2. 同济大学附属第十人民医院眼科,上海 200072
2. Department of Ophthalmology, Tenth People's Hospital of Tongji University, Shanghai 200072, China
外层渗出性视网膜病变由Coats首次发现并于1908年报道[1],故又名Coats病。其特征性表现为局限性黄色渗出及邻近视网膜血管异常,包括毛细血管扩张、迂曲,动脉瘤样扩张,毛细血管丢失及偶有新生血管[2-3]。该病好发于男性儿童,且多为单眼发病[4]。Shields等[5]详细分析了150例外层渗出性视网膜病变的临床特征并对该病进行分期:1期仅有视网膜毛细血管扩张(约占1%);2期为毛细血管扩张和渗出(约占14%),其中2A期为黄斑中心凹外渗出(约占8%),2B期为渗出累及黄斑中心凹(约占6%);3期为渗出性视网膜脱离(约占68%),其中3A期为视网膜次全脱离(约占38%,3A1期为黄斑中心凹外脱离、3A2期为黄斑中心凹区脱离,各占19%),3B期为视网膜全脱离(约占30%);4期为视网膜全脱离和青光眼(约占15%);5期为严重的终末期病变(约占2%)。外层渗出性视网膜病变尤其是2B期以上患者的治疗非常棘手[6]。1~2期外层渗出性视网膜病变的治疗方法包括视网膜激光光凝术(原理为凝固缺血缺氧区及异常血管[7-8])和视网膜冷凝术(原理为通过低温方法破坏异常血管[4, 9]),但伴有渗出性视网膜脱离的3期及以上病变对激光光凝术反应较差,而对经巩膜冷凝术不仅反应不佳,还会破坏血视网膜屏障,使视网膜下液体进一步增加、吸收困难而造成黄斑区渗出聚集及视网膜前增生膜[4, 10],最终造成患者视力预后差[11]。因此,对3期及以上外层渗出性视网膜病变患者常需联合玻璃体手术。近年来有报道外层渗出性视网膜病变患者玻璃体中血管内皮生长因子(vascular endothelial growth factor,VEGF)升高,使得玻璃体内注射抗VEGF药成为辅助治疗方法[12-17],研究表明该方法能减少毛细血管扩张及增加视网膜下液的吸收,并能改善黄斑区形态[18-20];但有文献报道贝伐单抗治疗会增加外层渗出性视网膜病变患者发生纤维增殖及牵引性视网膜脱离的风险[21]。本研究采用经巩膜视网膜下液引流及冷凝术联合玻璃体内注射抗VEGF药康柏西普用于3期外层渗出性视网膜病变的治疗,以达到在破坏异常扩张毛细血管的同时减少视网膜下液聚集的目的,现将其治疗效果报告如下。
1 资料和方法 1.1 一般资料本研究为回顾性病例系列分析。选择2017年1月至2020年1月在上海交通大学医学院附属上海儿童医学中心接受经巩膜视网膜下液引流及冷凝术联合玻璃体内注射抗VEGF药康柏西普治疗的3期外层渗出性视网膜病变患者。纳入标准:(1)患者经眼底及荧光素眼底血管造影术(fluorescein fundus angiography,FFA)检查有局限性黄色渗出及邻近视网膜血管异常表现,包括毛细血管扩张、迂曲,动脉瘤样扩张,毛细血管丢失及偶有新生血管;(2)疾病分期符合Shields分期标准中3期表现,即渗出性视网膜脱离(3A期为视网膜次全脱离,3B期为视网膜全脱离)[5]。排除标准:(1)其他白瞳症或视网膜血管异常疾病,如视网膜母细胞瘤、家族性渗出性玻璃体视网膜病变、永存原始玻璃体增生症及视网膜血管炎等;(2)曾接受过其他眼部治疗手术。
1.2 术前检查患者监护人被充分告知患者病情和经巩膜视网膜下液引流及冷凝术联合玻璃体内注射康柏西普的注意事项,并就治疗方案签署知情同意书。术前检查项目为常规术前检查,包括最佳矫正视力(best corrected visual acuity,BCVA)、眼压、裂隙灯检查、超声检查、眼底照相、FFA、眼位及眼球运动。BCVA采用最小分辨角对数(logarithm of the minimum angle of resolution,logMAR)法测定。不能配合眼科检查的患儿在水合氯醛或全身麻醉辅助下完成检查。
1.3 手术方法患者行全身麻醉。打开球结膜,置四直肌牵引线,选取经巩膜穿刺放液口(视网膜次全脱离患者放液口选在视网膜脱离最高位,视网膜全脱离患者在4个象限均做巩膜放液口),采用1 mL注射器针头(27 G)行视网膜下液外放液,避免放液速度过快,注意维持眼压。放液完成后,用冷冻头顶压眼球后在显微镜直视下经巩膜冷凝视网膜异常血管7~16个点,冷凝程度为视网膜出现白色反应并形成冰晶。所有患眼冷凝后行玻璃体内注射抗VEGF药康柏西普(成都康弘生物科技有限公司生产,批准文号为国药准字S20130012,规格为10 mg/mL、0.2 mL/支),每次剂量为0.5 mg(0.05 mL)。
1.4 术后检查和随访术后检查包括BCVA、眼压、裂隙灯检查、眼底照像及超声检查,观察测量指标为BCVA、眼压、视网膜复位情况、视网膜下液渗出吸收情况及并发症等;根据病情行黄斑光学相干断层扫描及FFA检查。术后第1天、第1周及每月定期复查,1个月后根据实际情况行视网膜激光光凝术或玻璃体内康柏西普注射等治疗。
2 结果 2.1 基线资料共11例患者(均为单眼发病)纳入本研究。患者年龄为2~16岁,男10例、女1例,右眼病变6例、左眼病变5例。11例患者中10例患者术前BCVA为眼前手动~0.2,其中2例为眼前手动,3例为眼前指数,5例为0.05~0.2;眼压为8~20 mmHg(1 mmHg=0.133 kPa);裂隙灯及眼底照相可见周边视网膜血管异常扩张,视网膜下黄色渗出,渗出性视网膜脱离,其中3A期7例、3B期4例,有2例视网膜脱离至晶状体后;FFA检查证明视网膜小血管异常扩张。
2.2 治疗结果治疗过程中患者眼压稳定,术后眼压为8.5~18 mmHg。术后补充行视网膜激光光凝术5例;玻璃体内注射康柏西普次数为1~5次,其中1例注射1次,4例注射2次,3例注射3次,2例注射4次,1例注射5次。所有患者资料汇总见表 1。术后随访6~20个月,11例患者中9例患者视网膜完全复位,2例患者出现局限性渗出性视网膜脱离予以观察随访。末次随访时,11例患者中视力提高8例,其中1例患者由0.2提高到0.4;视力无变化1例;视力下降1例;1例患者术前视力检查不配合,末次随访时视力为眼前指数30 cm。随访期间新发生斜视1例(病例11,患者年龄小、视力差),未观察到手术操作相关的并发症。图 1为1例患者治疗前后的眼底照相结果。
|
|
表 1 经巩膜视网膜下液引流及冷凝术联合康柏西普治疗的11例3期外层渗出性视网膜病变患者资料 |
|
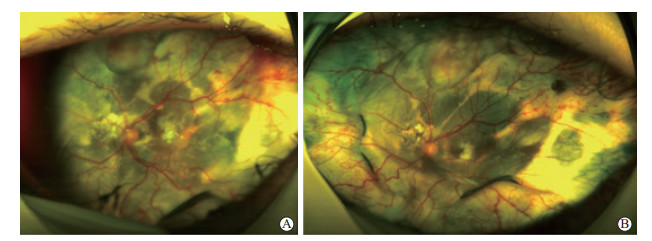
图 1 1例经巩膜视网膜下液引流及冷凝术联合康柏西普治疗的3期外层渗出性视网膜病变患者眼底照相结果 A:治疗前患者全视网膜渗出性视网膜脱离,颞上方可见大片状视网膜新生血管;B:治疗后6个月视网膜后极部下液吸收,视网膜复位,颞上方大片状新生血管明显消退. |
3 讨论
外层渗出性视网膜病变具体发病机制不明,特征性表现为视网膜毛细血管、微动脉扩张及视网膜层间和视网膜下硬性渗出,病理机制为视网膜血管内皮细胞和周细胞的丢失导致视网膜血管内屏障破坏和脂质性渗出[22]。该病发病率较低,根据发病年龄分为儿童型和成人型,其中儿童型占93%[23]。成人型外层渗出性视网膜病变通常无症状或单眼视力逐渐下降,可能与成年人患病能及时发现和就诊有关;儿童型外层渗出性视网膜病变的首发表现为单眼视力差、弱视、斜视,甚至白瞳症[24]。儿童型外层渗出性视网膜病变占比高,往往发现较晚,治疗困难且预后差,且疾病可能为终身发病,易复发、需要多次治疗。1~2期外层渗出性视网膜病变患者视网膜病变程度较轻,可采用直接视网膜激光光凝术或冷凝术治疗。对于不能配合体位的年龄较小患儿,常需要在全身麻醉下经双目间接眼底镜下行激光光凝术,如果患儿合并黄斑区的渗出,可以采用玻璃体内注射抗VEGF药辅助治疗。但当疾病发展到3期即视网膜发生脱离时,单纯的经外眼视网膜激光光凝术治疗无效,需行经巩膜的两切口眼内激光光凝术[25]、视网膜冷凝术治疗,甚至需行玻璃体切除手术联合视网膜激光光凝术;然而冷凝术造成的视网膜炎症反应较重,会进一步破坏血视网膜屏障[10]。由于儿童的玻璃体及增生膜与视网膜粘连紧密,存在视网膜下液黏稠、切开的视网膜对激光反应差、注射硅油后患儿体位配合难度大及硅油自身并发症多等因素,导致外层渗出性视网膜病变患儿玻璃体切除手术难度非常大、术后易复发[26-27];此外,即使脱离的视网膜经手术复位后,患儿的视网膜和黄斑区仍然残存有大量硬性渗出和水肿,导致视功能恢复不理想[28-29]。
本研究采用经巩膜视网膜下液引流及视网膜冷凝术联合玻璃体内注射抗VEGF药康柏西普治疗了11例3期外层渗出性视网膜病变患者,患者术后视网膜复位率高,视功能得到提高和保持。11例患者中,9例视网膜完全复位,2例出现局限性渗出性视网膜脱离予以观察。8例患者视力提高(其中1例患者视力由0.2提高到0.4),1例患者视力无变化,1例患者视力下降,1例患者因术前视力检查不配合而未获得视力变化结果。本联合治疗方法优势如下:(1)复位脱离的视网膜时需先经巩膜外放液减少视网膜下液的聚集,一方面增加了冷凝精准性及效能、减少了无效冷凝剂量,另一方面降低了在已发生视网膜脱位、隆起的儿童眼内直接行玻璃体内药物注射导致医源性视网膜裂孔的风险,此外还降低了冷凝和抗VEGF药并发症的发生,本组11例患者均未发生玻璃体增生和牵引性视网膜脱离;(2)冷凝术通过低温方法破坏异常血管,抑制异常血管进一步渗出,操作较为简单,效果较眼内激光光凝术确切;(3)联合抗VEGF药康柏西普治疗减轻了冷凝术带来的血视网膜屏障破坏,抑制了异常新生血管形成并促进了视网膜下液吸收;(4)通过视网膜下液引流达到较高视网膜复位率,从而避免玻璃体切除手术;(5)术后根据病情进行个体化处理,决定是否补充激光光凝术或玻璃体内注射康柏西普使患者病情达到稳定,这减少了患儿反复手术及全身麻醉次数。
本研究初步表明经巩膜视网膜下液引流和视网膜冷凝手术联合抗VEGF药康柏西普疗法综合了3种方法的优势,且手术操作简单、手术时间短、术后并发症较少、对体位无要求,是3期外层渗出性视网膜病变患者的一种有效的治疗方法。但本研究样本量较小,仍需要进一步扩大样本量并设立随机对照试验增强临床研究的证据强度,另一方面还需延长随访时间进一步观察该治疗方法的长期疗效。
| [1] |
COATS G. Forms of retinal dysplasia with massive exudation[J]. R LondOphthalmol Hosp Rep, 1908, 17: 440-525. |
| [2] |
YANG X Y, WANG C G, SU G F. Recent advances in the diagnosis and treatment of Coats' disease[J]. Int Ophthalmol, 2019, 39: 957-970. DOI:10.1007/s10792-019-01095-8 |
| [3] |
ZHANG J, RUAN L, JIANG C, YANG Q, JU Y Q, CHANG Q, et al. Updating understanding of macular microvascular abnormalities and their correlations with the characteristics and progression of macular edema or exudation in Coats' disease[C]. Front Med (Lausanne), 2022, 9: 788001. DOI: 10.3389/fmed.2022.788001.
|
| [4] |
SEN M, SHIELDS C L, HONAVAR S G, SHIELDS J A. Coats disease: an overview of classification, management and outcomes[J]. Indian J Ophthalmol, 2019, 67: 763-771. DOI:10.4103/ijo.IJO_841_19 |
| [5] |
SHIELDS J A, SHIELDS C L, HONAVAR S G, DEMIRCI H, CATER J. Classification and management of Coats disease: the 2 000 proctor lecture[J]. Am J Ophthalmol, 2001, 131: 572-583. DOI:10.1016/S0002-9394(01)00896-0 |
| [6] |
SHIELDS C L, UDYAVER S, DALVIN L A, LIM L A S, ATALAY H T, KHOO C, et al. Visual acuity outcomes in Coats disease by classification stage in 160 patients[J]. Br J Ophthalmol, 2020, 104: 422-431. DOI:10.1136/bjophthalmol-2019-314363 |
| [7] |
MULVIHILL A, MORRIS B. A population-based study of Coats disease in the United Kingdom Ⅱ: investigation, treatment, and outcomes[J]. Eye (Lond), 2010, 24: 1802-1807. DOI:10.1038/eye.2010.127 |
| [8] |
BENLAHBIB M, SEMOUN O, AMOROSO F, COLANTUONO D, SOUIED E H. Multimodal imaging of unusual macular macroaneurysm rupture after navigated retinal laser in a patient with adult onset Coats disease[C]. Am J Ophthalmol Case Rep, 2022, 26: 101458. DOI: 10.1016/j.ajoc.2022.101458.
|
| [9] |
OTANI T, YASUDA K, AIZAWA N, SAKAI F, NAKAZAWA T, SHIMURA M. Over 10 years follow-up of Coats' disease in adulthood[J]. Clin Ophthalmol, 2011, 5: 1729-1732. |
| [10] |
JACCOMA E H, CONWAY B P, CAMPOCHIARO P A. Cryotherapy causes extensive breakdown of the blood-retinal barrier. A comparison with Argon laser photocoagulation[J]. Arch Ophthalmol, 1985, 103: 1728-1730. DOI:10.1001/archopht.1985.01050110124039 |
| [11] |
SIGLER E J, RANDOLPH J C, CALZADA J I, WILSON M W, HAIK B G. Current management of Coats disease[J]. SurvOphthalmol, 2014, 59: 30-46. |
| [12] |
HE Y G, WANG H, ZHAO B R, LEE J, BAHL D, MCCLUSKEY J. Elevated vascular endothelial growth factor level in Coats' disease and possible therapeutic role of bevacizumab[J]. Graefes Arch Clin Exp Ophthalmol, 2010, 248: 1519-1521. DOI:10.1007/s00417-010-1366-1 |
| [13] |
LIANG T Y, XU Y, ZHU X Y, ZHANG X, LI J, ZHAO P Q. Aqueous humour cytokines profiles in eyes with Coats disease and the association with the severity of the disease[C]. BMC Ophthalmol, 2020, 20: 178. DOI: 10.1186/s12886-020-01421-0.
|
| [14] |
ZHANG J, JIANG C, RUAN L, HUANG X. Associations of cytokine concentrations in aqueous humour with retinal vascular abnormalities and exudation in Coats' disease[J]. Acta Ophthalmol, 2019, 97: 319-324. DOI:10.1111/aos.13971 |
| [15] |
ZHENG X X, JIANG Y R. The effect of intravitreal bevacizumab injection as the initial treatment for Coats' disease[J]. Graefes Arch Clin Exp Ophthalmol, 2014, 252: 35-42. DOI:10.1007/s00417-013-2409-1 |
| [16] |
RAY R, BARAÑANO D E, HUBBARD G B. Treatment of Coats' disease with intravitreal bevacizumab[J]. Br J Ophthalmol, 2013, 97: 272-277. DOI:10.1136/bjophthalmol-2012-302250 |
| [17] |
WU S, FANG J, YU F, HAN F, XIAO J. Clinical study of intravitreal injection of anti-VEGF drugs combined with triamcinolone acetonide in the treatment of coats disease[C]. Comput Math Methods Med, 2022, 2022: 9911549. DOI: 10.1155/2022/9911549.
|
| [18] |
JARIN R R, TEOH S C B, LIM T H. Resolution of severe macular oedema in adult Coat's syndrome with high-dose intravitreal triamcinolone acetonide[J]. Eye (Lond), 2006, 20: 163-165. DOI:10.1038/sj.eye.6701828 |
| [19] |
WU A L, WU W C. Anti-VEGF for ROP and pediatric retinal diseases[J]. Asia Pac J Ophthalmol (Phila), 2018, 7: 145-151. |
| [20] |
PETRONI S, CATENA G, IAROSSI G, FEDERICI M, ZINZANELLA G, PARRILLA R, et al. Treatment of advanced Coats' disease with combination therapy of laser photocoagulation, intravitreal ranibizumab, and sub-tenon methylprednisolone acetate[J]. J Pediatr Ophthalmol Strabismus, 2022, 59: 187-191. DOI:10.3928/01913913-20211110-02 |
| [21] |
RAMASUBRAMANIAN A, SHIELDS C L. Bevacizumab for Coats' disease with exudative retinal detachment and risk of vitreoretinal traction[J]. Br J Ophthalmol, 2012, 96: 356-359. DOI:10.1136/bjophthalmol-2011-300141 |
| [22] |
FERNANDES B F, ODASHIRO A N, MALONEY S, ZAJDENWEBER M E, LOPES A G, BURNIER M N. Clinical-histopathological correlation in a case of Coats' disease[C]. DiagnPathol, 2006, 1: 24. DOI: 10.1186/1746-1596-1-24.
|
| [23] |
RISHI E, RISHI P, APPUKUTTAN B, UPARKAR M, SHARMA T, GOPAL L. Coats' disease of adult-onset in 48 eyes[J]. Indian J Ophthalmol, 2016, 64: 518-523. DOI:10.4103/0301-4738.190141 |
| [24] |
SHIELDS J A, SHIELDS C L, HONAVAR S G, DEMIRCI H. Clinical variations and complications of Coats disease in 150 cases: the 2000 Sanford Gifford Memorial Lecture[J]. Am J Ophthalmol, 2001, 131: 561-571. DOI:10.1016/S0002-9394(00)00883-7 |
| [25] |
CAI X, ZHAO P Q, ZHANG Q, JIN H Y. Treatment of stage 3 Coats' disease by endolaser photocoagulation via a two-port pars plana nonvitrectomy approach[J]. Graefes Arch Clin Exp Ophthalmol, 2015, 253: 999-1004. DOI:10.1007/s00417-015-2984-4 |
| [26] |
NUCCI P, BANDELLO F, SERAFINO M, WILSON M E. Selective photocoagulation in Coats disease: ten-year follow-up[J]. Eur J Ophthalmol, 2002, 12: 501-505. DOI:10.1177/112067210201200609 |
| [27] |
KARACORLU M, HOCAOGLU M, SAYMAN MUSLUBAS I, ARF S. Long-term anatomical and functional outcomes following vitrectomy for advanced Coats disease[J]. Retina, 2017, 37: 1757-1764. DOI:10.1097/IAE.0000000000001415 |
| [28] |
ONG S S, BUCKLEY E G, MCCUEN B W 2nd, JAFFE G J, POSTEL E A, MAHMOUD T H, et al. Comparison of visual outcomes in Coats' disease: a 20-year experience[J]. Ophthalmology, 2017, 124: 1368-1376. DOI:10.1016/j.ophtha.2017.03.051 |
| [29] |
DALVIN L A, UDYAVER S, LIM L A S, MAZLOUMI M, ATALAY H T, KHOO C T L, et al. Coats disease: clinical features and outcomes by age category in 351 cases[J]. J PediatrOphthalmol Strabismus, 2019, 56: 288-296. |
 2022, Vol. 43
2022, Vol. 43


