2. 中国人民解放军联勤保障部队988医院麻醉科,郑州 450042
2. Department of Anesthesiology, No. 988 Hospital of Logistic Support Force of PLA, Zhengzhou 450042, Henan, China
阿片类药物被广泛应用于各种急慢性疼痛的治疗,镇痛效果强,但长期应用导致的成瘾、戒断反应是限制其临床应用的一大障碍。吗啡成瘾后突然撤药导致的戒断反应包括流涕、恶心、呕吐、药物耐受、痛觉过敏等,严重降低患者生活质量,其中痛觉过敏也是导致吗啡镇痛效果下降的重要因素[1]。因此,探索吗啡戒断反应中痛觉过敏的发生机制并减缓其进展对提高临床镇痛效果至关重要。小胶质细胞是神经系统内的一种免疫巨噬细胞,在脊髓的生理功能中发挥重要作用。神经损伤、感染和促炎因子的释放均能诱导小胶质细胞过度激活,通过降钙素基因相关肽(calcitonin gene-related peptide,CGRP)等途径激活神经炎症反应,改变神经元的功能和可塑性,在疼痛调节过程中发挥重要作用[2]。右美托咪定(dexmedetomidine,Dex)作为临床上常用的镇静药物,具有良好的抗炎、抗焦虑、抗交感神经活性等功能。本研究利用吗啡戒断反应痛觉过敏模型小鼠,探讨Dex在脊髓层面调控小胶质细胞活化对痛觉过敏的影响。
1 材料和方法 1.1 实验试剂吗啡溶液、纳洛酮(美国Sigma公司),盐酸Dex注射液(江苏恩华药业股份有限公司,国药准字H20110085),小胶质细胞激活抑制剂米诺环素(minocycline,Min;美国Sigma公司),CGRP抑制剂MK8825(杭州科莱普科技有限公司)。兔来源钙离子结合调节因子1(ionized calcium-binding adapter molecule 1,IBA-1)抗体(日本WAKO公司),小鼠来源CGRP抗体(英国Abcam公司),小鼠来源GAPDH抗体(美国CST公司),抗兔二抗、抗小鼠二抗(美国CST公司),山羊抗兔IgG(美国Invitrogen公司)。
1.2 实验动物分组及给药健康雄性C57/BL小鼠168只(体重20~30 g)购自上海市实验动物研究中心[实验动物生产许可证号:SCXK(沪)2017-0012],于恒温、恒湿条件下饲养。将小鼠随机分为空白对照组、吗啡戒断反应痛觉过敏模型组(M组)、M+Dex组、M+Min组、M+Dex+Min组、M+MK8825组、M+Min+MK8825组,每组6只。空白对照组、M组、M+Dex组、M+Min组及M+Dex+Min组共120只小鼠用于机械性痛阈检测、免疫荧光检测、蛋白质印迹检测及PCR检测,空白对照组、M组、M+Dex组、M+Min组、M+Dex+Min组、M+MK8825组、M+Min+MK8825组共42只小鼠用于微透析技术检测,电生理检测使用6只M组小鼠。空白对照组小鼠不予任何干预;其他6组小鼠每天上午8:00和下午6:00各腹腔注射吗啡溶液(5 mg/kg)1次,连续注射6 d,随后腹腔注射纳洛酮(10 μg/kg)构建吗啡戒断反应痛觉过敏模型[3]。M组为单纯吗啡戒断反应痛觉过敏小鼠,M+Dex、M+Min、M+Dex+Min、M+MK8825、M+Min+MK8825组小鼠在纳洛酮给药前30 min通过鞘内导管分别单次给予人工脑脊液稀释的盐酸Dex注射液(1 μg/kg)、Min(10 μg/kg)、盐酸Dex注射液(1 μg/kg)+Min(10 μg/kg)、MK8825(5 mg/kg)、Min(10 μg/kg)+MK8825(5 mg/kg)。
1.3 鞘内置管在构建吗啡戒断反应痛觉过敏模型前行鞘内置管手术。小鼠腹腔注射戊巴比妥钠30 mg/kg麻醉后腰中线备皮、消毒后切开长1~2 cm的小口,剪开筋膜,一手托起小鼠,尽量暴露L5/L6椎间隙,用小剪刀垂直L5棘突剪开,再在L6棘突上斜向L5方向向下剪开肌肉,剪除部分L6棘突,暴露L5/L6椎间的三角间隙,用小针穿破硬膜,可见动物甩尾反射。夹取PE-10导管沿穿刺孔插入,插入时可见动物甩尾或蹬腿,置入约1 cm后可见脑脊液流出,即可封闭管口。导管周围敷以氨苄青霉素粉末,依次缝合,妥善固定导管于肌肉和皮肤,在皮下再敷以氨苄青霉素粉末,缝合皮肤对合完整,腹腔注射0.3 mL氨苄青霉素(100 mg/mL)预防感染,术后单笼饲养。
1.4 机械性痛阈测定在注射吗啡前测定基础痛阈,并分别在模型构建2、4、6 h后测定痛阈。在安静的房间内将小鼠放在一组茶色丙烯酸树脂笼中,底部为金属网,用von Frey纤维丝(美国North Coast Medical公司)尖端刺激小鼠后足五趾间柔软部位,缓慢加力,出现缩足反应后即停止接触,此时记为阳性。每次测试至少间隔5 min,测3次取平均值为小鼠的机械性痛阈值。为避免造成小鼠足底机械性损伤,实验采用的von Frey纤维丝最大值为10 g,接触时间为5 s[4]。
1.5 免疫荧光检测注射纳洛酮4 h后取脊髓背角组织,采用免疫荧光观察小胶质细胞激活标志物IBA-1的表达情况。小鼠腹腔注射戊巴比妥钠50 mg/kg深麻醉后用0.1 mol/L PBS和4%多聚甲醛溶液灌流,将L4~L5段脊髓取出后用4%多聚甲醛溶液后固定,并移入30%蔗糖溶液中脱水。用Leica冷冻切片机切片,厚度为20 μm,用5%山羊血清室温下封闭2 h后在4 ℃下加入兔来源IBA-1抗体(稀释比例为1∶500)孵育过夜,PBS洗3次;加入山羊抗兔IgG(稀释比例为1∶1 000)孵育2 h。用荧光显微镜拍摄,并使用ImageJ 1.8软件对目标区域阳性细胞进行计数。
1.6 蛋白质印迹法注射纳洛酮4 h后取小鼠脊髓背角组织用蛋白质印迹法测定IBA-1和CGRP的表达。小鼠腹腔注射戊巴比妥钠50 mg/kg深麻醉后迅速取出L4~L5段脊髓背角组织,加入到200 μL RIPA裂解液超声匀浆,裂解完成后高速离心(12 000×g,15 min,4 ℃)取上清。随后用BCA法测定总蛋白浓度,并在99 ℃条件下变性10 min。取30 μg蛋白样本用8% SDS-PAGE分离蛋白,采用湿转法将蛋白转移至PVDF膜上,用5%脱脂奶粉溶液封闭2 h后,分别加入兔来源IBA-1抗体和小鼠来源CGRP、GAPDH抗体(稀释比例分别为1∶1 000、1∶1 000、1∶5 000)4 ℃孵育过夜。TBST洗膜3次后加入二抗(稀释比例均为1∶5 000),室温孵育2 h后进行化学发光显影。采用Photoshop CS6软件测量条带的灰度值。目的蛋白的相对表达量=目的条带灰度值/GAPDH条带灰度值。
1.7 qPCR注射纳洛酮4 h后取小鼠脊髓背角组织,用PCR检测CGRP mRNA的表达。小鼠腹腔注射戊巴比妥钠50 mg/kg深麻醉后取L4~L5段脊髓背角组织,加入1 mL TRIzol充分匀浆,室温静置10 min,加入0.2 mL氯仿,剧烈震荡15 s后高速离心(12 000×g,15 min,4 ℃)取上清液;加入等体积异丙醇,吹打混匀后高速离心(12 000×g,10 min,4 ℃)弃上清;加入1 mL的75%乙醇洗涤沉淀后弃上清;加入DEPC水充分溶解RNA沉淀,采用分光光度计测量RNA浓度和纯度。获取的RNA在37 ℃ 15 min、85 ℃ 15 s、4 ℃ 10 min条件下进行反转录,获得cDNA,并以cDNA为模版在95 ℃ 15 min,95 ℃ 15 s、60 ℃ 15 s、72 ℃ 40 s(40个循环)条件下扩增。扩增引物序列如下:CGRP正向引物5'-CCTTTCCTGGTTGTCAGCATCTT-3',反向引物5'-GTAGGCGAGCTTCTTCTTCACTGA-3';内参照GAPDH正向引物5'-CCACTCCTCCACCTTTGAC- G-3',反向引物5'-TAGCCAAATTCGTTGTCATA- CCAGG-3'。计算目的基因的相对表达量。
1.8 微透析采用微透析技术检测小鼠脊髓背角内炎症因子TNF-α、IL-1β的水平。注射纳洛酮4 h后腹腔注射戊巴比妥钠30 mg/kg麻醉小鼠并固定于立体定位仪上,于腰部中间皮肤切口,摘除椎板暴露部分脊髓,插入透析针,保持透析管同脊髓在同一平面并固定。打开微透析泵并填充透析液,平衡2 h后收集透析液。通过高效液相层析色谱法[5]测定透析液中TNF-α、IL-1β的浓度。
1.9 电生理测定6只M组小鼠腹腔注射戊巴比妥钠50 mg/kg深麻醉后迅速取出L4~L5段脊髓放入预冷人工脑脊液中,用95% O2+5% CO2的混合气将人工脑脊液充分饱和。在显微镜下清除软脊膜和神经根,在琼脂糖槽中使用振动切片机将脊髓切片,厚度为400 μm,并迅速转移切片至灌流槽中,33 ℃恒温下孵育1 h。将脊髓切片转移到记录玻片上,室温下用人工脑脊液持续灌流。在透明相差显微镜下找到半透明脊髓胶状质神经元,将含有电极内液的电极移至细胞表面,直到出现电极电阻增加,然后负压吸破细胞膜。通过MultiClamp 700B放大器和pClamp10.2数据采集软件,在0 mV电位下以电压钳模式记录自发性抑制性突触后电流(spontaneous inhibitory postsynaptic current,sIPSC)。所有药物均使用给药系统进行给药,给药系统注射尖端置于记录玻片的灌流槽中,将配制好的Dex溶液加入给药系统中,灌流前和Dex溶液灌流30 s后分别用人工脑脊液冲洗2 min[6]。
1.10 统计学处理应用SPSS 21.0软件进行统计学分析。服从正态分布且方差齐的计量资料以x±s表示,两组间比较采用t检验,多组间比较采用单因素方差分析,多重比较采用Tukey检验;不同组别和时间点的机械性痛阈分析采用多因素重复方差分析;电生理结果用Clampfit 11软件进行分析。检验水准(α)为0.05。
2 结果 2.1 Dex减轻吗啡戒断反应痛觉过敏如图 1所示,5组小鼠的基础机械性痛阈差异无统计学意义(P>0.05),M组小鼠机械性痛阈在注射纳洛酮后2、4、6 h时均低于空白对照组(P均<0.05),说明吗啡戒断反应痛觉过敏模型小鼠构建成功。与M组相比,M+Dex、M+Min、M+Dex+Min组小鼠机械性痛阈在注射纳洛酮后4 h时均升高(P均<0.05),而M+Dex+Min组小鼠痛阈与M+Dex组、M+Min相比差异均无统计学意义(P均>0.05)。提示Dex鞘内注射或小胶质细胞功能抑制均可减轻小鼠吗啡戒断反应的痛觉过敏症状。由于小鼠在注射纳洛酮后4 h痛觉过敏最为明显,因此后续实验均采用此时为时间点。
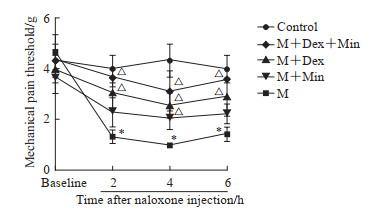
|
图 1 Dex对小鼠吗啡戒断反应痛觉过敏的影响 Fig 1 Effects of Dex on pain thresholds of morphine withdrawal mice *P < 0.05 vs control group at the same time point; △P < 0.05 vs M group at the same time point. n=6, x±s. Dex: Dexmedetomidine; M: Morphine; Min: Minocycline (a microglia inhibitor). |
2.2 Dex抑制脊髓小胶质细胞激活
如图 2所示,M组小胶质细胞活化数量及IBA-1蛋白表达均高于空白对照组(P均<0.05),M+Dex、M+Min、M+Dex+Min组小胶质细胞活化数量及IBA-1蛋白表达均低于M组(P均<0.05),M+Dex、M+Min、M+Dex+Min组间小胶质细胞活化数量及IBA-1蛋白表达差异无统计学意义(P均>0.05)。说明Dex可抑制脊髓背角小胶质细胞的活化。
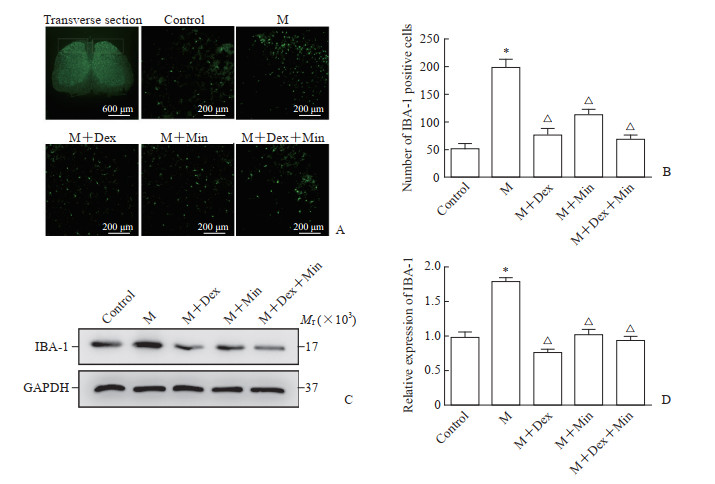
|
图 2 Dex对小鼠脊髓背角小胶质细胞的影响 Fig 2 Effect of Dex on microglia in spinal dorsal horn of mice A: Representative immunofluorescent staining pictures of IBA-1 in spinal dorsal horn of mice. The white box indicates the observed target spinal dorsal horn. B: Quantitative analysis of number of IBA-1 positive cells in spinal dorsal horn. C: Western blotting of IBA-1 protein expression in spinal dorsal horn. D: Quantitative analysis of IBA-1 protein expression in spinal dorsal horn. *P < 0.05 vs control group; △P < 0.05 vs M group. n=6, x±s. Dex: Dexmedetomidine; M: Morphine; Min: Minocycline (a microglia inhibitor); IBA-1: Ionized calcium-binding adapter molecule 1; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. |
2.3 Dex减少脊髓背角CGRP表达
蛋白质印迹法检测结果(图 3A、3B)显示,M组小鼠脊髓背角CGRP蛋白表达高于空白对照组(P<0.05),M+Dex、M+Min、M+Dex+Min组CGRP蛋白表达均低于M组(P均<0.05),M+Dex、M+Min、M+Dex+Min组间CGRP蛋白表达差异无统计学意义(P>0.05)。qPCR检测结果(图 3C)显示,各组CGRP mRNA的表达与CGRP蛋白表达趋势相同。以上结果提示Dex鞘内注射或小胶质细胞功能抑制均可减少脊髓背角CGRP的表达。
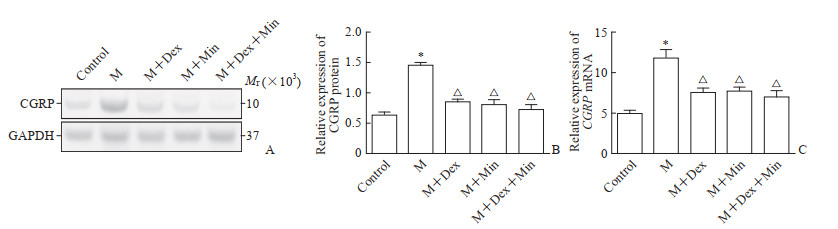
|
图 3 Dex对小鼠脊髓背角CGRP表达的影响 Fig 3 Effect of Dex on CGRP expression in spinal dorsal horn of mice A: Representative Western blotting images of CGRP protein expression in spinal dorsal horn; B: Quantitative analysis of CGRP protein expression in spinal dorsal horn detected by Western blotting; C: Quantitative analysis of CGRP mRNA expression in spinal dorsal horn detected by quantitative polymerase chain reaction. *P < 0.05 vs control group; △P < 0.05 vs M group. n=6, x±s. Dex: Dexmedetomidine; CGRP: Calcitonin gene-related peptide; M: Morphine; Min: Minocycline (a microglia inhibitor); GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. |
2.4 Dex减少脊髓背角炎症因子水平
由图 4可见,M组脊髓背角TNF-α、IL-1β水平高于空白对照组(P均<0.05),M+Dex、M+Min、M+Dex+Min、M+MK8825、M+Min+MK8825组脊髓背角TNF-α、IL-1β水平均低于M组(P均<0.05),M+Dex、M+Min、M+Dex+Min、M+MK8825、M+Min+MK8825组间脊髓背角TNF-α、IL-1β水平差异均无统计学意义(P均>0.05)。以上结果提示Dex鞘内注射、小胶质细胞或CGRP功能抑制均可减少脊髓背角炎症因子表达。
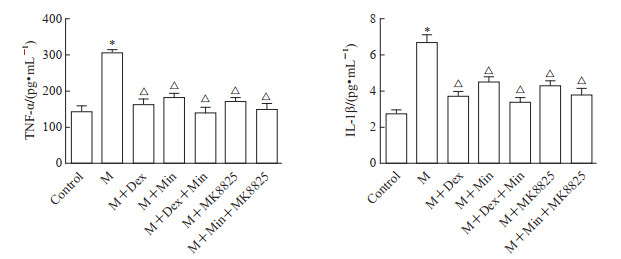
|
图 4 Dex对小鼠脊髓背角中TNF-α和IL-1β水平的影响 Fig 4 Effects of Dex on TNF-α and IL-1β in spinal dorsal horn of mice *P < 0.05 vs control group; △P < 0.05 vs M group. n=6, x±s. Dex: Dexmedetomidine; TNF-α: Tumor necrosis factor α; IL-1β: Interleukin-1β; M: Morphine; Min: Minocycline (a microglia inhibitor); MK8825: Calcitonin gene-related peptide inhibitor. |
2.5 Dex增强脊髓背角sIPSC
电生理测定结果显示,与灌流人工脑脊液相比,灌流Dex后小鼠脊髓背角sIPSC的频率[(5.68±1.04)Hz vs(4.13±0.97)Hz]和振幅[(66.33±8.61)pA vs(40.98±7.88)pA]均增强,差异均有统计学意义(P均<0.05)。这一结果表明Dex可增强脊髓背角抑制性神经元的电活动,从而改善吗啡戒断反应中的痛觉过敏。
3 讨论阿片类药物长期使用导致的戒断综合征严重影响患者的生活质量,其主要症状之一是患者处于痛觉过敏状态,即对轻微刺激表现出明显的疼痛反应,严重影响镇痛效果。本研究结果显示,Dex通过抑制脊髓背角小胶质细胞激活介导的信号通路、减轻炎症反应及增强sIPSC抑制吗啡戒断反应中痛觉过敏的出现。
吗啡戒断反应中痛觉过敏涉及多种生理过程,脊髓背角神经元的突触重塑和长时程增强发挥重要作用[7-8]。小胶质细胞在中枢神经系统分布广泛,能够介导疼痛信号转导过程。中枢小胶质细胞对外界病理性损伤尤为敏感,如感染、炎症、神经损伤等刺激都能够激活小胶质细胞,改变神经元突触的结构和功能,导致TNF-α、IL-1β、IL-6、生长因子等物质释放,对中枢产生多种效应,其中的内源性IL-1β能够减少γ-氨基丁酸能神经元的sIPSC[9]。
Toll样受体4(Toll-like receptor 4,TLR4)作为一种体内固有免疫受体,在小胶质细胞膜上广泛分布,是吗啡引起小胶质细胞活化和脊髓神经炎症反应的关键分子。持续的吗啡应用可诱导神经元释放热休克蛋白,触发TLR4介导的小胶质细胞激活并诱发炎症反应,导致MAPK氧化磷酸化,也在一定程度上参与戒断反应痛觉过敏的进展[10-11]。脊髓背角小胶质细胞激活后可调节脊髓突触信息传递,通过促进突触前CGRP等释放增强细胞间兴奋性突触传递,也能部分减少抑制性突触传递导致脊髓神经元兴奋性增加[12]。
CGRP是参与中枢、外周性伤害性信息传递的神经活性物质,并且能够介导神经炎症反应。小胶质细胞激活后能够释放脑源性神经营养因子,后者进入突触间隙作用于相邻神经元,促进CGRP的生成、释放和转运,导致突触后易化和长时程增强使突触重塑和中枢敏化,从而引发和维持痛觉过敏[13]。本研究通过连续腹腔注射吗啡后应用纳洛酮诱发小鼠出现戒断反应痛觉过敏,结果证实模型中小胶质细胞激活和CGRP表达、炎症因子TNF-α及IL-1β释放增加,在应用Min后CGRP表达和炎症因子释放均减少,提示小胶质细胞能够调控下游CGRP的表达、促进神经炎症反应,从而影响痛觉过敏的进展。
Dex作为高选择性α2受体激动剂,具有较强的镇静、抗焦虑作用,被广泛应用于围手术期[14]。Dex通过结合脊髓突触前膜的α2受体产生突触前抑制,减少钙离子内流,抑制ATP等物质介导的伤害性信息传递及谷氨酸、P物质等兴奋性神经递质释放[15-16]。同时,Dex具有强大的抗炎作用,在脊髓背角能够抑制TLR4介导的神经胶质细胞激活,减少小胶质细胞标志物IBA-1表达,缓解神经元凋亡,一定程度上抑制突触重塑和长时程增强的发生,延缓痛觉过敏的进展,尤其是通过鞘内给药直接作用于脊髓背角效果更加显著[17-18]。本研究中吗啡戒断反应痛觉过敏模型小鼠的小胶质细胞激活、CGRP表达和炎症反应能被Dex抑制,且Dex增强了脊髓背角的sIPSC,提示Dex通过多种途径减缓突触重塑的发生提高模型小鼠痛阈。Dex和Min联合应用并未增强其作用效果,与单独应用Dex作用相当,提示Dex对痛觉过敏的抑制作用主要是通过调控小胶质细胞介导的CGRP释放影响炎症反应,或直接影响脊髓背角抑制性电活动。
综上所述,Dex能够通过抑制脊髓背角小胶质细胞活化、减少CGRP的表达、减轻炎症反应并能够增强脊髓抑制性电活动,缓解吗啡戒断反应中的痛觉过敏,这为临床合理使用阿片类镇痛药物、减少戒断反应痛觉过敏的发生提供了新的治疗思路。
| [1] |
ZHANG G L, WU X, ZHANG Y M, LIU H, JIANG Q, PANG G, et al. Activation of serotonin 5-HT2C receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-dependent mice[J]. Neuropharmacology, 2016, 101: 246-254. DOI:10.1016/j.neuropharm.2015.09.031 |
| [2] |
CHEN G, ZHANG Y Q, QADRI Y J, SERHAN C N, JI R R. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain[J]. Neuron, 2018, 100: 1292-1311. DOI:10.1016/j.neuron.2018.11.009 |
| [3] |
ELHABAZI K, AYACHI S, ILIEN B, SIMONIN F. Assessment of morphine-induced hyperalgesia and analgesic tolerance in mice using thermal and mechanical nociceptive modalities[J/OL]. J Vis Exp, 2014(89): e51264. DOI: 10.3791/51264.
|
| [4] |
TRUIN M, VAN KLEEF M, VERBOEKET Y, DEUMENS R, HONIG W, JOOSTEN E A J. The effect of Spinal Cord Stimulation in mice with chronic neuropathic pain after partial ligation of the sciatic nerve[J]. Pain, 2009, 145: 312-318. DOI:10.1016/j.pain.2009.06.034 |
| [5] |
WANG W Y, WANG W, WU H, LI S P, WANG R, DAI L, et al. Microdialysis sampling combined with ultra-high-performance liquid chromatography-tandem mass spectrometry for the determination of geniposide in dialysate of joint cavities in adjuvant arthritis rats[J/OL]. Rapid Commun Mass Spectrom, 2018 (2018-01-05)[2021-09-21]. DOI: 10.1002/rcm.8056.
|
| [6] |
NASHMI R, VELUMIAN A A, CHUNG I, ZHANG L, AGRAWAL S K, FEHLINGS M G. Patch-clamp recordings from white matter glia in thin longitudinal slices of adult rat spinal cord[J]. J Neurosci Methods, 2002, 117: 159-166. DOI:10.1016/S0165-0270(02)00096-1 |
| [7] |
FAN Y C, LIANG X, WANG R R, SONG L. Role of endogenous melatoninergic system in development of hyperalgesia and tolerance induced by chronic morphine administration in rats[J]. Brain Res Bull, 2017, 135: 105-112. DOI:10.1016/j.brainresbull.2017.10.005 |
| [8] |
JIN H, SUN Y T, GUO G Q, CHEN D L, LI Y J, XIAO G P, et al. Spinal TRPC6 channels contributes to morphine-induced antinociceptive tolerance and hyperalgesia in rats[J]. Neurosci Lett, 2017, 639: 138-145. DOI:10.1016/j.neulet.2016.12.062 |
| [9] |
YAN X S, LI F, MAIXNER D W, YADAV R, GAO M, ALI M W, et al. Interleukin-1β released by microglia initiates the enhanced glutamatergic activity in the spinal dorsal horn during paclitaxel-associated acute pain syndrome[J]. Glia, 2019, 67: 482-497. DOI:10.1002/glia.23557 |
| [10] |
ELLIS A, GRACE P M, WIESELER J, FAVRET J, SPRINGER K, SKARDA B, et al. Morphine amplifies mechanical allodynia via TLR4 in a rat model of spinal cord injury[J]. Brain Behav Immun, 2016, 58: 348-356. DOI:10.1016/j.bbi.2016.08.004 |
| [11] |
JURGA A M, ROJEWSKA E, PIOTROWSKA A, MAKUCH W, PILAT D, PRZEWLOCKA B, et al. Blockade of toll-like receptors (TLR2, TLR4) attenuates pain and potentiates buprenorphine analgesia in a rat neuropathic pain model[J/OL]. Neural Plast, 2016, 2016: 5238730. DOI: 10.1155/2016/5238730.
|
| [12] |
YANG H Y, WU Z Y, BIAN J S. Hydrogen sulfide inhibits opioid withdrawal-induced pain sensitization in rats by down-regulation of spinal calcitonin gene-related peptide expression in the spine[J]. Int J Neuropsychopharmacol, 2014, 17: 1387-1395. DOI:10.1017/S1461145714000583 |
| [13] |
ZHOU L J, PENG J Y, XU Y N, ZENG W J, ZHANG J, WEI X, et al. Microglia are indispensable for synaptic plasticity in the spinal dorsal horn and chronic pain[J]. Cell Rep, 2019, 27: 3844-3859. DOI:10.1016/j.celrep.2019.05.087 |
| [14] |
GRAPE S, KIRKHAM K R, FRAUENKNECHT J, ALBRECHT E. Intra-operative analgesia with remifentanil vs. dexmedetomidine: a systematic review and meta-analysis with trial sequential analysis[J]. Anaesthesia, 2019, 74: 793-800. DOI:10.1111/anae.14657 |
| [15] |
CHEN J, LI H, LIM G, MCCABE M F, ZHAO W, YANG Y, et al. Different effects of dexmedetomidine and midazolam on the expression of NR2B and GABAA-α1 following peripheral nerve injury in rats[J]. IUBMB Life, 2018, 70: 143-152. DOI:10.1002/iub.1713 |
| [16] |
LIN J P, CHEN C Q, HUANG L E, LI N N, YANG Y, ZHU S M, et al. Dexmedetomidine attenuates neuropathic pain by inhibiting P2X7R expression and ERK phosphorylation in rats[J]. Exp Neurobiol, 2018, 27: 267-276. DOI:10.5607/en.2018.27.4.267 |
| [17] |
LU Y, LIN B, ZHONG J. The therapeutic effect of dexmedetomidine on rat diabetic neuropathy pain and the mechanism[J]. Biol Pharm Bull, 2017, 40: 1432-1438. DOI:10.1248/bpb.b17-00224 |
| [18] |
SUN Z, ZHAO T, LV S, GAO Y, MASTERS J, WENG H. Dexmedetomidine attenuates spinal cord ischemia-reperfusion injury through both anti-inflammation and anti-apoptosis mechanisms in rabbits[J/OL]. J Transl Med, 2018, 16: 209. DOI: 10.1186/s12967-018-1583-7.
|
 2022, Vol. 43
2022, Vol. 43


