2. 复旦大学附属肿瘤医院中西医结合科,上海 200032;
3. 上海交通大学附属第六人民医院麻醉科,上海 200233;
4. 华南理工大学生物科学与工程学院,广州 510720;
5. 云南省第一人民医院转化医学中心,昆明 650034
2. Department of TCM-WM Integrated Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, China;
3. Department of Anesthesiology, Shanghai Sixth People's Hospital, Shanghai Jiao Tong University, Shanghai 200233, China;
4. School of Biology and Biological Engineering, South China University of Technology, Guangzhou 510720, Guangdong, China;
5. Center for Translational Medicine, The First People's Hospital of Yunnan Province, Kunming 650034, Yunnan, China
压疮又称压力性损伤,是发生于皮肤与皮下软组织的局限性损伤,损伤通常发生于活动受限患者或老年患者的骨隆突处,常与临床医疗及治疗设备的使用相关[1-2]。压疮院内患病率高,危重症患者的压疮患病率常年高于13%[3-5]。国际上将压疮分为6期,长期卧床患者多并发3、4期压疮[6]。3、4期压疮治愈率低、预后差,目前尚无有效的治疗方法,长期发展可能引起脓毒血症甚至危及生命。
干细胞疗法是当前皮肤损伤修复领域的研究热点。人脐带间充质干细胞(human umbilical cord mesenchymal stem cell,hucMSC)因增殖分化能力强、免疫原性低、获取方便、无伦理学争议等优点,是较理想的种子细胞。目前干细胞治疗仍存在体内存活时间短、过分化、致瘤性等问题,临床应用受到限制。干细胞的治疗作用依赖于旁分泌机制释放的生物活性因子[7]。干细胞来源外泌体是干细胞在静息或应激状态下分泌的参与细胞信号传递的细胞外囊泡,可通过传递特定蛋白质、RNA、转录因子等重新编程损伤细胞,促进组织再生。目前,hucMSC来源外泌体(hucMSC-derived exosome,hucMSC-Exo)已被广泛应用于皮肤损伤修复研究[8-10],但尚未见hucMSC-Exo治疗压疮的报道。本研究观察了hucMSC-Exo治疗小鼠4期压疮的疗效,并对其治疗机制进行了初步探索,旨在为压疮治疗提供新思路。
1 材料和方法 1.1 主要试剂及仪器α-MEM培养基、FBS(美国Hyclone公司); Ⅱ型胶原蛋白酶、透明质酸酶、TrypLETM胰蛋白酶替代物(美国Gibco公司); 小鼠抗人CD44-FITC、CD105-藻红蛋白(phycoerythrin,PE)、CD90-PE-Cy5、CD73-PE-Cy7、人类白细胞抗原DR(human leukocyte antigen DR,HLA-DR)-FITC、CD34-PE、CD19-Texas red、CD11b-PE-Cy5、CD45-PE-Cy7抗体(美国Beckman公司); 间充质干细胞诱导分化试剂、去外泌体FBS(以色列Biological Industries公司); CD63抗体(英国Abcam公司); 肿瘤易感基因101(tumor suppressor gene 101,TSG101)抗体、钙联蛋白(calnexin)抗体(美国Proteintech公司); α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)抗体(武汉赛维尔生物科技有限公司); 超氧化物歧化酶(superoxide dismutase,SOD)、丙二醛(malondialdehyde,MDA)检测试剂盒(南京建成生物工程研究所); 圆形铁氧体磁铁(深圳市友达磁铁制品有限公司); 流式细胞仪(美国Beckman公司); CP100NX超速冷冻离心机(日本Hitachi公司); NanoSight纳米粒径分析仪(英国Malvern仪器有限公司); 透射电子显微镜(日本JEOL公司)。
1.2 实验动物8周龄SPF级雄性BALB/c小鼠购于珠海百试通生物科技有限公司[动物生产许可证号SCXK(粤)2020-0051],体重(26±3)g,全部单笼饲养于屏障设施环境中,构建压疮模型前适应性饲养1周。涉及的动物实验在华腾生物医药科技有限公司[动物使用许可证号SYXK(粤)2020-0237]完成,并通过动物伦理委员会批准。
1.3 hucMSC分离培养与鉴定实验用hucMSC采自足月妊娠剖宫产健康新生儿新鲜脐带标本,由佛山复兴禅诚医院产科提供,脐带的采集经过医院伦理委员会审批(CYIRB-LCYJ-2021066-PJ-20210629),产妇签署知情同意书。将24 h内采集的脐带置于0.9%氯化钠溶液中,剥离脐动脉、脐静脉,分离出华通胶(Wharton’s jelly)组织,充分清洗后剪碎成约0.5~1 mm3的组织块,转入离心管中,加入0.2%Ⅱ型胶原酶与透明胶质酶于37 ℃消化1 h,以孔径为70 μm的滤膜过滤,滤液经400×g室温离心5 min,获得细胞沉淀; 加入含10% FBS和1%青霉素-链霉素的α-MEM培养基重悬细胞沉淀,接种于T25培养瓶内,置于37 ℃、5% CO2培养箱中培养(标记为P0代); 每2~3 d换液1次,细胞生长至80%融合时进行传代,每个T75瓶接种4×105~6×105个细胞。第1代(P1代)后各代细胞融合达90%以上时,按1∶3比例分瓶传代,于倒置显微镜下观察细胞形态。第4代(P4代)细胞用于鉴定,第5代(P5代)细胞用于后续实验。
取P4代对数生长期细胞,分别用成骨、成脂及成软骨分化培养基对细胞进行诱导培养,成骨分化培养10 d后行茜素红染色,成脂分化培养至14 d行油红O染色,成软骨分化培养至21 d行阿辛蓝染色,镜下观察分化效果; 通过流式细胞术检测hucMSC表面阳性标志物CD44、CD105、CD90、CD73及阴性标志物CD34、CD19、CD11 b、CD45、HLA-DR表达,采用CXP Cytometer软件分析检测结果。
1.4 hucMSC-Exo提取与鉴定取P5代生长状态良好的hucMSC,当融合度达60%~70%时,弃去培养基,用PBS润洗2~3次,用含10%(体积分数)去外泌体血清的α-MEM培养基培养48 h后收集细胞上清。采用差速离心法提取外泌体,收集的上清以每管40 mL分装,4 ℃条件下300×g离心10 min去除悬浮细胞,收集上清以2 000×g离心20 min去除细胞碎片,再收集上清以10 000×g离心30 min去除大囊泡及凋亡小体,剩余上清经0.22 μm针头滤器过滤后转移至超速离心管中,100 000×g离心2 h,小心去除上清,取适量无菌PBS轻轻重悬离心管底部沉淀,获得外泌体,-80 ℃保存并用于后续实验。
对所提取的外泌体进行形态、群体特征及表面蛋白标志物鉴定。利用透射电子显微镜观察外泌体形态特征; 采用纳米粒子跟踪分析技术观察外泌体布朗运动速率,计算其数目及大小分布; 采用蛋白质印迹法检测外泌体表面蛋白阳性标志物CD63、TSG101及阴性标志物calnexin表达。
1.5 小鼠4期压疮模型制备采用磁铁夹压法制备小鼠4期压疮模型[11-13]。取9只雄性BALB/c小鼠,建模前1 d称重,腹腔注射3%戊巴比妥钠(10 mg/kg)后褪毛,标记操作区域; 建模当日小鼠制动,以背部中线为界轻拉起背部皮肤,用2个圆形铁氧体磁铁板(直径12 mm,厚5.0 mm,平均质量2.4 g,磁力0.1 T)挤压皮肤全层,不挤压肌肉; 以磁铁每挤压12 h后放松12 h为1个循环,共作用3个循环后挤压结束。常规喂养7 d后对创面进行清创,评估建模效果。
4期压疮建模成功标准:大体观全层皮肤及皮下组织缺失,暴露肌层; H-E染色见大量炎症细胞浸润,皮肤、皮下脂肪及肌层变性坏死[1]。
1.6 实验分组及创面干预取27只BALB/c小鼠成功建立4期压疮模型,然后按随机数字表法分为hucMSC-Exo治疗组、PBS对照组及模型对照组,每组9只,清创后1 d给药治疗。hucMSC-Exo治疗组:将hucMSC-Exo沉淀用无菌PBS按1 μg∶1 μL比例充分重悬,用1 mL胰岛素注射器抽吸后,沿创面周围0.5 cm处4个位点均匀皮下注射至创缘及基底,每个位点25 μL,共注射100 μL; PBS对照组:用1 mL胰岛素注射器沿创面周围0.5 cm处4个位点均匀皮下注射100 μL无菌PBS; 模型对照组:清创后未给予任何处理。
1.7 观测指标 1.7.1 创面愈合情况观察治疗后每天常规观察,直至创面愈合。于治疗后0、3、7、10、14 d对创面进行拍照(拍摄时于创面边缘放一最小刻度为1 mm的直尺,镜头与创面平行),用ImageJ图像分析软件计算创面面积(创面面积=长轴半径×短轴径×π)。创面完全被上皮组织或新生皮肤覆盖视为伤口愈合,计算创面愈合率[创面愈合率(%)=(初始创面面积-当日测量创面面积)/初始创面面积×100%]。
1.7.2 组织学观察治疗后7、14 d每组各取3只小鼠,经腹腔麻醉处死后,以创面为中心取15 mm2的正方形皮肤,用4%多聚甲醛溶液室温固定48 h后脱水、石蜡包埋、切片,通过H-E染色观察创面皮肤结构特点、肉芽组织新生及炎症细胞浸润情况,通过Masson染色观察创面胶原纤维沉积及排列情况。
1.7.3 α-SMA蛋白表达观察通过免疫组织化学染色观察创面α-SMA蛋白表达。取治疗后7 d小鼠皮肤组织石蜡包埋切片,脱蜡至水,经EDTA修复抗原,双氧水阻断内源性过氧化物酶,血清室温封闭,加入一抗4 ℃孵育过夜,加入二抗(HRP标记)37 ℃孵育1 h,DAB显色,苏木精复染细胞核,脱水、透明后封片,镜下观察。
1.7.4 SOD、MDA测定于治疗后7、14 d,每组分别取6只小鼠腹腔麻醉后经眼静脉丛取血,室温下1 000×g离心15 min后取血清,通过黄嘌呤氧化酶法(羟胺法)测定SOD活力,硫代巴比妥酸比色法测定MDA含量,参考试剂盒说明书进行操作。
1.8 统计学处理采用GraphPad Prism 7软件进行统计学分析。计量资料以x±s表示,组间比较采用单因素方差分析,两两比较采用最小显著性差异法。检验水准(α)为0.05。
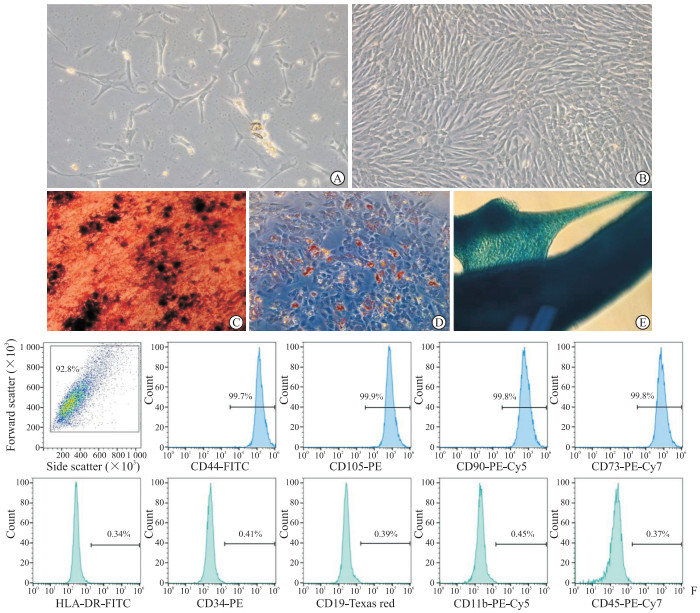
2 结果 2.1 hucMSC体外培养与鉴定结果P0代细胞培养48 h后可见少数贴壁,呈短棒状或多角形; 72 h后贴壁完全,细胞多呈长梭形; 随后梭形贴壁细胞逐渐增多,6~7 d形成细胞集落(图 1A); 10~12 d细胞融合度可达80%以上。传代至P5代后,细胞为大小相对均一的梭形,呈平行排列或漩涡状生长(图 1B),符合间充质干细胞的形态特征。
|
图 1 hucMSC培养及鉴定结果 Fig 1 Culture and identification of hucMSCs A: Morphological observation of passage 0 hucMSCs (100×); B: Morphological observation of passage 5 hucMSCs (100×); C: Alizarin red staining after osteogenic induction for 10 d (200×); D: Oil red O staining after adipogenic induction for 14 d (200×); E: Alcian blue staining after chondrogenic induction for 21 d (200×); F: Expression of extracted CD44+, CD105+, CD90+, CD73+, HLA-DR-, CD34-, CD19-, CD11b- and CD45- of hucMSCs identified by flow cytometry. hucMSC: Human umbilical cord mesenchymal stem cell; FITC: Fluorescein isothiocyanate; PE: Phycoerythrin; HLA-DR: Human leukocyte antigen DR. |
成骨诱导培养至10 d,显微镜下可见钙结节,茜素红染色后呈深红色(图 1C); 成脂诱导培养至14 d,细胞周围出现环形脂滴,油红O染色后呈红色串珠样(图 1D); 成软骨诱导培养至21 d,显微镜下可见球状细胞团,阿辛蓝染色后沉积的蛋白多糖呈蓝色(图 1E)。综上观察结果提示,所培养的细胞具备多向分化潜能。流式细胞术鉴定hucMSC表面标志物结果(图 1F)显示,CD44、CD105、CD90、CD73均为阳性表达(>95.0%),HLA-DR、CD34、CD19、CD11b、CD45均为阴性表达(<2.0%),符合《临床研究用人脐带来源间充质干细胞制剂规范》[14]。
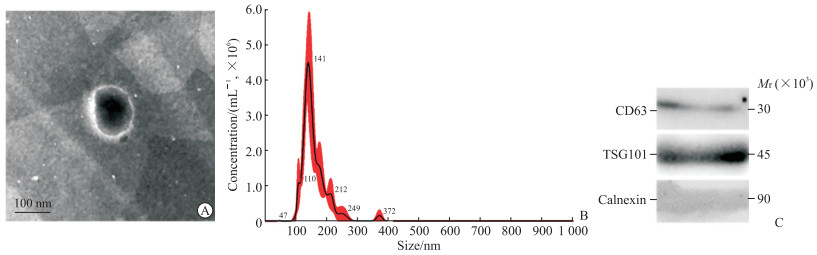
2.2 hucMSC-Exo的获取和鉴定结果hucMSC培养至P5代,细胞数量达4×108,收集细胞上清1 200 mL,通过差速离心法提取约2 mg外泌体。透射电镜观察所提取的外泌体直径为30~150 nm,结构完整,具有典型双层膜结构,形态呈类圆形杯盘状(图 2A); 粒径分析结果显示,外泌体直径主峰为141 nm,浓度为(2.41±0.37)×108/mL(图 2B); 蛋白质印迹法检测到提取的外泌体表达CD63、TSG101,不表达内质网蛋白calnexin(图 2C)。综上鉴定结果显示,提取的外泌体符合标准特征。
|
图 2 hucMSC-Exo鉴定结果 Fig 2 Identification of hucMSC-Exo A: Morphology of hucMSC-Exo observed by transmission electron microscopy; B: Particle size and diameter distribution of hucMSC-Exo measured by qNano analysis; C: Western blotting showed the presence of exosomal markers CD63 and TSG101, but exosomes were negative for calnexin. hucMSC-Exo: Human umbilical cord mesenchymal stem cell-derived exosome; TSG101: Tumor suppressor gene 101. |
2.3 BALB/c小鼠4期压疮模型成功制备
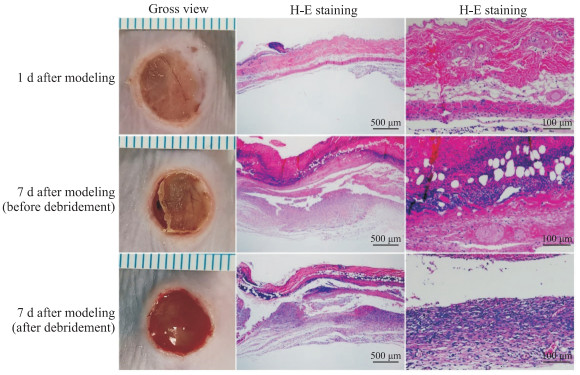
小鼠建模后1 d,大体观背部挤压区域出现同磁铁大小一致的缺血创面,皮肤全层明显变薄; H-E染色结果显示组织大范围损伤,皮肤及皮下组织局部坏死,伴少量炎症细胞浸润。建模后7 d,大体观清创前创面被坏死组织及焦痂覆盖,边缘内卷,肌层部分暴露; 清创后创面全层皮肤及皮下组织缺失、肌层完全暴露; H-E染色显示清创前皮肤全层坏死、部分脱落,清创后皮肤全层完全脱落,均伴肌层、肌层下结缔组织坏死及大量炎性物渗出。见图 3。上述观察结果提示,建模后7 d,小鼠4期压疮模型制备成功。
|
图 3 BALB/c小鼠4期压疮模型制备 Fig 3 Preparation of BALB/c mouse model with stage 4 pressure ulcers Gross view (scale: 1 mm per grid) and H-E staining were used to observe the skin damage 1 and 7 d after modeling. The mouse model with stage 4 pressure ulcers was successfully established 7 d after modeling. H-E: Hematoxylin-eosin. |
2.4 hucMSC-Exo促进压疮创面愈合 2.4.1 hucMSC-Exo加速压疮创面愈合
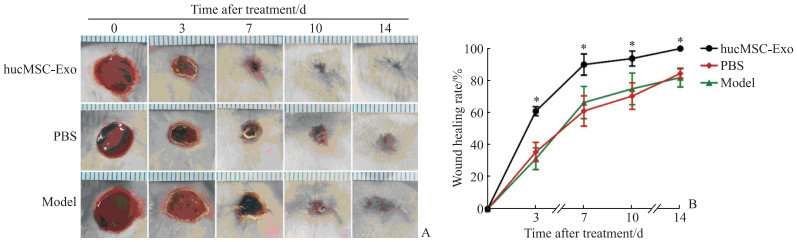
治疗后0 d,各组小鼠压疮创面无明显差异。3 d时,hucMSC-Exo治疗组创面覆盖薄痂,痂皮内卷,创面干燥无渗出; PBS对照组及模型对照组小鼠创面可见少许炎性渗出,痂皮覆盖不完全。7 d时,hucMSC-Exo治疗组小鼠痂皮脱落,新生上皮组织形成,残余创面最小; PBS对照组及模型对照组小鼠创面仍覆盖焦痂,炎症反应明显。10 d时,hucMSC-Exo治疗组小鼠创面已基本愈合,边缘毛发生长; PBS对照组及模型对照组小鼠创面仍有少许焦痂覆盖,创面肉芽组织生长。14 d时,hucMSC-Exo治疗组小鼠创面愈合,皮肤颜色接近正常; PBS对照组及模型对照组小鼠创面呈瘢痕愈合,创面发红。统计学分析结果显示,治疗后3、7、10、14 d时,hucMSC-Exo治疗组小鼠创面愈合率均高于PBS对照组及模型对照组(P均<0.05),PBS对照组与模型对照组之间愈合率差异无统计学意义(P均>0.05)。见图 4。
|
图 4 治疗后各组小鼠创面愈合大体观及创面愈合率分析 Fig 4 Gross view of wound healing and wound healing rate of mice after treatment in each group A: Gross view (scale: 1 mm per grid); B: The wound healing rate. *P < 0.05 vs PBS and model groups at the same time point. n=6, x±s. hucMSC-Exo: Human umbilical cord mesenchymal stem cell-derived exosome; PBS: Phosphate-buffered saline. |
2.4.2 hucMSC-Exo减轻压疮创面皮肤病理损伤及炎症细胞浸润
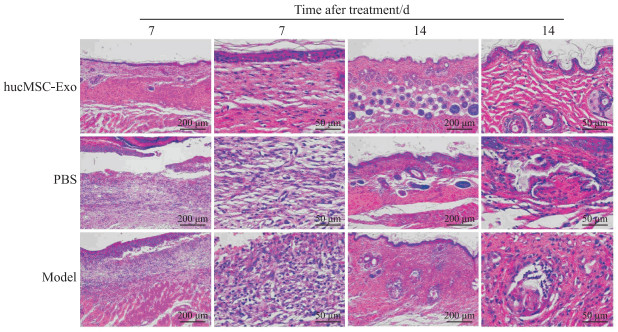
H-E染色结果显示,治疗后7 d,hucMSC-Exo治疗组小鼠压疮处表皮完整,小范围轻度增厚,真皮层毛囊及皮脂腺减少,未见其他明显异常; PBS对照组与模型对照组小鼠压疮处皮肤仍见大范围损伤,表皮增厚、断裂、局部与真皮分离,损伤处未见真皮层胶原、毛囊、皮脂腺及皮下脂肪,可见大量新生肉芽组织,伴较多炎症细胞浸润。治疗后14 d,各组小鼠表皮均完整,其中hucMSC-Exo治疗组小鼠皮肤各层结构清晰,厚度均匀,真皮层胶原、毛囊及皮脂腺丰富,形态正常,组织未见明显异常; PBS对照组及模型对照组小鼠局部表皮增厚,可见散在毛囊、皮脂腺结构,细胞排列紊乱,肌层肌细胞坏死,伴少量炎症细胞浸润。见图 5。
|
图 5 治疗后各组小鼠创面组织H-E染色结果 Fig 5 H-E staining of wound tissues of mice after treatment in each group H-E: Hematoxylin-eosin; hucMSC-Exo: Human umbilical cord mesenchymal stem cell-derived exosome; PBS: Phosphate-buffered saline. |
2.4.3 hucMSC-Exo促进压疮创面胶原沉积及重组
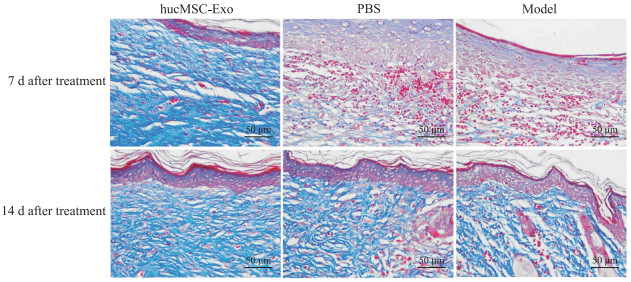
Masson染色结果显示,治疗后7 d,hucMSC-Exo治疗组小鼠压疮处可见真皮层大量胶原沉积,排列较疏松; PBS对照组及模型对照组小鼠压疮处皮肤真皮层胶原较少,着色浅且分布不均。治疗后14 d,各组小鼠压疮处皮肤均可见大量胶原沉积,其中hucMSC-Exo治疗组胶原排列规律,分布均一; PBS对照组及模型对照组胶原排列紊乱,胶原纤维纵横交错、粗细不均。见图 6。
|
图 6 治疗后各组小鼠创面组织Masson染色结果 Fig 6 Masson staining of wound tissues of mice after treatment in each group hucMSC-Exo: Human umbilical cord mesenchymal stem cell-derived exosome; PBS: Phosphate-buffered saline. |
2.5 hucMSC-Exo治疗压疮创面愈合的机制 2.5.1 hucMSC-Exo可降低压疮创面α-SMA蛋白表达
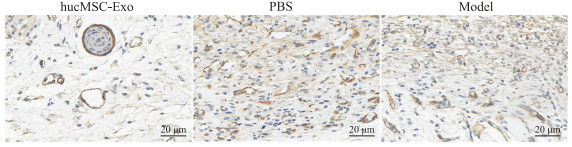
免疫组织化学染色结果显示,治疗后7 d,各组小鼠创面组织细胞质中均可见α-SMA表达,呈棕黄色环状或斑点样聚合。与PBS对照组及模型对照组比较,hucMSC-Exo治疗组α-SMA蛋白着色较浅,阳性表达明显减少。见图 7。
|
图 7 治疗后7 d各组小鼠创面组织α-SMA蛋白表达 Fig 7 Expression of α-SMA protein in wound tissues of mice on day 7 after treatment in each group Immunohistochemistry staining. SMA: α-smooth muscle actin; hucMSC-Exo: Human umbilical cord mesenchymal stem cell-derived exosome; PBS: Phosphate-buffered saline. |
2.5.2 hucMSC-Exo可改善体内氧化应激水平
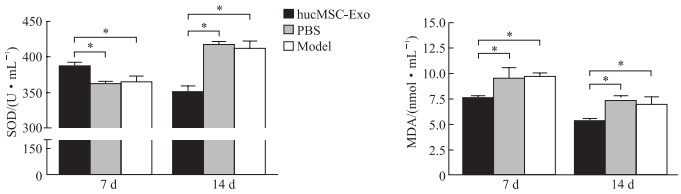
治疗后7 d,hucMSC-Exo治疗组小鼠血清SOD活力较PBS对照组与模型对照组升高(P均<0.05); 治疗后14 d,hucMSC-Exo治疗组小鼠SOD活力下降至接近正常水平,而PBS对照组及模型对照组SOD活力上升且高于hucMSC-Exo治疗组(P均<0.05)。治疗后7 d,hucMSC-Exo治疗组MDA含量低于PBS对照组和模型对照组(P均<0.05); 治疗后14 d,各组MDA含量均下降,但PBS对照组与模型对照组仍高于hucMSC-Exo治疗组(P均<0.05)。治疗后各时间点PBS对照组与模型对照组之间SOD活力和MDA含量差异均无统计学意义(P均>0.05)。见图 8。
|
图 8 治疗后各组小鼠血清SOD活力及MDA含量变化 Fig 8 Changes of serum SOD activity and MDA content of mice after treatment in each group *P < 0.05. n=6, x±s. SOD: Superoxide dismutase; MDA: Malondialdehyde; hucMSC-Exo: Human umbilical cord mesenchymal stem cell-derived exosome; PBS: Phosphate-buffered saline. |
3 讨论
压疮是临床常见的皮肤损伤疾病,目前常用的治疗方法有中医药疗法、湿性愈合敷料、负压封闭引流技术、皮肤再生医疗技术等,但均存在治愈率较低、易复发、治疗时间长等问题,一旦发生压疮,患者住院时间延长、生活质量下降,给家庭带来巨大经济负担的同时也大大增加了医疗资源消耗。因此,寻找新型有效的压疮治疗方法非常重要。
近年来,细胞相关疗法飞速发展,作为一种新型治疗方式,其临床转化潜力引起科学界极大关注。细胞外囊泡是细胞分泌的具有磷脂双分子层的膜结合囊泡,根据细胞起源和生物学发生可将其分为微囊泡、外泌体、凋亡小体及其他亚群[15]。其中外泌体是细胞多囊泡体与质膜融合分泌的直径为30~150 nm、大小均一的细胞外囊泡,其内含特异脂质、蛋白质、核酸等多种成分,能特异性靶向受体细胞,调节细胞间通讯及信号转导。当前应用的外泌体分离手段很难获得高纯度且单一的样品,尽管细胞外囊泡和外泌体是通过不同机制产生,但它们大小、内容物存在重叠,目前尚无可独立证明外泌体存在的方法[16]。本研究采用的hucMSC-Exo指代与外泌体生物学形态、大小相符,且鉴定结果满足2018年国际囊泡协会颁布的最新指南要求的细胞外囊泡[17]。
伤口愈合包括凝血、炎症、增殖和重塑4个阶段。目前已有研究证明hucMSC-Exo可在伤口愈合各阶段发挥作用:在凝血期,hucMSC-Exo可通过释放磷脂酰丝氨酸促进血液凝固; 在炎症期,hucMSC-Exo可分泌多种细胞因子,促进炎症细胞渗出,加快成纤维细胞及血管内皮细胞增生; 在增殖期,hucMSC-Exo可进一步促进成纤维细胞增殖、肉芽组织形成及新生血管生成; 在重塑期,hucMSC-Exo可加快再上皮化进程,促进胶原蛋白重组,进而促进伤口愈合、减少瘢痕形成[18-22]。针对多种啮齿类动物模型的安全性实验证明,hucMSC-Exo无免疫原性、致瘤性、栓塞等风险,静脉滴注400 μg hucMSC-Exo不会导致肝肾功能异常、肌肉和血管刺激、全身过敏反应等并发症[23]。因此,hucMSC-Exo有望成为压疮治疗的新方法。本研究采用皮下注射100 μg hucMSC-Exo治疗小鼠4期压疮,与PBS对照组及模型对照组比较,hucMSC-Exo治疗组创面愈合速度加快,愈合质量提高,瘢痕面积较小,到治疗后14 d时hucMSC-Exo治疗组小鼠新生皮肤已同周围正常皮肤无明显差异。
伤口愈合过程中成纤维细胞增生、细胞外基质合成速率高于降解速率、创面炎症等因素共同作用会导致病理性瘢痕形成[24]。α-SMA是肌成纤维细胞的特异性骨架蛋白,可通过调节细胞骨架构象影响肌成纤维细胞增殖,被认为是肌成纤维细胞收缩的标志,也是决定瘢痕形成的关键[25-26]。大量研究表明,伤口愈合初期α-SMA表达增高有利于缩小细胞间距,促进伤口闭合; 到后期组织中α-SMA表达降低可减少肉芽组织血管生成,促进胶原重组,进而减少瘢痕形成[27-29]。研究人员通过高通量测序确定了一组包括miRNA-21、miRNA-23a、miRNA-125b和miRNA-145在内的hucMSC-Exo特异性miRNA,这些miRNA可通过介导TGF-β/Smad2信号通路,抑制α-SMA表达及胶原过量沉积,减少瘢痕形成[21]。同既往报道一致,本研究的免疫组织化学染色结果显示治疗后7 d hucMSC-Exo治疗组α-SMA蛋白表达明显低于PBS对照组及模型对照组。结合大体观及组织学观察结果可知,hucMSC-Exo治疗可减少压疮创面瘢痕增生、缩小瘢痕面积。
缺血再灌注损伤机制是目前公认引起压疮最重要的发病机制,氧化应激在压疮形成中起重要推动作用。间充质干细胞来源外泌体已被证明可通过多种途径改善氧化应激所致损伤[30]。在外力作用下,局部组织长期受压可导致组织灌注减少,而反复缺血-再灌注会引起微循环受损,组织抗氧化能力下降,引发过度氧化应激反应,致使大量氧自由基释放及相关代谢产物堆积,进一步加剧组织损伤[31-33]。研究表明,hucMSC-Exo可通过释放谷胱甘肽过氧化酶1诱导ERK1/2磷酸化及Bcl-2表达,进而抑制核因子κB抑制因子(inhibitor of nuclear factor κB,IκB)/NF-κB/caspase信号通路的激活,减轻氧化应激诱导的细胞凋亡[34]。此外,hucMSC-Exo释放的14-3-3ζ蛋白可通过调节沉默信息调节因子1依赖的抗氧化途径,抑制炎症反应,发挥细胞保护功能[35]。SOD是体内重要的抗氧化酶,能及时清除氧自由基,保护细胞免受损伤,从而促进伤口愈合[36]。MDA则是脂质过氧化反应的产物,其含量可反映组织内脂质过氧化情况及组织含氧自由基水平,与损伤程度成正比[37]。本研究中,hucMSC-Exo治疗组小鼠血清SOD活力在治疗后7 d高于PBS对照组及模型对照组并且到14 d时接近正常水平,而MDA含量始终低于PBS对照组及模型对照组,表明hucMSC-Exo可通过改善氧化应激水平促进小鼠压疮创面愈合。
综上所述,hucMSC-Exo可促进小鼠压疮创面愈合,减少瘢痕形成,其机制可能与改善体内氧化应激水平有关。下一步我们将研究hucMSC-Exo调控氧化应激的信号通路,从而进一步探讨hucMSC-Exo治疗压疮的机制。
| [1] |
KOTTNER J, CUDDIGAN J, CARVILLE K, BALZER K, BERLOWITZ D, LAW S, et al. Prevention and treatment of pressure ulcers/injuries: the protocol for the second update of the international clinical practice guideline 2019[J]. J Tissue Viability, 2019, 28: 51-58. DOI:10.1016/j.jtv.2019.01.001 |
| [2] |
LIU L, MOODY J, GALL A. A quantitative, pooled analysis and systematic review of controlled trials on the impact of electrical stimulation settings and placement on pressure ulcer healing rates in persons with spinal cord injuries[J]. Ostomy Wound Manage, 2016, 62: 16-34. |
| [3] |
LIU Y, WU X J, MA Y F, LI Z, CAO J, JIAO J, et al. The prevalence, incidence, and associated factors of pressure injuries among immobile inpatients: a multicentre, cross-sectional, exploratory descriptive study in China[J]. Int Wound J, 2019, 16: 459-466. DOI:10.1111/iwj.13054 |
| [4] |
JAKOBSEN T B T, PITTURERI C, SEGANTI P, BORISSOVA E, BALZANI I, FABBRI S, et al. Incidence and prevalence of pressure ulcers in cancer patients admitted to hospice: a multicentre prospective cohort study[J]. Int Wound J, 2020, 17: 641-649. DOI:10.1111/iwj.13317 |
| [5] |
ARTICO M, PIREDDA M, D'ANGELO D, LUSIGNANI M, GIANNARELLI D, MARCHETTI A, et al. Prevalence, incidence and associated factors of pressure injuries in hospices: a multicentre prospective longitudinal study[J/OL]. Int J Nurs Stud, 2020, 111: 103760. DOI: 10.1016/j.ijnurstu.2020.103760.
|
| [6] |
ARTICO M, DANTE A, D'ANGELO D, LAMARCA L, MASTROIANNI C, PETITTI T, et al. Prevalence, incidence and associated factors of pressure ulcers in home palliative care patients: a retrospective chart review[J]. Palliat Med, 2018, 32: 299-307. DOI:10.1177/0269216317737671 |
| [7] |
LIANG X T, DING Y, ZHANG Y L, TSE H F, LIAN Q Z. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives[J]. Cell Transplant, 2014, 23: 1045-1059. DOI:10.3727/096368913X667709 |
| [8] |
YANG J Y, CHEN Z Y, PAN D Y, LI H Z, SHEN J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration[J]. Int J Nanomedicine, 2020, 15: 5911-5926. DOI:10.2147/IJN.S249129 |
| [9] |
LI Q J, GONG S Q, YAO W F, YANG Z T, WANG R J, YU Z J, et al. Exosome loaded genipin crosslinked hydrogel facilitates full thickness cutaneous wound healing in rat animal model[J]. Drug Deliv, 2021, 28: 884-893. DOI:10.1080/10717544.2021.1912210 |
| [10] |
SHI H, XU X, ZHANG B, XU J H, PAN Z J, GONG A H, et al. 3, 3'-Diindolylmethane stimulates exosomal Wnt11 autocrine signaling in human umbilical cord mesenchymal stem cells to enhance wound healing[J]. Theranostics, 2017, 7: 1674-1688. DOI:10.7150/thno.18082 |
| [11] |
STADLER I, ZHANG R Y, OSKOUI P, WHITTAKER M S, LANZAFAME R J. Development of a simple, noninvasive, clinically relevant model of pressure ulcers in the mouse[J]. J Invest Surg, 2004, 17: 221-227. DOI:10.1080/08941930490472046 |
| [12] |
SAITO Y, HASEGAWA M, FUJIMOTO M, MATSUSHITA T, HORIKAWA M, TAKENAKA M, et al. The loss of MCP-1 attenuates cutaneous ischemia-reperfusion injury in a mouse model of pressure ulcer[J]. J Invest Dermatol, 2008, 128: 1838-1851. DOI:10.1038/sj.jid.5701258 |
| [13] |
SEKIGUCHI A, MOTEGI S I, UCHIYAMA A, UEHARA A, FUJIWARA C, YAMAZAKI S, et al. Botulinum toxin B suppresses the pressure ulcer formation in cutaneous ischemia-reperfusion injury mouse model: possible regulation of oxidative and endoplasmic Reticulum stress[J]. J Dermatol Sci, 2018, 90: 144-153. DOI:10.1016/j.jdermsci.2018.01.006 |
| [14] |
深圳市细胞治疗技术协会. SZTT/SSCT 002-2018: 临床研究用人脐带来源间充质干细胞制剂规范[S/OL]. (2018-06-22) [2022-03-30]. http://szbz.sist.org.cn/Home/Detail?gid=ee92dcf6-8c4b-4ae8-aa52-99a2ddeeff12.
|
| [15] |
LÖTVALL J, HILL A F, HOCHBERG F, BUZÁS E I, DI VIZIO D, GARDINER C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles[J/OL]. J Extracell Vesicles, 2014, 3: 26913. DOI: 10.3402/jev.v3.26913.
|
| [16] |
LAWSON C, VICENCIO J M, YELLON D M, DAVIDSON S M. Microvesicles and exosomes: new players in metabolic and cardiovascular disease[J/OL]. J Endocrinol, 2016, 228: R57-R71. DOI: 10.1530/JOE-15-0201.
|
| [17] |
THÉRY C, WITWER K W, AIKAWA E, ALCARAZ M J, ANDERSON J D, ANDRIANTSITOHAINA R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines[J/OL]. J Extracell Vesicles, 2018, 7: 1535750. DOI: 10.1080/20013078.2018.1535750.
|
| [18] |
BAKHTYAR N, JESCHKE M G, HERER E, SHEIKHOLESLAM M, AMINI-NIK S. Exosomes from acellular Wharton's jelly of the human umbilical cord promotes skin wound healing[J/OL]. Stem Cell Res Ther, 2018, 9: 193. DOI: 10.1186/s13287-018-0921-2.
|
| [19] |
SILACHEV D N, GORYUNOV K V, SHPILYUK M A, BEZNOSCHENKO O S, MOROZOVA N Y, KRAEVAYA E E, et al. Effect of MSCs and MSC-derived extracellular vesicles on human blood coagulation[J/OL]. Cells, 2019, 8: 258. DOI: 10.3390/cells8030258.
|
| [20] |
ZHANG X F, WANG T, WANG Z X, HUANG K P, ZHANG Y W, WANG G L, et al. Hypoxic ucMSC-secreted exosomal miR-125b promotes endothelial cell survival and migration during wound healing by targeting TP53INP1[J]. Mol Ther Nucleic Acids, 2021, 26: 347-359. DOI:10.1016/j.omtn.2021.07.014 |
| [21] |
FANG S, XU C, ZHANG Y T, XUE C Y, YANG C, BI H D, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing[J]. Stem Cells Transl Med, 2016, 5: 1425-1439. DOI:10.5966/sctm.2015-0367 |
| [22] |
LIU J W, YAN Z X, YANG F J, HUANG Y, YU Y, ZHOU L P, et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate cutaneous wound healing by enhancing angiogenesis through delivering angiopoietin-2[J]. Stem Cell Rev Rep, 2021, 17: 305-317. DOI:10.1007/s12015-020-09992-7 |
| [23] |
SUN L, XU R M, SUN X X, DUAN Y P, HAN Y W, ZHAO Y Y, et al. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell[J]. Cytotherapy, 2016, 18: 413-422. DOI:10.1016/j.jcyt.2015.11.018 |
| [24] |
VARKEY M, DING J, TREDGET E E. Differential collagen-glycosaminoglycan matrix remodeling by superficial and deep dermal fibroblasts: potential therapeutic targets for hypertrophic scar[J]. Biomaterials, 2011, 32: 7581-7591. DOI:10.1016/j.biomaterials.2011.06.070 |
| [25] |
HARIHARAN S, KELM R J Jr, STRAUCH A R. The Purα/Purβ single-strand DNA-binding proteins attenuate smooth-muscle actin gene transactivation in myofibroblasts[J]. J Cell Physiol, 2014, 229: 1256-1271. DOI:10.1002/jcp.24564 |
| [26] |
SHINDE A V, HUMERES C, FRANGOGIANNIS N G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling[J]. Biochim Biophys Acta BBA Mol Basis Dis, 2017, 1863: 298-309. DOI:10.1016/j.bbadis.2016.11.006 |
| [27] |
CHU H H, WANG Y P, WANG X C, SONG X H, LIU H Q, LI X. Effects of transplanted adipose derived stem cells on the expressions of α-SMA and DCN in fibroblasts of hypertrophic scar tissues in rabbit ears[J]. Exp Ther Med, 2018, 16: 1729-1734. |
| [28] |
PUTRA A, ALIF I, HAMRA N, SANTOSA O, KUSTIYAH A R, MUHAR A M, et al. MSC-released TGF-β regulate α-SMA expression of myofibroblast during wound healing[J]. J Stem Cells Regen Med, 2020, 16: 73-79. DOI:10.46582/jsrm.1602011 |
| [29] |
ELBIALY Z I, ASSAR D H, ABDELNABY A, ASA S A, ABDELHIEE E Y, IBRAHIM S S, et al. Healing potential of Spirulina platensis for skin wounds by modulating bFGF, VEGF, TGF-ß1 and α-SMA genes expression targeting angiogenesis and scar tissue formation in the rat model[J/OL]. Biomed Pharmacother, 2021, 137: 111349. DOI: 10.1016/j.biopha.2021.111349.
|
| [30] |
翟骁, 王一然, 朱明向, 王奇金. 间充质干细胞来源外泌体在氧化应激损伤中的研究进展[J]. 第二军医大学学报, 2018, 39: 716-721. ZHAI X, WANG Y R, ZHU M X, WANG Q J. Research progress of mesenchymal stem cell-derived exosomes in oxidative stress injury[J]. Acad J Sec Mil Med Univ, 2018, 39: 716-721. |
| [31] |
WANG Y Y, PU L H, LI Z, HU X Y, JIANG L P. Hypoxia-inducible factor-1α gene expression and apoptosis in ischemia-reperfusion injury: a rat model of early-stage pressure ulcer[J]. Nurs Res, 2016, 65: 35-46. DOI:10.1097/NNR.0000000000000132 |
| [32] |
DONATO-TRANCOSO A, MONTE-ALTO-COSTA A, ROMANA-SOUZA B. Olive oil-induced reduction of oxidative damage and inflammation promotes wound healing of pressure ulcers in mice[J]. J Dermatol Sci, 2016, 83: 60-69. DOI:10.1016/j.jdermsci.2016.03.012 |
| [33] |
陈赟. 牡丹叶湿敷对Ⅲ期压疮抗氧化机制研究[D]. 广州: 广州中医药大学, 2015.
|
| [34] |
YAN Y M, JIANG W Q, TAN Y W, ZOU S Q, ZHANG H G, MAO F, et al. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury[J]. Mol Ther, 2017, 25: 465-479. DOI:10.1016/j.ymthe.2016.11.019 |
| [35] |
WU P P, ZHANG B, HAN X Y, SUN Y X, SUN Z X, LI L L, et al. HucMSC exosome-delivered 14-3-3ζ alleviates ultraviolet radiation-induced photodamage via SIRT1 pathway modulation[J]. Aging (Albany NY), 2021, 13: 11542-11563. |
| [36] |
ZHANG L, MA Y N, PAN X C, CHEN S Y, ZHUANG H H, WANG S F. A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing[J]. Carbohydr Polym, 2018, 180: 168-174. DOI:10.1016/j.carbpol.2017.10.036 |
| [37] |
ERSEL M, UYANIKGIL Y, KARBEK AKARCA F, OZCETE E, ALTUNCI Y A, KARABEY F, et al. Effects of silk sericin on incision wound healing in a dorsal skin flap wound healing rat model[J]. Med Sci Monit, 2016, 22: 1064-1078. DOI:10.12659/MSM.897981 |
 2022, Vol. 43
2022, Vol. 43


