2. 口腔疾病与生物医学重庆市重点实验室,重庆 401147;
3. 重庆市高校市级口腔生物医学工程重点实验室,重庆 401147
2. Chongqing Key Laboratory of Oral Diseases and Biomedical Sciences, Chongqing 401147, China;
3. Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education, Chongqing 401147, China
糖尿病是一种以血糖升高为特征的慢性代谢性疾病[1]。葡萄糖会通过多种机制对细胞特性产生不利影响,晚期糖基化终产物(advanced glycation end product,AGE)不可逆的形成和沉积可能是糖尿病性骨病发病的重要机制。糖尿病患者体内的高血糖及各种氧化应激反应都会加速AGE在组织中的形成和积累[2],对许多组织器官的生物物理特性和功能产生负面影响,如皮肤、骨骼和血管等[3-4]。已有研究证实,AGE在成骨细胞内外聚集均能破坏细胞功能[5],但其相关机制仍未明确。
研究表明,在糖尿病患者中,AGE与其受体结合后经过一系列信号转导通路参与介导细胞骨架的变化,而肌动蛋白重塑可调节Yes相关蛋白(Yes-associated protein,YAP)/PDZ结合基序转录共激活因子(transcriptional coactivator with PDZ-binding motif,TAZ)的活性[6]。YAP是Hippo信号转导通路的主要效应分子,以往对Hippo通路的研究多集中在肿瘤的发生、发展等方面,近年研究表明YAP在成骨细胞分化中也起着十分重要的作用[7-8]。YAP与β-联蛋白(β-catenin)在基因和功能上存在诸多相互作用,研究表明YAP可通过促进β-联蛋白入核及维持其核内水平促进间充质细胞成骨分化[9]。本研究拟通过观察AGE对小鼠胚胎成骨细胞(MC3T3-E1细胞)增殖和成骨分化的影响,以及细胞骨架蛋白纤丝状肌动蛋白(filamentous actin,F-actin)、YAP和β-联蛋白在此过程中的变化,为糖尿病性骨病的预防和治疗提供新思路。
1 材料和方法 1.1 细胞和试剂小鼠胚胎成骨细胞系MC3T3-E1细胞购自中国科学院上海细胞库。FBS购自乌拉圭Lonsera公司;MEM-α培养基购自美国HyClone公司;AGE蛋白质购自北京博奥森生物技术有限公司;CCK-8试剂盒、膜联蛋白Ⅴ-FITC细胞凋亡检测试剂盒、氯化硝基四氮唑蓝/5-溴-4-氯-3-吲哚基磷酸盐(nitrotetrazolium blue chloride/5-bromo-4-chloro-3-indolyl phosphate,NBT/BCIP)碱性磷酸酶(alkaline phosphatase,ALP)染色试剂盒、BCA蛋白质定量试剂盒、HRP标记的二抗(山羊抗兔IgG)均购自上海碧云天生物技术有限公司;罗丹明-鬼笔环肽染色液购自北京索莱宝科技有限公司;兔YAP单克隆抗体、兔β-联蛋白单克隆抗体、兔GAPDH单克隆抗体均购自美国Cell Signaling Technology公司;SYBR Green PCR预混液试剂盒购自德国Qiagen公司;反转录试剂盒和萤光素酶活性检测试剂盒均购自美国Promega公司。
1.2 细胞培养MC3T3-E1细胞用含10% FBS、1%青霉素/链霉素的MEM-α培养基于5% CO2、37 ℃培养箱中培养。每2~3 d换液1次,培养至细胞融合度为95%时用0.25%胰酶消化、传代。
1.3 CCK-8法测定细胞增殖活性将细胞接种于96孔板,常规培养24 h后吸弃原培养基并用PBS洗涤,于各孔中加入含100 mg/L牛血清白蛋白或含不同质量浓度(0、100、200、300 mg/L)AGE的MEM-α培养基继续培养,每组设置3个复孔。分别于培养12、24、36、48、60 h后行CCK-8检测。每孔加入10 μL CCK-8反应液和100 μL培养基,孵育1 h后用酶标仪测定450 nm波长处的光密度(D450)值。以时间为横坐标、D450为纵坐标绘制生长曲线。
1.4 流式细胞术检测细胞凋亡用100、200、300 mg/L的AGE处理细胞24 h后,将细胞消化、离心,弃上清,加入195 μL膜联蛋白Ⅴ-FITC结合液轻轻重悬细胞,随后依次加入5 μL膜联蛋白Ⅴ-FITC结合液和10 μL PI染色液,室温避光孵育20 min后上机检测。
1.5 ALP染色观察细胞成骨能力细胞用不含AGE或含100 mg/L AGE的成骨诱导培养基(加入0.1 μmol/L地塞米松、10 mmol/L β-甘油磷酸钠、50 mg/L抗坏血酸的MEM-α培养基)培养7 d后,吸弃细胞培养基并用PBS清洗,室温下用4%甲醛固定液固定细胞15 min;吸弃甲醛固定液,用PBS清洗,加入NBT/BCIP ALP染色剂,室温下孵育2 h,置于显微镜下观察。
1.6 qPCR测定成骨相关基因、YAP和β-联蛋白的mRNA表达细胞用不含AGE或含100 mg/L AGE的成骨诱导培养基培养7 d后检测成骨相关基因骨钙素、ALP、Runx2的mRNA表达。细胞用不含AGE或含100 mg/L AGE的常规培养基培养24 h后检测YAP和β-联蛋白的mRNA表达。按照TRIzol试剂盒说明书提取细胞总RNA,按照反转录试剂盒的操作说明书将RNA反转录成cDNA,然后用SYBR Green PCR预混液试剂盒进行实时PCR扩增,反应体系为20 μL。采用荧光qPCR仪(型号CFX Connect,美国Bio-Rad公司)检测和记录数据,结果由仪器自带数据分析软件CFX Maestro 2.0自动计算获得。各基因引物均由生工生物工程(上海)股份有限公司进行合成及质量检测。引物序列如下:骨钙素上游引物序列为5'-AGCAGCTTGGCCCAGACCTA-3',下游引物序列为5'-TAGCGCCGGAGTCTGTTCACTAC-3';ALP上游引物序列为5'-TGGCTCTGCCTTTATTC- CCTAGT-3',下游引物序列为5'-AAATAAGGTGC- TTTGGGAATCTGT-3';Runx2上游引物序列为5'-TGCAAGCAGTATTTACAACAGAGG-3',下游引物序列为5'-GGCTCACGTCGCTCATCTT-3';YAP上游引物序列为5'-TGAGATCCCTGATGATG-TACCAC-3',下游引物序列为5'-TGTTGTTGTCT-GATCGTTGTGAT-3';β-联蛋白上游引物序列为5'-TGGAGCCGGACAGAAAAGC-3',下游引物序列为5'-CTTGCCACTCAGGGAAGGA-3';内参照基因GAPDH上游引物序列为5'-GGCTGCCCAG-AACATCAT-3',下游引物序列为5'-CGGACACA-TTGGGGGTAG-3'。
1.7 免疫荧光染色观察细胞骨架蛋白F-actin表达和分布细胞用不含AGE或含100 mg/L AGE的常规培养基培养24 h后,室温下用4%甲醛固定液固定15 min,PBS清洗3次;用0.5% Triton X-100处理30 min后,PBS清洗3次;加入提前配制好的鬼笔环肽染色工作液,室温下孵育30 min后用PBS清洗3次;滴加含DAPI的防荧光淬灭剂,封片,于激光共聚焦显微镜(型号Leica Application Suite X,德国Leica公司)下观察细胞染色情况。
1.8 蛋白质印迹法检测YAP和β-联蛋白的蛋白质表达细胞用不含AGE或含100 mg/L AGE的常规培养基培养24 h后,采用RIPA裂解液充分裂解细胞,提取总蛋白质,BCA法测定蛋白质浓度。按照每孔20 μg的上样量进行SDS-PAGE,转膜,采用5%脱脂牛奶室温封闭2 h后,加入一抗于4 ℃孵育过夜。次日加入HRP标记的二抗于室温孵育1 h,然后进行ECL反应,使用化学发光成像仪(型号Bio-Rad ChemiDoc,美国Bio-Rad公司)曝光显影。
1.9 免疫荧光染色观察YAP和β-联蛋白的入核情况细胞用不含AGE或含100 mg/L AGE的常规培养基培养24 h后,室温下用4%甲醛固定液固定细胞15 min,PBS清洗3次;用封闭缓冲液封闭1 h,吸弃封闭缓冲液,分别加入一抗YAP抗体和β-联蛋白抗体(稀释比例均为1∶100),于4 ℃孵育过夜。次日于室温下加入二抗孵育2 h,PBS清洗3次后,滴加含DAPI的防荧光淬灭剂、封片,于激光共聚焦显微镜下观察细胞染色情况。
1.10 统计学处理采用GraphPad Prism 7软件进行统计学分析。计量资料以x±s表示,多组间比较采用单因素方差分析,多重比较采用最小显著性差异法。检验水准(α)为0.05。
2 结果 2.1 AGE对MC3T3-E1细胞增殖的影响CCK-8检测结果(图 1)显示,与对照组相比,100 mg/L牛血清白蛋白及100 mg/L AGE对MC3T3-E1细胞的增殖均无明显影响,200、300 mg/L AGE处理36、48、60 h时MC3T3-E1细胞的增殖均受到抑制(P均<0.05)。
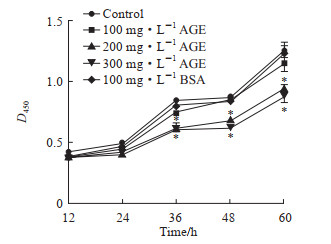
|
图 1 CCK-8检测不同质量浓度AGE对MC3T3-E1细胞增殖的影响 Fig 1 Effects of different mass concentrations of AGE on proliferation of MC3T3-E1 cells detected by CCK-8 *P < 0.05 vs control group at same time point. n=3, x±s. CCK-8: Cell counting kit 8; AGE: Advanced glycation end product; BSA: Bovine serum albumin. |
2.2 AGE对MC3T3-E1细胞凋亡的影响
流式细胞术检测结果(图 2)显示,100 mg/L AGE组MC3T3-E1细胞凋亡率[(6.19±0.43)%,n=3]与对照组[(5.49±0.55)%,n=3]相比差异无统计学意义(P>0.05),200 mg/L AGE组[(13.82±3.16)%,n=3]、300 mg/L AGE组[(28.85±3.90)%,n=3]细胞凋亡率均较对照组增高(P均<0.05),且浓度越高细胞凋亡率越高(P<0.05)。结合AGE对细胞增殖和凋亡的影响,后续实验选取质量浓度为100 mg/L的AGE。
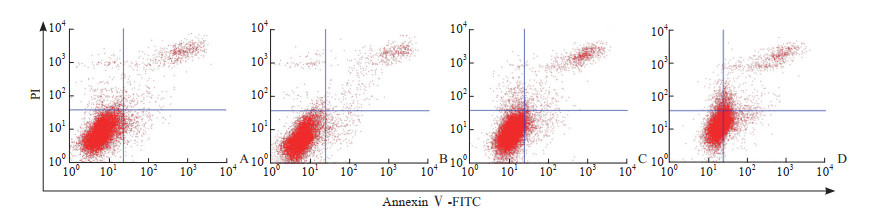
|
图 2 流式细胞术检测不同质量浓度AGE对MC3T3-E1细胞凋亡的影响 Fig 2 Effects of different mass concentrations of AGE on apoptosis of MC3T3-E1 cells detected by flow cytometry A: Control group; B: 100 mg/L AGE group; C: 200 mg/L AGE group; D: 300 mg/L AGE group. AGE: Advanced glycation end product; PI: Propidium iodide; FITC: Fluorescein isothiocyanate. |
2.3 AGE对MC3T3-E1细胞成骨分化能力和成骨相关基因表达的影响
ALP染色(图 3A)和qPCR检测结果(图 3B)显示,在成骨诱导培养条件下,与对照组相比,MC3T3-E1细胞经100 mg/L AGE处理后ALP染色较浅,成骨相关基因骨钙素、ALP和Runx2 mRNA的表达量均较低(P均<0.05)。

|
图 3 AGE对MC3T3-E1细胞成骨分化能力和成骨相关基因表达的影响 Fig 3 Effects of AGE on osteogenic differentiation and osteogenesis-related gene expression of MC3T3-E1 cells A: ALP staining (40×); B: Quantitative polymerase chain reaction results. *P < 0.05. n=3, x±s. AGE: Advanced glycation end product; ALP: Alkaline phosphatase; OCN: Osteocalcin. |
2.4 AGE对MC3T3-E1细胞骨架蛋白F-actin的影响
免疫荧光染色结果(图 4)显示,对照组MC3T3-E1细胞的F-actin分布在细胞周边,线条完整且连续;100 mg/L AGE作用24 h后MC3T3-E1细胞F-actin形态和分布发生变化,且细胞周边的F-actin明显较少。
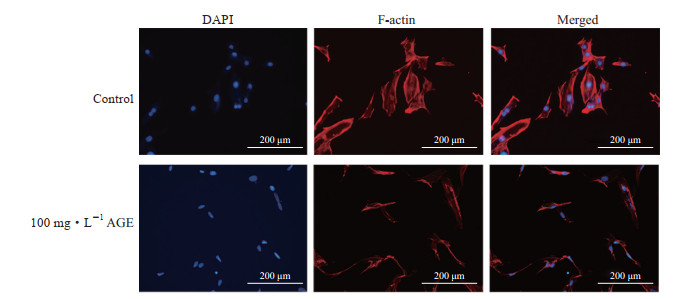
|
图 4 免疫荧光染色检测AGE对MC3T3-E1细胞骨架蛋白F-actin的影响 Fig 4 Effects of AGE on cytoskeletal protein F-actin of MC3T3-E1 cells detected by immunofluorescence staining AGE: Advanced glycation end product; F-actin: Filamentous actin; DAPI: 4', 6-diamidino-2-phenylindole. |
2.5 AGE对MC3T3-E1细胞中YAP和β-联蛋白表达的影响
qPCR和蛋白质印迹法检测结果(图 5)显示,与对照组相比,100 mg/L AGE处理后MC3T3-E1细胞中YAP的mRNA和蛋白质表达均无明显变化(P均>0.05),而β-联蛋白的mRNA和蛋白质表达均减少(P均<0.05)。

|
图 5 AGE对MC3T3-E1细胞中YAP和β-联蛋白表达的影响 Fig 5 Effects of AGEs on expression levels of YAP and β-catenin in MC3T3-E1 cells A: Expression of YAP and β-catenin mRNA detected by quantitative polymerase chain reaction; B: Representative Western blotting images of YAP and β-catenin proteins; C: Western blotting analytical results of protein expression levels of YAP and β-catenin. *P < 0.05. n=3, x±s. AGE: Advanced glycation end product; YAP: Yes-associated protein; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. |
2.6 AGE对MC3T3-E1细胞YAP和β-联蛋白核移位的影响
免疫荧光染色结果(图 6)显示,与对照组相比,100 mg/L AGE处理后MC3T3-E1细胞中YAP的核内含量减少,而β-联蛋白的核内含量无明显变化。

|
图 6 免疫荧光染色检测AGE对MC3T3-E1细胞YAP和β-联蛋白核内含量的影响 Fig 6 Effects of AGE on nuclear contents of YAP and β-catenin in MC3T3-E1 cells detected by immunofluorescence staining A: YAP; B: β-catenin. AGE: Advanced glycation end product; YAP: Yes-associated protein; DAPI: 4', 6-diamidino-2-phenylindole. |
3 讨论
AGE的积累在糖尿病和衰老引起的各种器官和组织损伤中起着重要作用[10]。AGE主要通过与细胞表面受体相互作用,或引起细胞内蛋白质的AGE修饰导致细胞稳态紊乱,从而使细胞功能受损。此外,AGE与其受体结合还可引起炎症、氧化应激和细胞凋亡等反应。有研究表明,细胞外AGE通过AGE/AGE受体信号转导诱导成骨细胞凋亡,从而对骨形成造成不良影响[11-12]。此外,成骨细胞中不断累积的AGE也会诱导细胞凋亡,破坏成骨细胞功能[5]。本研究结果显示,AGE能抑制MC3T3-E1细胞增殖、诱导凋亡,且呈现浓度依赖性,还能抑制MC3T3-E1细胞的成骨分化能力。以上结果提示,体内AGE的不断积累可能是老年人和糖尿病患者骨愈合能力较差及这类人群骨折发生率较高的原因之一。
细胞骨架对于维持细胞正常的生命活动及其功能起着至关重要的作用,AGE可通过改变细胞骨架蛋白F-actin纤维的形态和分布,影响细胞功能[13-14]。本研究与文献结果一致,经AGE作用后MC3T3-E1细胞中F-actin形态和分布发生变化,且细胞周边的F-actin明显变少,这提示AGE对MC3T3-E1细胞成骨分化能力的抑制可能与细胞骨架的变化有关。Hippo信号转导通路在许多生物学过程(如细胞增殖、凋亡、分化等)中发挥着至关重要的作用,YAP是该信号通路中的关键调节因子,在某些条件下YAP可以从细胞质转移到细胞核而调节基因表达。研究表明,在间充质干细胞和成骨细胞中,YAP可通过调节转录因子Runx2表达促进成骨分化[15-16]。有学者认为,通过调节细胞骨架张力可促进YAP核转移,当阻断F-actin或Rho家族时YAP活性会受到抑制[7]。本研究结果显示,MC3T3-E1细胞经AGE作用后YAP的核内含量减少,这可能是导致细胞成骨分化能力降低的主要原因之一,同时也提示YAP核转移减少与细胞骨架蛋白F-actin的变化有关。
β-联蛋白是一种具备结构分子和信号转导双重功能的蛋白质,它可通过与α-联蛋白结合间接参与肌动蛋白纤维的重组[17]。Azzolin等[18]研究发现,Wnt/β-联蛋白信号转导会增加YAP/TAZ的水平,相关研究还表明转录调节因子YAP/TAZ是Wnt信号通路的关键下游因子,也是经典Wnt/β-联蛋白信号的负调控因素[19]。研究表明,YAP可通过促进β-联蛋白入核及维持其核内水平增强间充质细胞的成骨分化能力[9];Wnt3a可促进TAZ去磷酸化,阻止其与14-3-3蛋白结合,促进TAZ入核,从而促进细胞成骨分化[20]。以上这些结果表明,Hippo/YAP通路和Wnt/β-联蛋白信号转导可通过多种机制相互调节,具体取决于生物学环境[19, 21]。本研究结果表明,在AGE作用下MC3T3-E1细胞内β-联蛋白表达减少,YAP表达无明显变化但其核内含量减少,提示YAP和β-联蛋白均参与AGE对MC3T3-E1细胞成骨分化的调控过程,YAP的核内含量减少是否与β-联蛋白表达减少有关及两者间的相互调控机制仍有待进一步研究。
| [1] |
MURRAY, COLEMAN. Impact of diabetes mellitus on bone health[J/OL]. Int J Mol Sci, 2019, 20: 4873. DOI: 10.3390/ijms20194873.
|
| [2] |
KURT A, ANDICAN G, SIVA Z O, ANDICAN A, BURCAK G. The effects of n-3 long-chain polyunsaturated fatty acid supplementation on AGEs and sRAGE in type 2 diabetes mellitus[J]. J Physiol Biochem, 2016, 72: 679-687. DOI:10.1007/s13105-016-0506-4 |
| [3] |
THOMAS C J, CLELAND T P, SROGA G E, VASHISHTH D. Accumulation of carboxymethyl-lysine (CML) in human cortical bone[J]. Bone, 2018, 110: 128-133. DOI:10.1016/j.bone.2018.01.028 |
| [4] |
POUNDARIK A A, WU P C, EVIS Z, SROGA G E, URAL A, RUBIN M, et al. A direct role of collagen glycation in bone fracture[J]. J Mech Behav Biomed Mater, 2015, 52: 120-130. DOI:10.1016/j.jmbbm.2015.08.012 |
| [5] |
SUZUKI R, FUJIWARA Y, SAITO M, ARAKAWA S, SHIRAKAWA J I, YAMANAKA M, et al. Intracellular accumulation of advanced glycation end products induces osteoblast apoptosis via endoplasmic reticulum stress[J]. J Bone Miner Res, 2020, 35: 1992-2003. DOI:10.1002/jbmr.4053 |
| [6] |
ZHENG L F, XIANG C X, LI X M, GUO Q Q, GAO L L, NI H W, et al. STARD13-correlated ceRNA network-directed inhibition on YAP/TAZ activity suppresses stemness of breast cancer via co-regulating Hippo and Rho-GTPase/F-actin signaling[J/OL]. J Hematol Oncol, 2018, 11: 72. DOI: 10.1186/s13045-018-0613-5.
|
| [7] |
PAN H H, XIE Y T, ZHANG Z Q, LI K, HU D D, ZHENG X B, et al. YAP-mediated mechanotransduction regulates osteogenic and adipogenic differentiation of BMSCs on hierarchical structure[J]. Colloids Surf B Biointerfaces, 2017, 152: 344-353. DOI:10.1016/j.colsurfb.2017.01.039 |
| [8] |
LI L J, YANG S, XU L, LI Y Z, FU Y R, ZHANG H, et al. Nanotopography on titanium promotes osteogenesis via autophagy-mediated signaling between YAP and β-catenin[J]. Acta Biomater, 2019, 96: 674-685. DOI:10.1016/j.actbio.2019.07.007 |
| [9] |
PAN J X, XIONG L, ZHAO K, ZENG P, WANG B, TANG F L, et al. YAP promotes osteogenesis and suppresses adipogenic differentiation by regulating β-catenin signaling[J/OL]. Bone Res, 2018, 6: 18. DOI: 10.1038/s41413-018-0018-7.
|
| [10] |
BASTA G, SCHMIDT A M, DE CATERINA R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes[J]. Cardiovasc Res, 2004, 63: 582-592. DOI:10.1016/j.cardiores.2004.05.001 |
| [11] |
MENG H Z, ZHANG W L, LIU F, YANG M W. Advanced glycation end products affect osteoblast proliferation and function by modulating autophagy via the receptor of advanced glycation end products/raf protein/mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase (RAGE/raf/MEK/ERK) pathway[J]. J Biol Chem, 2015, 290: 28189-28199. DOI:10.1074/jbc.M115.669499 |
| [12] |
MAO Y X, CAI W J, SUN X Y, DAI P P, LI X M, WANG Q, et al. RAGE-dependent mitochondria pathway: a novel target of silibinin against apoptosis of osteoblastic cells induced by advanced glycation end products[J/OL]. Cell Death Dis, 2018, 9: 674. DOI: 10.1038/s41419-018-0718-3.
|
| [13] |
王吉萍, 郭晓华, 王陵军, 李强, 陈波, 吴炜, 等. Rho/ROCK信号通路参与晚期糖基化终产物诱导的人皮肤微血管内皮细胞骨架结构改变[J]. 生理学报, 2009, 61: 132-138. |
| [14] |
LI Z Y, ZHONG Q Q, YANG T L, XIE X M, CHEN M F. The role of profilin-1 in endothelial cell injury induced by advanced glycation end products (AGEs)[J/OL]. Cardiovasc Diabetol, 2013, 12: 141. DOI: 10.1186/1475-2840-12-141.
|
| [15] |
XIAO Z S, QUARLES L D. Physiological mechanisms and therapeutic potential of bone mechanosensing[J]. Rev Endocr Metab Disord, 2015, 16: 115-129. DOI:10.1007/s11154-015-9313-4 |
| [16] |
ZHANG Y Y, GONG H, SUN Y, HUANG Y, FAN Y B. Enhanced osteogenic differentiation of MC3T3-E1 cells on grid-topographic surface and evidence for involvement of YAP mediator[J]. J Biomed Mater Res A, 2016, 104: 1143-1152. DOI:10.1002/jbm.a.35648 |
| [17] |
DREES F, POKUTTA S, YAMADA S, NELSON W J, WEIS W I. α-catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly[J]. Cell, 2005, 123: 903-915. DOI:10.1016/j.cell.2005.09.021 |
| [18] |
AZZOLIN L, PANCIERA T, SOLIGO S, ENZO E, BICCIATO S, DUPONT S, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response[J]. Cell, 2014, 158: 157-170. DOI:10.1016/j.cell.2014.06.013 |
| [19] |
PARK H W, KIM Y C, YU B, MOROISHI T, MO J S, PLOUFFE S W, et al. Alternative Wnt signaling activates YAP/TAZ[J]. Cell, 2015, 162: 780-794. DOI:10.1016/j.cell.2015.07.013 |
| [20] |
BYUN M R, HWANG J H, KIM A R, KIM K M, HWANG E S, YAFFE M B, et al. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation[J]. Cell Death Differ, 2014, 21: 854-863. DOI:10.1038/cdd.2014.8 |
| [21] |
WANG Y, PAN P, WANG Z H, ZHANG Y, XIE P, GENG D C, et al. β-catenin-mediated YAP signaling promotes human glioma growth[J/OL]. J Exp Clin Cancer Res, 2017, 36: 136. DOI: 10.1186/s13046-017-0606-1.
|
 2022, Vol. 43
2022, Vol. 43


