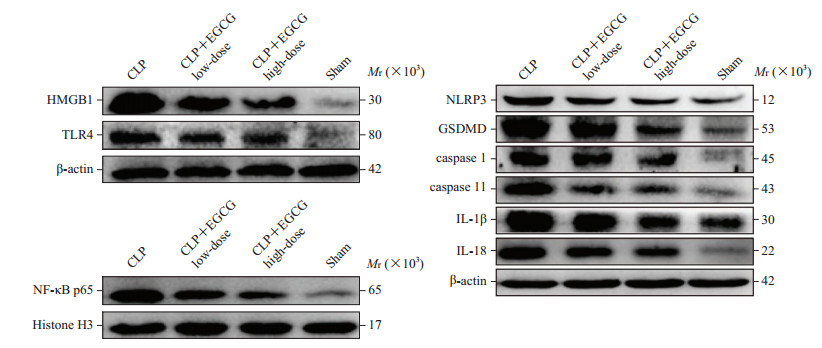
2.5 各组小鼠术后肝组织中HMGB1、TLR4、NF-κB p65、NLRP3及焦亡相关蛋白的表达情况
蛋白质印迹法检测结果(图 2、表 2)显示,术后24 h,CLP组、CLP+EGCG低剂量组及CLP+EGCG高剂量组小鼠肝组织细胞核中NF-κB p65的表达量及肝组织中HMGB1、TLR4、NLRP3、GSDMD、caspase 1、caspase 11、IL-1β和IL-18的表达量均高于假手术组(P均<0.01);CLP+EGCG低剂量组和CLP+EGCG高剂量组上述蛋白质的表达量均低于CLP组(P均<0.05),且CLP+EGCG高剂量组中上述蛋白质的表达量均较CLP+EGCG低剂量组更低(P均<0.05)。以上结果提示EGCG对脓毒症后急性肝损伤的保护作用可能与通过减弱HMGB1/TLR4/NF-κB/NLRP3途径降低肝细胞焦亡有关。
|
图 2 蛋白质印迹法检测小鼠肝组织中HMGB1、TLR4、NF-κB p65、NLRP3及焦亡相关蛋白的表达 Fig 2 Expression of HMGB1, TLR4, NF-κB p65, NLRP3 and pyroptosis-related proteins of mouse liver tissues detected by Western blotting The intervention concentrations of low-and high-dose EGCG were 4 and 8 mg/kg, respectively. HMGB1: High mobility group protein B1; TLR4: Toll-like receptor 4; NF-κB p65: Nuclear factor κB p65; NLRP3: Nucleotide binding oligomerization domain-like receptor protein 3; CLP: Cecal ligation and puncture; EGCG: Epigallocatechin gallate; GSDMD: Gasdermin D; caspase: Cysteine aspartic acid specific protease; IL: Interleukin. |
|
|
表 2 各组小鼠肝组织中HMGB1、TLR4、NF-κB p65、NLRP3及焦亡相关蛋白的表达情况 Tab 2 Expression of HMGB1, TLR4, NF-κB p65, NLRP3 and pyroptosis-related proteins of mouse liver tissues in each group |
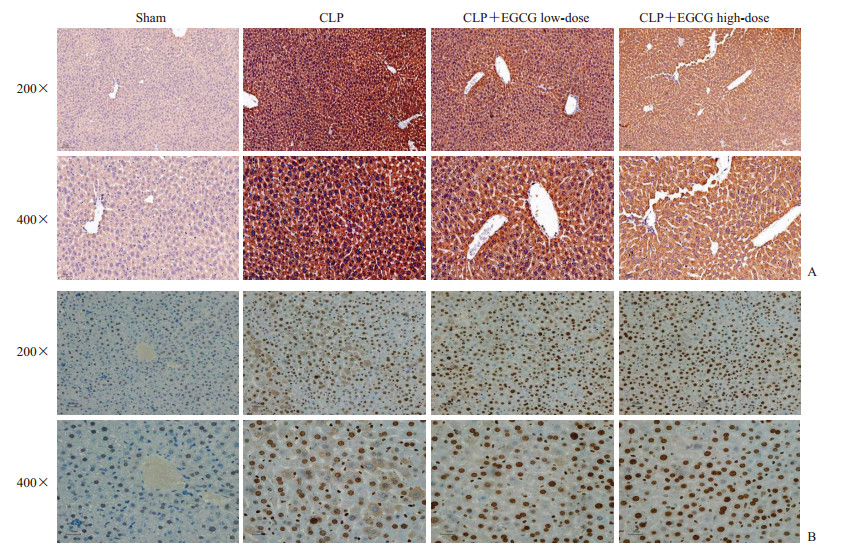
2.6 各组小鼠肝组织中HMGB1、GSDMD表达与定位情况
术后24 h免疫组织化学染色检测结果(图 3)显示,GSDMD主要表达于肝细胞的细胞质内,而HMGB1在细胞核和细胞质均有表达;CLP组、CLP+EGCG低剂量组及CLP+EGCG高剂量组小鼠肝组织中GSDMD的表达量分别为(18.21±1.71)%、(11.14±1.27)%、(6.81±1.31)%,HMGB1的表达量分别为(25.54±1.02)%、(16.69±1.13)%、(13.62±0.76)%,均高于假手术组[(0.14±0.02)%、(0.16±0.07)%,P均<0.05],且CLP+EGCG高剂量组肝组织中GSDMD和HMGB1的表达量均较CLP+EGCG低剂量组更低(P均<0.05)。
|
图 3 各组小鼠肝组织免疫组织化学染色结果 Fig 3 Immunohistochemical staining results of mouse liver tissues in each group A: The expression of GSDMD in liver tissues; B: The expression of HMGB1 in liver tissues. The intervention concentrations of low-and high-dose EGCG were 4 and 8 mg/kg, respectively. CLP: Cecal ligation and puncture; EGCG: Epigallocatechin gallate; GSDMD: Gasdermin D; HMGB1: High mobility group protein B1. |
2.7 L02细胞培养上清液中炎症因子HMGB1、TNF-α、IL-6的表达情况
ELISA检测结果显示,脂多糖组、脂多糖+HMGB1组及脂多糖+EGCG组L02细胞培养上清液中HMGB1的表达量分别为(874.44±96.84)、(1 858.15±67.94)、(540.63±121.78)pg/mL,TNF-α的表达量分别为(233.30±24.95)、(327.24±27.84)、(170.93±15.75)pg/mL,IL-6的表达量分别为(285.76±29.97)、(413.03±21.37)、(170.68±21.87)pg/mL,均高于对照组[HMGB1、TNF-α、IL-6表达量分别为(21.92±1.64)pg/mL、(8.96±1.41)pg/mL、(39.12±4.82)pg/mL,P均<0.01];脂多糖+HMGB1组细胞培养上清液中上述炎症因子的表达水平均高于脂多糖组(P均<0.05),而脂多糖+EGCG组细胞培养上清液中上述炎症因子的表达水平较脂多糖组和脂多糖+HMGB1组下降(P均<0.05)。以上结果提示EGCG有抗炎作用。
2.8 L02细胞中HMGB1、TLR4、NF-κB p65、NLRP3及焦亡相关蛋白的表达情况蛋白质印迹法检测结果(图 4、表 3)显示,脂多糖组、脂多糖+HMGB1组及脂多糖+EGCG组L02细胞核中NF-κB p65的表达量及细胞中HMGB1、TLR4、NLRP3、GSDMD、caspase 1、caspase 11、IL-1β、IL-18的表达量均较对照组增加(P均<0.05),脂多糖+HMGB1组上述蛋白质的表达量较脂多糖组增加(P均<0.05),而脂多糖+EGCG组上述蛋白质的表达量较脂多糖组、脂多糖+HMGB1组下降(P均<0.05),提示EGCG可能通过增强HMGB1/TLR4/NF-κB/NLRP3途径促进肝细胞焦亡。

|
图 4 蛋白质印迹法检测L02细胞中HMGB1、TLR4、NF-κB p65、NLRP3及焦亡相关蛋白的表达 Fig 4 Expression of HMGB1, TLR4, NF-κB p65, NLRP3 and pyroptosis-related proteins in L02 cells detected by Western blotting HMGB1: High mobility group protein B1; TLR4: Toll-like receptor 4; NF-κB p65: Nuclear factor κB p65; NLRP3: Nucleotide binding oligomerization domain-like receptor protein 3; LPS: Lipopolysaccharide; EGCG: Epigallocatechin gallate; GSDMD: Gasdermin D; caspase: Cysteine aspartic acid specific protease; IL: Interleukin. |
|
|
表 3 各组L02细胞中HMGB1、TLR4、NF-κB p65、NLRP3及焦亡相关蛋白的表达情况 Tab 3 Expression of HMGB1, TLR4, NF-κB p65, NLRP3 and pyroptosis-related proteins in L02 cells of each group |
3 讨论
脓毒症可引起多器官功能障碍,肝脏作为一个重要的免疫器官,容易在脓毒症中发生损伤。脓毒症后急性肝损伤与脓毒症患者的严重程度和预后密切相关[3]。CLP模型被认为是脓毒症研究的首选模型,其通过模拟人类阑尾穿孔使肠内容物漏入腹腔形成混合菌群感染,导致革兰阴性菌相关的多菌性脓毒症,该模型具有坏死组织炎症来源明确、重复性好、与人类脓毒症的进展过程相似等特点[9-11]。由于缺乏通用于人类患者的生理监测机制,脓毒症暂无评定标准,常通过实验组的死亡率来评估脓毒症的严重程度并调整需要的动物数量。有证据表明血清IL-6可能是C57/BL6及BALB/c小鼠脓毒症严重程度和死亡的预测指标[10]。本研究术后24 h CLP组小鼠的死亡率及血常规、AST、ALT、IL-6水平的检测结果证实脓毒症后急性肝损伤模型构建成功。
EGCG具有强大的生物活性及抗氧化和抗炎效果,可显著抑制促炎因子TNF-α、IL-1β、IL-6的表达,本实验对细胞培养上清液中HMGB1、TNF-α及IL-6的检测结果也证实EGCG的抗炎作用显著。研究表明EGCG对药物性肝损伤和脂多糖诱导的急性肝损伤均有较好的保护作用[12-13],然而有关EGCG对脓毒症后急性肝损伤是否有保护作用及可能机制的研究较少。本研究中组织病理学及血清HMGB1、TNF-α、IL-6、ALT、AST检测结果表明,EGCG可减轻脓毒症所致的急性肝损伤,对脓毒症后急性肝损伤有保护作用。
研究证实细胞焦亡与脓毒症后急性肝损伤密切相关,抑制肝细胞焦亡可影响CLP诱导的脓毒症后急性肝损伤的严重程度[14]。细胞焦亡是由多种病理因素触发并由caspase家族蛋白介导的炎症性细胞死亡,其特点是细胞膜快速破裂及细胞内促炎物质释放[15-16]。GSDMD是细胞焦亡过程中的主要执行蛋白,主要表达于细胞质,是caspase 1/4/5/11的底物[17-18]。病原体入侵宿主时,NLRP3炎症小体可直接募集caspase 1前体,激活的caspase 1特异性裂解GSDMD,而活化的GSDMD可与细胞膜特异性结合发挥“打孔”作用,导致IL-1β、IL-18等炎症因子释放。caspase 11可直接识别细菌脂多糖并与之结合,而结合了脂多糖的caspase 11可发生水解并直接裂解GSDMD,引发细胞焦亡[7, 19]。本实验结果显示,CLP组小鼠肝组织中焦亡相关蛋白的表达量升高,而在EGCG干预后焦亡相关蛋白的表达量降低,提示细胞焦亡与脓毒症后急性肝损伤的发生密切相关。
HMGB1在细胞核中参与核转录、重组、DNA复制和修复,而在细胞质或细胞外可导致广泛的炎症反应和多器官功能障碍;HMGB1还可与TLR4结合,激活核转录因子NF-κB,进一步增强炎症反应[20]。NF-κB是免疫反应和细胞增殖转化的重要调控转录因子,它控制着细胞因子的产生和细胞的存活,还在固有免疫反应的启动和随后促炎介质的产生中发挥着核心作用,而促炎介质的产生与释放会加重器官损伤[8]。研究发现脓毒症常伴有NF-κB的激活上调,抑制NF-κB激活可减轻脓毒症实验动物的炎症反应,提高其存活率[21]。NF-κB是激活NLRP3炎症小体和焦亡的关键因子,调节NLRP3炎症小体的激活可影响小鼠的细胞焦亡水平,例如在非小细胞肺癌中,重楼皂苷Ⅵ(polyphyllin Ⅵ)通过活性氧/NF-κB/NLRP3/GSDMD信号通路诱导caspase 1介导的细胞焦亡[22]。辅酶Q10联合七叶皂苷可通过HMGB1/TLR4/NF-κB p65/NLRP3信号通路抑制NLRP3炎症小体的激活和细胞焦亡,预防脓毒症诱导的急性肺损伤[23]。本实验结果显示,使用不同质量浓度的EGCG调低HMGB1水平后,小鼠肝组织中TLR4、NF-κB p65、NLRP3及焦亡相关蛋白的表达水平均较CLP组降低,表明EGCG对脓毒症后急性肝损伤的保护作用可能与通过减弱HMGB1/TLR4/NF-κB/NLRP3途径降低肝细胞焦亡有关。为了进一步证明这一结论,本实验进行了体外实验,利用脂多糖诱导L02细胞建立脓毒症后急性肝损伤细胞模型,实验结果显示增加HMGB1水平后细胞中TLR4、NF-κB p65、NLRP3及焦亡相关蛋白的表达量均增加。
综上所述,EGCG对脓毒症后急性肝损伤有保护作用,其作用机制可能与通过减弱HMGB1/TLR4/NF-κB/NLRP3途径减轻肝细胞焦亡有关。这一结论或许可为将来探索脓毒症后急性肝损伤的治疗方法提供新的方向。
| [1] |
DU X J, WU M, TIAN D, ZHOU J L, WANG L, ZHAN L Y. MicroRNA-21 contributes to acute liver injury in LPS-induced sepsis mice by inhibiting PPARα expression[J/OL]. PPAR Res, 2020, 2020: 6633022. DOI: 10.1155/2020/6633022.
|
| [2] |
SU H L, MA Z S, GUO A X, WU H, YANG X M. Salvianolic acid B protects against sepsis-induced liver injury via activation of SIRT1/PGC-1α signaling[J/OL]. Exp Ther Med, 2020, 20: 2675-2683. DOI: 10.3892/etm.2020.9020.
|
| [3] |
YAN J, LI S, LI S L. The role of the liver in sepsis[J]. Int Rev Immunol, 2014, 33: 498-510. DOI:10.3109/08830185.2014.889129 |
| [4] |
SECRETAN P H, THIRION O, SADOU YAYÉ H, DAMY T, ASTIER A, PAUL M, et al. Simple approach to enhance green tea epigallocatechin gallate stability in aqueous solutions and bioavailability: experimental and theoretical characterizations[J/OL]. Pharmaceuticals (Basel), 2021, 14: 1242. DOI: 10.3390/ph14121242.
|
| [5] |
LI W, ASHOK M, LI J H, YANG H, SAMA A E, WANG H C. A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1[J/OL]. PLoS One, 2007, 2: e1153. DOI: 10.1371/journal.pone.0001153.
|
| [6] |
SONG X, DU J, ZHAO W, GUO Z. Epigallocatechin-3-gallate (EGCG): mechanisms and the combined applications[J]. Comb Chem High Throughput Screen, 2017, 20: 872-885. |
| [7] |
RODRÍGUEZ-ANTONIO I, LÓPEZ-SÁNCHEZ G N, URIBE M, CHÁVEZ-TAPIA N C, NUÑO-LÁMBARRI N. Role of the inflammasome, gasdermin D, and pyroptosis in non-alcoholic fatty liver disease[J]. J Gastroenterol Hepatol, 2021, 36: 2720-2727. DOI:10.1111/jgh.15561 |
| [8] |
ABOYOUSSEF A M, MOHAMMAD M K, ABO-SAIF A A, MESSIHA B A S. Granisetron attenuates liver injury and inflammation in a rat model of cecal ligation and puncture-induced sepsis[J]. J Pharmacol Sci, 2021, 147: 358-366. DOI:10.1016/j.jphs.2021.08.005 |
| [9] |
SONG Y, WU Q, JIANG H J, HU A H, XU L Q, TAN C P, et al. The effect of shionone on sepsis-induced acute lung injury by the ECM1/STAT5/NF-κB pathway[J/OL]. Front Pharmacol, 2021, 12: 764247. DOI: 10.3389/fphar.2021.764247.
|
| [10] |
BURAS J A, HOLZMANN B, SITKOVSKY M. Animal models of sepsis: setting the stage[J]. Nat Rev Drug Discov, 2005, 4: 854-865. DOI:10.1038/nrd1854 |
| [11] |
ZHANG Y L, CHEN L W, LUO Y N, WANG K, LIU X Y, XIAO Z, et al. Pink1/parkin-mediated mitophagy regulated the apoptosis of dendritic cells in sepsis[J]. Inflammation, 2022, 45: 1374-1387. DOI:10.1007/s10753-022-01628-x |
| [12] |
YU S J, JIANG R, MAZZU Y Z, WEI C B, SUN Z L, ZHANG Y Z, et al. Epigallocatechin-3-gallate prevents triptolide-induced hepatic injury by restoring the Th17/Treg balance in mice[J]. Am J Chin Med, 2016, 44: 1221-1236. DOI:10.1142/S0192415X16500683 |
| [13] |
MA Y, LIU G, TANG M Y, FANG J, JIANG H M. Epigallocatechin gallate can protect mice from acute stress induced by LPS while stabilizing gut microbes and serum metabolites levels[J/OL]. Front Immunol, 2021, 12: 640305. DOI: 10.3389/fimmu.2021.640305.
|
| [14] |
CHEN Y L, XU G, LIANG X, WEI J, LUO J, CHEN G N, et al. Inhibition of hepatic cells pyroptosis attenuates CLP-induced acute liver injury[J]. Am J Transl Res, 2016, 8: 5685-5695. |
| [15] |
谢天裕, 刘云, 廖世杰, 罗晓婷, 冯文宇, 韦昌武, 等. 细胞焦亡与恶性肿瘤治疗的研究进展[J]. 南昌大学学报(医学版), 2020, 60: 94-98. |
| [16] |
石建霞, 刘奇, 彭永德. 细胞焦亡在代谢性疾病中的研究进展[J]. 第二军医大学学报, 2020, 41: 1250-1254. SHI J X, LIU Q, PENG Y D. Pyroptosis in metabolic diseases: recent progress[J]. Acad J Sec Mil Med Univ, 2020, 41: 1250-1254. |
| [17] |
LIU Z H, WANG C P, YANG J, ZHOU B W, YANG R, RAMACHANDRAN R, et al. Crystal structures of the full-length murine and human gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization[J/OL]. Immunity, 2019, 51: 43-49. e4. DOI: 10.1016/j.immuni.2019.04.017.
|
| [18] |
HUANG X L, FENG Y, XIONG G Q, WHYTE S, DUAN J, YANG Y C, et al. Caspase-11, a specific sensor for intracellular lipopolysaccharide recognition, mediates the non-canonical inflammatory pathway of pyroptosis[J/OL]. Cell Biosci, 2019, 9: 31. DOI: 10.1186/s13578-019-0292-0.
|
| [19] |
WANG X Y, SHI J, LI Z Z, LI L, ZHANG R, BAI Y, et al. An 8-hydroxy-quinoline derivative protects against lipopolysaccharide-induced lethality in endotoxemia by inhibiting HMGB1-mediated caspase-11 signaling[J/OL]. Front Pharmacol, 2021, 12: 673818. DOI: 10.3389/fphar.2021.673818.
|
| [20] |
LI Q, XU M P, LI Z Q, LI T T, WANG Y L, CHEN Q, et al. Maslinic acid attenuates ischemia/reperfusion injury-induced myocardial inflammation and apoptosis by regulating HMGB1-TLR4 axis[J/OL]. Front Cardiovasc Med, 2021, 8: 768947. DOI: 10.3389/fcvm.2021.768947.
|
| [21] |
LIU F J, GU T J, WEI D Y. Emodin alleviates sepsis-mediated lung injury via inhibition and reduction of NF-κB and HMGB1 pathways mediated by SIRT1[J]. Kaohsiung J Med Sci, 2022, 38: 253-260. DOI:10.1002/kjm2.12476 |
| [22] |
TENG J F, MEI Q B, ZHOU X G, TANG Y, XIONG R, QIU W Q, et al. Polyphyllin Ⅵ induces caspase-1-mediated pyroptosis via the induction of ROS/NF-κB/NLRP3/GSDMD signal axis in non-small cell lung cancer[J/OL]. Cancers, 2020, 12: 193. DOI: 10.3390/cancers12010193.
|
| [23] |
ALI F E M, AHMED S F, ELTRAWY A H, YOUSEF R S, ALI H S, MAHMOUD A R, et al. Pretreatment with coenzyme Q10 combined with aescin protects against sepsis-induced acute lung injury[J]. Cells Tissues Organs, 2021, 210: 195-217. DOI:10.1159/000516192 |
 2022, Vol. 43
2022, Vol. 43


