2. 上海交通大学附属第六人民医院老年科,上海 200233
2. Department of Geriatrics, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai 200233, China
全球每年有超过700万人发生急性心肌梗死,其中急性ST段抬高型心肌梗死(ST segment elevation myocardial infarction,STEMI)是冠心病最严重的亚型[1]。急诊经皮冠状动脉介入(primary percutaneous coronary intervention,PPCI)是目前挽救受损心肌最有效的治疗手段,其大大降低了心肌梗死的早期死亡率,但是STEMI后期坏死心肌重构引发的心力衰竭是导致远期临床事件的主要原因[2]。如何预测和防治心肌梗死后心肌重构是临床治疗的关键和研究热点,在STEMI早期预测是否发生心肌重构也极为重要。目前公认STEMI起病6个月后,患者左心室舒张末期容积(left ventricular end-diastolic volume,LVEDV)较基线增加≥20%,即为急性心肌梗死后发生了心肌负性重构[3-4]。心脏磁共振(cardiac magnetic resonance,CMR)可以无创、准确地评估心脏的结构和功能,并可通过造影剂首过灌注和延迟强化序列定量判断心肌缺血程度、区分心肌梗死区和远端非梗死区。组织追踪技术在不增加扫描序列的前提下,通过分析电影序列定量评估心肌形变的程度及达峰时间等变化。本研究拟利用CMR组织追踪技术比较心脏整体和局部应变的改变,探究能够灵敏预测STEMI心肌负性重构的参数。
1 资料和方法 1.1 病例资料及分组序贯选择2018年1月1日至2019年1月31日在上海交通大学附属第六人民医院心血管诊疗中心确诊为STEMI且成功接受PPCI治疗的83例患者。所有患者均分别于STEMI急性期(发病7 d内)和起病6个月后进行3.0 T CMR检查。急性心肌梗死左心室重构(left ventricular remodeling,LVR)定义为第2次CMR检查测量的LVEDV较基线增加≥20%[∆LVEDV≥20%,∆LVEDV=(第2次CMR检查测量的LVEDV-第1次CMR检查测量的LVEDV)/第1次CMR检查测量的LVEDV][5]。纳入标准:(1)符合STEMI诊断标准[6],即急性心肌生物学标志物血清肌钙蛋白高于正常值参考值上限的99%,并有心肌缺血临床症状或心电图出现ST段抬高;(2)出现胸痛12 h内成功接受PPCI治疗,或因心源性休克等血流动力学不稳定而适当延长PPCI时间窗至36 h的患者。排除标准:(1)既往有陈旧性心肌梗死、稳定型心绞痛、不稳定型心绞痛或非ST段抬高型心肌梗死;(2)有活动性心肌炎或心肌病(扩张型心肌病、肥厚型心肌病、限制型心肌病);(3)心脏起搏器植入;(4)有恶性心律失常;(5)有慢性阻塞性肺疾病、严重贫血、营养不良、严重肾功能不全[估算肾小球滤过率≤30 mL/(min·1.73 m2)]或肿瘤等疾病;(6)血流动力学不稳定者;(7)有幽闭恐惧症、造影剂过敏等MRI检查禁忌证者。2例患者因CMR图像质量较差无法进行后续分析被排除,共81例STEMI患者入组,根据起病6个月后是否发生LVR分为LVR组(33例)和非LVR组(48例),收集两组患者的人口学数据、入院时实验室检查结果、合并疾病、药物治疗方案等资料。本研究通过上海交通大学附属第六人民医院伦理委员会审批[2017-KY-003(K)],并获得患者知情同意。
1.2 检查方法应用3.0 T MRI仪(荷兰Philips公司)进行CMR检查。患者取仰卧位,首先采用稳态进动快速成像序列于呼气末采集图像,获得左心室长轴两腔、三腔、四腔心3个切面的电影图像(每个切面沿左心室中轴间隔60°)。心脏短轴扫描范围为心室基底部至心尖部,覆盖全部左心室。扫描参数:重复时间为3.2 ms,回波时间为1.5 ms,翻转角为45°,视野为340 mm×360 mm,采集矩阵为232×219,层厚为8 mm。静脉注射造影剂钆喷酸葡胺(德国Bayer公司)及生理盐水8~10 min后进行心肌延迟强化(late gadolinium enhancement,LGE)序列扫描,采集左心室3个长轴切面及连续短轴各切面图像。
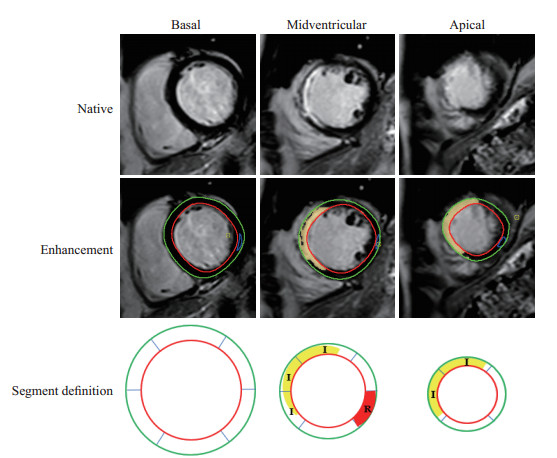
1.3 图像处理采用心功能分析软件CVI42(加拿大Circle Cardiovascular Imaging公司)对图像进行分析,主要分析指标包括LVEDV、左心室收缩末期容积(left ventricular end-systolic volume,LVESV)、LVEF等心功能参数。分别在左心室舒张末期和收缩末期,通过自动跟踪和手动校正勾画出左心室心内膜和心外膜边界。通过特征追踪功能,根据心动周期内心肌体素的变化主动追踪心肌运动形变信息。同时采用美国心脏病协会划分的17节段法,以牛眼图和各种应变指标定量心肌整体及节段应变功能,统计参数包括整体纵向应变(global longitudinal strain,GLS)、整体圆周应变(global circumferential strain,GCS)、整体径向应变(global radial strain,GRS)。同时获得梗死区纵向应变达峰时间和远端非梗死区纵向应变达峰时间。梗死区定义为在LGE序列图像中信号高于正常心肌信号5个标准差的区域[7];远端非梗死区定义为与梗死节段心肌间隔1个正常心肌切面的心肌区域,且该区域无缺血、水肿、室壁运动异常,无延迟强化[8](图 1)。按以下公式计算心肌梗死大小百分比:心肌梗死大小百分比(%)=梗死心肌容积/左心室心肌容积×100%。
|
图 1 根据心肌延迟强化序列和美国心脏病协会17节段法标注梗死区及远端非梗死区 Fig 1 Infarct zone and remote non-infarct zone classified by late gadolinium enhancement sequence and American Heart Association 17-segment model The endocardial and epicardial contours of left ventricular were outlined in red and green, respectively. After the healthy myocardium was outlined in blue, myocardium was divided into infarct zone (I, yellow) and remote non-infarct zone (R, red). Remote non-infarct zone was defined as unenhanced zone with a unenhanced border zone between it and infarct zone. |
1.4 统计学处理
应用SPSS 25.0软件进行统计学分析。符合正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验;呈偏态分布的计量资料以中位数(下四分位数,上四分位数)表示,两组间比较采用Mann-Whitney U检验;计数资料以例数和百分数表示,两组间比较采用χ2检验。将可能预测STEMI患者发生LVR的因素纳入logistic回归分析,计算OR和95% CI。采用GraphPad Prism 9软件绘制ROC曲线,评价相关参数对于STEMI患者发生LVR的预测价值,计算AUC值并根据约登指数估算最佳临界值及所对应的灵敏度和特异度。检验水准(α)为0.05。
2 结果 2.1 患者基线资料81例STEMI患者的年龄为27~75(55.07±12.50)岁,男69例(85.2%),女12例(14.8%)。两组患者在性别、年龄、心率、BMI、冠心病危险因素、峰值脑钠肽前体(peak pro-brain natriuretic peptide,peak proBNP)、病变累及血管及药物治疗方案等方面差异均无统计学意义(P均>0.05),而LVR组峰值超敏血清肌钙蛋白I(peak hypersensitive serum cardiac troponin I,peak hs-cTnI)水平高于非LVR组(P<0.01)。两组间基线CMR常规心功能参数LVEF、LVEDV、LVESV及心肌梗死大小百分比差异均无统计学意义(P均>0.05)。见表 1。
|
|
表 1 两组STEMI患者的基线资料比较 Tab 1 Comparison of baseline indicators of STEMI patients between 2 groups |
2.2 心肌应变参数分析
LVR组患者的GLS低于非LVR组(P<0.01),而两组间GCS、GRS差异均无统计学意义(P均>0.05)。LVR组梗死区纵向应变达峰时间短于非LVR组(P<0.01)。见表 2。
|
|
表 2 两组STEMI患者的心肌应变参数比较 Tab 2 Comparison of myocardial strain parameters of STEMI patients between 2 groups |
2.3 STEMI患者发生LVR影响因素的logistic回归及ROC曲线分析
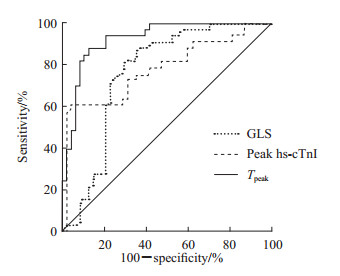
以peak hs-cTnI、peak proBNP、GLS等参数为自变量,STEMI患者在起病6个月后是否发生LVR为因变量进行logistic回归分析。单因素分析显示peak hs-cTnI、GLS和梗死区纵向应变达峰时间差异均有统计学意义(P均<0.05)。进一步行多因素logistic回归分析,采用前向Wald法进行变量筛选,结果显示peak hs-cTnI、梗死区纵向应变达峰时间是STEMI患者起病6个月后发生LVR的独立影响因素[b=0.025,标准误=0.010,OR=1.026(95% CI 1.006~1.046),P=0.012;b=-0.034,标准误=0.009,OR=0.967(95% CI 0.950~0.983),P<0.001]。ROC曲线(图 2)分析结果显示,peak hs-cTnI预测STEMI患者起病6个月后发生LVR的AUC值为0.795(P<0.001),最佳临界值为85.54 μg/L,灵敏度为60.60%,特异度为97.90%;GLS的AUC值为0.761(P<0.001),最佳临界值为-10.56%,灵敏度为81.80%,特异度为70.80%;梗死区纵向应变达峰时间的AUC值为0.926(P<0.001),最佳临界值为309.12 ms,灵敏度为87.90%,特异度为87.50%。
|
图 2 STEMI患者发生LVR影响因素的ROC曲线分析 Fig 2 ROC curve analysis of influencing factors of LVR in STEMI patients STEMI: ST segment elevation myocardial infarction; LVR: Left ventricular remodeling; ROC: Receiver operating characteristic; GLS: Global longitudinal strain; hs-cTnI: Hypersensitive serum cardiac troponin I; Tpeak: Time to peak longitudinal strain in infarct zone. |
3 讨论
本研究通过组织追踪技术,分析左心室整体应变(GLS、GRS、GCS)及梗死区、远端非梗死区应变达峰时间预测STEMI患者发生左心室心肌负性重构的价值,结果表明在STEMI急性期,LVEF在LVR组和非LVR组之间差异无统计学意义,而peak hs-cTnI、GLS和梗死区纵向应变达峰时间对发生LVR有较好的预测价值。
急性心肌梗死是严重的心血管事件,及时再灌注治疗提高了急性心肌梗死患者的早期生存率,但急性心肌梗死后1~3年患者的全因死亡及心血管不良事件(心肌梗死复发、心源性死亡)的发生风险仍较正常人高30%[9]。急性心肌梗死后心肌坏死和水肿诱导炎症细胞增殖与浸润,导致心室腔扩张、室壁应力增加,从而促进纤维沉积;同时也会使交感神经兴奋性代偿性升高,心率加快,远端非梗死区心肌代偿性做功增加,导致梗死区与远端非梗死区心肌不协调运动,引起心肌负性重构[10]。心肌应变是评价心肌运动的灵敏指标,可通过比较梗死区与远端非梗死区心肌的收缩幅度、速度、达峰时间判断左心室机械离散度[11],既往研究发现不同方向应变达峰时间不仅可以用于区分梗死区与非梗死区心肌[12],而且可以用于判断梗死区域的范围[13]。本研究结果显示在STEMI急性期,在心肌梗死大小百分比、心率无明显差异的前提下,发生LVR患者的梗死区纵向应变达峰时间较未发生LVR患者短,随后的logistic回归分析结果也提示梗死区纵向应变达峰时间是STEMI患者起病6个月后发生LVR的独立影响因素。推测这与梗死区心肌纵向应变峰值显著降低相关[14]。远端非梗死区纵向应变达峰时间在LVR组与非LVR组之间差异无统计学意义,可能是由于STEMI急性期力学改变主要发生在梗死区,心肌梗死发生后28 d才会累及邻近及远端非梗死区[15]。
LVEF是评估心肌梗死后发生心力衰竭的常用指标,LVEF的下降预示着心血管预后不良。研究发现心肌梗死急性期LVEF轻度下降(为40%~45%),与急性心肌梗死远期预后无关[16]。在射血分数保留型心力衰竭的急性心肌梗死患者中,纵向应变可以预测左心室的负性重构[17]。多项研究表明左心室的应变是判断急性心肌梗死预后的重要指标[18-19],而左心室纵向应变受损不仅与左心室纤维化相关,而且可以预测梗死面积[13]。本研究结果显示,在急性期梗死面积无明显差异的前提下,LVR组的GLS低于非LVR组,表明对于预测LVR,GLS较梗死面积更灵敏。该结果与Reindl等[20]的研究结果一致。
肌钙蛋白I或T作为心肌细胞重要的结构蛋白,在心肌细胞发生坏死后被从心肌细胞释放[21],诊断急性心肌梗死的灵敏度较高[22],并对心血管事件具有独立预测价值[23]。多项研究表明高敏肌钙蛋白T和高敏肌钙蛋白I与急性心肌梗死PPCI术后发生心力衰竭等密切相关[24-25]。本研究结果与上述结果一致,且logistic回归分析结果显示peak hs-cTnI是STEMI预后的独立影响因素,peak hs-cTnI水平越高STEMI患者起病6个月后发生LVR的概率越高。Gohar等[26]研究发现,高敏肌钙蛋白T或I和N末端脑钠肽前体均可以预测射血分数降低型心力衰竭,但高敏肌钙蛋白I无论是在射血分数保留型心力衰竭还是在射血分数降低型心力衰竭的预测作用中,均是男性高于女性,造成这种性别差异的原因目前尚不清楚。虽然肌钙蛋白I或T具有较高的灵敏度和特异度,但其水平还受肾功能、感染、自身免疫性抗体等多种因素影响。CMR检查是公认的评价心肌结构变化最灵敏的影像学手段,其中心肌应变参数能够直观、定量评估心脏整体和梗死区局部室壁运动能力,相较于心肌损伤标志物提供的信息更具象和直观。本研究中peak hs-cTnI、梗死区纵向应变达峰时间在预测STEMI患者发生LVR方面相辅相成,且心肌应变参数对远期预后有一定提示价值。
本研究为单中心研究,入组的诊断为STEMI且成功接受PPCI治疗的患者仅81例,样本量较小,可能会对多因素分析中临界值的准确性造成影响。后续将进一步扩大样本量,延长随访时间,以获得更可靠的临床结论。
| [1] |
REED G W, ROSSI J E, CANNON C P. Acute myocardial infarction[J]. Lancet, 2017, 389: 197-210. DOI:10.1016/S0140-6736(16)30677-8 |
| [2] |
ONG S B, HERNÁNDEZ-RESÉNDIZ S, CRESPO-AVILAN G E, MUKHAMETSHINA R T, KWEK X Y, CABRERA-FUENTES H A, et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities[J]. Pharmacol Ther, 2018, 186: 73-87. DOI:10.1016/j.pharmthera.2018.01.001 |
| [3] |
BOLOGNESE L, CERISANO G, BUONAMICI P, SANTINI A, SANTORO G M, ANTONIUCCI D, et al. Influence of infarct-zone viability on left ventricular remodeling after acute myocardial infarction[J]. Circulation, 1997, 96: 3353-3359. DOI:10.1161/01.CIR.96.10.3353 |
| [4] |
ZHONG J D, LIU P, LI S, HUANG X M, ZHANG Q H, HUANG J Y, et al. A comparison of three-dimensional speckle tracking echocardiography parameters in predicting left ventricular remodeling[J/OL]. J Healthc Eng, 2020, 2020: 8847144. DOI: 10.1155/2020/8847144.
|
| [5] |
LEGALLOIS D, HODZIC A, ALEXANDRE J, DOLLADILLE C, SALOUX E, MANRIQUE A, et al. Definition of left ventricular remodelling following ST-elevation myocardial infarction: a systematic review of cardiac magnetic resonance studies in the past decade[J]. Heart Fail Rev, 2022, 27: 37-48. DOI:10.1007/s10741-020-09975-3 |
| [6] |
中华医学会心血管病学分会, 中华心血管病杂志编辑委员会. 急性ST段抬高型心肌梗死诊断和治疗指南(2019)[J]. 中华心血管病杂志, 2019, 47: 766-783. |
| [7] |
CARRICK D, HAIG C, RAUHALAMMI S, AHMED N, MORDI I, MCENTEGART M, et al. Pathophysiology of LV remodeling in survivors of STEMI: inflammation, remote myocardium, and prognosis[J]. JACC Cardiovasc Imaging, 2015, 8: 779-789. DOI:10.1016/j.jcmg.2015.03.007 |
| [8] |
LANGE T, STIERMAIER T, BACKHAUS S J, BOOM P C, KOWALLICK J T, DE WAHA-THIELE S, et al. Functional and prognostic implications of cardiac magnetic resonance feature tracking-derived remote myocardial strain analyses in patients following acute myocardial infarction[J]. Clin Res Cardiol, 2021, 110: 270-280. DOI:10.1007/s00392-020-01747-1 |
| [9] |
JOHANSSON S, ROSENGREN A, YOUNG K, JENNINGS E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: a systematic review[J/OL]. BMC Cardiovasc Disord, 2017, 17: 53. DOI: 10.1186/s12872-017-0482-9.
|
| [10] |
PEET C, IVETIC A, BROMAGE D I, SHAH A M. Cardiac monocytes and macrophages after myocardial infarction[J]. Cardiovasc Res, 2020, 116: 1101-1112. DOI:10.1093/cvr/cvz336 |
| [11] |
ABOU R, GOEDEMANS L, VAN DER BIJL P, FORTUNI F, PRIHADI E A, MERTENS B, et al. Correlates and long-term implications of left ventricular mechanical dispersion by two-dimensional speckle-tracking echocardiography in patients with ST-segment elevation myocardial infarction[J]. J Am Soc Echocardiogr, 2020, 33: 964-972. DOI:10.1016/j.echo.2020.03.010 |
| [12] |
MARET E, LIEHL M, BRUDIN L, TODT T, EDVARDSEN T, ENGVALL J E. Phase analysis detects heterogeneity of myocardial deformation on cine MRI[J]. Scand Cardiovasc J, 2015, 49: 149-158. DOI:10.3109/14017431.2015.1023343 |
| [13] |
DANNENBERG V, CHRISTIANSEN F, SCHNEIDER M, KASTL S, HOFBAUER T M, SCHERZ T, et al. Exploratory echocardiographic strain parameters for the estimation of myocardial infarct size in ST-elevation myocardial infarction[J]. Clin Cardiol, 2021, 44: 925-931. DOI:10.1002/clc.23608 |
| [14] |
STATHOGIANNIS K, MOR-AVI V, RASHEDI N, LANG R M, PATEL A R. Regional myocardial strain by cardiac magnetic resonance feature tracking for detection of scar in ischemic heart disease[J]. Magn Reson Imaging, 2020, 68: 190-196. DOI:10.1016/j.mri.2020.02.009 |
| [15] |
TORRES W M, JACOBS J, DOVIAK H, BARLOW S C, ZILE M R, SHAZLY T, et al. Regional and temporal changes in left ventricular strain and stiffness in a porcine model of myocardial infarction[J]. Am J Physiol Heart Circ Physiol, 2018, 315: H958-H967. DOI:10.1152/ajpheart.00279.2018 |
| [16] |
POTTER E, MARWICK T H. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction[J]. JACC Cardiovasc Imaging, 2018, 11: 260-274. DOI:10.1016/j.jcmg.2017.11.017 |
| [17] |
HSIAO J F, CHUNG C M, CHU C M, LIN Y S, PAN K L, CHANG S T, et al. Two-dimensional speckle tracking echocardiography predict left ventricular remodeling after acute myocardial infarction in patients with preserved ejection fraction[J/OL]. PLoS One, 2016, 11: e0168109. DOI: 10.1371/journal.pone.0168109.
|
| [18] |
REINDL M, TILLER C, HOLZKNECHT M, LECHNER I, BECK A, PLAPPERT D, et al. Prognostic implications of global longitudinal strain by feature-tracking cardiac magnetic resonance in ST-elevation myocardial infarction[J/OL]. Circ Cardiovasc Imaging, 2019, 12: e009404. DOI: 10.1161/CIRCIMAGING.119.009404.
|
| [19] |
LUVSANSUREN B, CHIMED S. Significance of left ventricular global longitudinal strain assessment in patients with preserved ejection fraction after acute myocardial infarction[J/OL]. Eur Heart J, 2020, 41: ehaa946.1212. DOI: 10.1093/ehjci/ehaa946.1212.
|
| [20] |
REINDL M, TILLER C, HOLZKNECHT M, LECHNER I, EISNER D, RIEPL L, et al. Global longitudinal strain by feature tracking for optimized prediction of adverse remodeling after ST-elevation myocardial infarction[J]. Clin Res Cardiol, 2021, 110: 61-71. DOI:10.1007/s00392-020-01649-2 |
| [21] |
SOETKAMP D, RAEDSCHELDERS K, MASTALI M, SOBHANI K, BAIREY MERZ C N, VAN EYK J. The continuing evolution of cardiac troponin I biomarker analysis: from protein to proteoform[J]. Expert Rev Proteomics, 2017, 14: 973-986. DOI:10.1080/14789450.2017.1387054 |
| [22] |
COLLET J P, THIELE H. The 'Ten Commandments' for the 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation[J]. Eur Heart J, 2020, 41: 3495-3497. DOI:10.1093/eurheartj/ehaa624 |
| [23] |
LYNGBAKKEN M N, RØSJØ H, HOLMEN O L, DALEN H, HVEEM K, OMLAND T. Temporal changes in cardiac troponin I are associated with risk of cardiovascular events in the general population: the Nord-Trøndelag Health Study[J]. Clin Chem, 2019, 65: 871-881. DOI:10.1373/clinchem.2018.301069 |
| [24] |
STELZLE D, SHAH A S V, ANAND A, STRACHAN F E, CHAPMAN A R, DENVIR M A, et al. High-sensitivity cardiac troponin I and risk of heart failure in patients with suspected acute coronary syndrome: a cohort study[J]. Eur Heart J Qual Care Clin Outcomes, 2018, 4: 36-42. DOI:10.1093/ehjqcco/qcx022 |
| [25] |
SHAH A S, ANAND A, SANDOVAL Y, LEE K K, SMITH S W, ADAMSON P D, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study[J]. Lancet, 2015, 386: 2481-2488. DOI:10.1016/S0140-6736(15)00391-8 |
| [26] |
GOHAR A, CHONG J P C, LIEW O W, DEN RUIJTER H, DE KLEIJN D P V, SIM D, et al. The prognostic value of highly sensitive cardiac troponin assays for adverse events in men and women with stable heart failure and a preserved vs. reduced ejection fraction[J]. Eur J Heart Fail, 2017, 19: 1638-1647. DOI:10.1002/ejhf.911 |
 2022, Vol. 43
2022, Vol. 43


