2. 海军军医大学(第二军医大学)第一附属医院麻醉学部,上海 200433
2. Department of Anesthesiology, The First Affiliated Hospital of Naval Medical University (Second Military Medical University), Shanghai 200433, China
帕金森病是老年患者最常见的神经系统退行性疾病之一,临床主要表现为静止性震颤、僵直、运动迟缓和姿势不稳等,严重影响患者生活质量,增加社会医疗负担[1-3]。帕金森病的具体发病机制目前仍不明确,研究提示中枢神经炎症、线粒体功能障碍等与帕金森病发生和发展相关,尤其中枢神经炎症与帕金森病密切相关[4-5]。
短链脂肪酸主要包括乙酸盐(acetate,Ace)、丙酸盐和丁酸盐,可通过血脑屏障,因此其在神经系统疾病中的作用备受关注。有研究指出丁酸可减少神经退行性疾病小鼠模型中的小胶质细胞炎症因子基因表达和小胶质细胞活化[6-8]。Ace具有调控免疫反应及炎症反应等功能[9-10],可抑制小胶质细胞活化,降低神经炎症反应及氧化应激,从而改善围手术期神经认知功能障碍[11]。目前Ace对帕金森病的神经保护作用尚未见报道。本研究利用小鼠模型初步探讨Ace在帕金森病中的神经保护作用,为帕金森病的干预和治疗提供潜在的研究方向。
1 材料和方法 1.1 实验动物8周龄SPF级雄性C57BL/6小鼠,体重22 g左右,购自江苏集萃药康生物科技股份有限公司[实验动物生产许可证号:SCXK(苏)2018-0008]。实验动物饲养条件:室温(18~22 ℃),维持正常昼夜节律(12 h/12 h),自由进食、饮水。动物实验经海军军医大学(第二军医大学)动物伦理审查委员会审查批准。
1.2 主要试剂与仪器1-甲基-4-苯基-1,2,3,6-四氢吡啶(1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine,MPTP)购自上海吉至生化科技有限公司,Ace购自美国Sigma公司,ELISA试剂盒、蛋白质印迹法试剂盒(包括相应抗体)均购自美国CST公司,小胶质细胞标志物离子钙接头蛋白1(ionized calcium binding adaptor molecule 1,IBA-1)抗体购自美国ThermoFisher公司。水迷宫实验装置购自上海欣软信息科技有限公司,爬杆实验装置购自江苏赛昂斯生物科技有限公司。
1.3 动物分组及模型构建24只小鼠随机分为对照组、MPTP组、MPTP+Ace组,每组8只。对照组和MPTP组小鼠饮用正常饮用水,MPTP+Ace组饮用添加1 mol/L Ace的饮用水连续7 d后更换为正常饮用水。7 d后,MPTP组和MPTP+Ace组腹腔注射30 mg/kg MPTP,每天1次,连续7 d,构建亚急性帕金森病小鼠模型[8];对照组同法注射等体积生理盐水。Ace的剂量选择:在预实验中,先参考既往报道[11]选择200 mmol/L Ace进行实验,发现其不能改善帕金森病模型小鼠的运动功能;后将Ace浓度调整为1 mol/L,发现其可以改善帕金森病模型小鼠的运动和认知功能,故本研究中选择1 mol/L Ace进行实验。
1.4 小鼠行为学实验 1.4.1 震颤麻痹评分造模结束(MPTP连续腹腔注射7 d)后对小鼠进行震颤麻痹评分[12]。评分标准:1分,无任何症状;2分,出现竖毛、弓背、间断性细小震颤;3分,出现吞咽频繁、频繁性震颤,后肢张开,颤尾,活动逐渐受限;4分,出现流涎、持续性震颤,四肢僵硬,活动受限;5分,因全身麻痹而死亡。
1.4.2 爬杆实验分别于造模前和造模后进行爬杆实验。小鼠爬杆实验装置由底座、杆体和球部组成,调整杆体与底座水平的夹角为45°,将被测小鼠置于球部,以小鼠双前上肢接触杆底平台和触碰中点黑线认定为爬完全程,记录爬完全程所需时间。
1.4.3 Morris水迷宫实验造模前所有小鼠进行连续5 d的隐蔽平台实验训练:将每只小鼠分别从4个象限中点面向池壁放入水中,小鼠自由游泳60 s,记录小鼠到达平台的时间(潜伏期)和运动轨迹,计算游泳速度。造模结束后进行空间探索实验:移除平台,将小鼠于目标象限对面的象限放入水中,记录小鼠的运动轨迹,计算小鼠在目标象限停留时间百分比、路程百分比,统计穿台次数,计算游泳速度。
1.5 小鼠海马组织小胶质细胞活化情况检测采用免疫组织化学染色检测小鼠海马组织小胶质细胞活化情况。行为学实验结束后,每组随机选取2只小鼠取完整脑组织,用4%多聚甲醛溶液固定。将固定好的脑组织进行石蜡包埋切片,脱蜡、水化,修复抗原,阻断内源性过氧化物酶,血清封闭,先后加入IBA-1抗体(稀释比例为1∶1 000)和二抗(稀释比例为1∶3 000)进行孵育,DAB显色,复染细胞核,脱水封片后进行图像采集。
1.6 小鼠外周血及海马组织促炎因子检测以七氟烷吸入麻醉各组剩余6只小鼠,取心脏血,肝素抗凝,3 000×g离心10 min,取上清液。小鼠心脏取血完毕后,取海马制作组织匀浆,12 000×g 4 ℃离心15 min。用ELISA试剂盒检测外周血和海马炎症因子TNF-α、IL-6及IL-1β的水平。
1.7 小鼠海马组织炎症信号通路检测取小鼠海马组织匀浆上清液,BCA法测定蛋白质浓度,依次进行电泳、转膜、5%脱脂奶粉溶液封闭,加入一抗(内参照β-肌动蛋白、p65、磷酸化p65、p38、磷酸化p38抗体,稀释比例均为1∶1 000)4 ℃孵育过夜,TBST洗膜3次,加入二抗(稀释比例为1∶3 000)孵育2 h,TBST洗涤3次。ECL显影,通过ImageJ软件分析目的蛋白的相对表达量。
1.8 统计学处理使用GraphPad Prism 8.0软件对数据进行分析。计量资料以x±s表示,水迷宫训练阶段潜伏期和游泳速度的比较采用双因素方差分析,其余指标组间比较采用单因素方差分析,多重比较采用最小显著性差异法。检验水准(α)为0.05。
2 结果 2.1 Ace对MPTP诱导亚急性帕金森病模型小鼠运动和认知功能的保护作用对照组所有小鼠均无震颤麻痹,震颤麻痹评分均为1分。MPTP组震颤麻痹评分为(3.5±0.5)分,其中4只小鼠为3分、4只为4分,与对照组相比增高(P < 0.05);MPTP+Ace组震颤麻痹评分为(2.1±1.0)分,其中2只小鼠为1分、4只为2分、1只为3分、1只为4分,与MPTP组相比降低(P < 0.05)。该结果表明Ace能改善MPTP诱导的亚急性帕金森病模型小鼠的震颤麻痹。
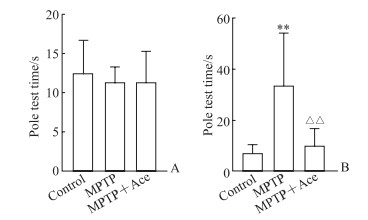
由图 1可见,造模前3组小鼠爬杆时间差异无统计学意义(P>0.05);造模后MPTP组爬杆时间较对照组延长(P < 0.01),而MPTP+Ace组爬杆时间较MPTP组缩短(P < 0.01)。该结果表明Ace能改善MPTP诱导的亚急性帕金森病模型小鼠的运动功能障碍。
|
图 1 Ace对MPTP诱导的亚急性帕金森病模型小鼠爬杆时间的影响 Fig 1 Effect of Ace on pole test time of subacute Parkinson disease mice induced by MPTP A: Before modeling; B: After modeling. **P < 0.01 vs control group; △△P < 0.01 vs MPTP group. n=8, x±s. Ace: Acetate; MPTP: 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. |
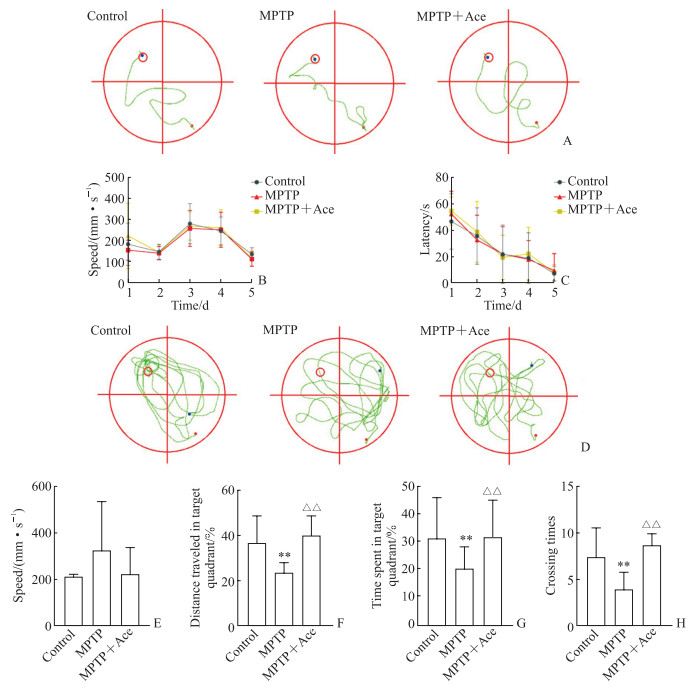
由图 2可见,造模前连续5 d进行Morris水迷宫隐蔽平台实验训练时,3组小鼠潜伏期和游泳速度差异均无统计学意义(P均>0.05);造模后空间探索实验结果显示,3组小鼠游泳速度差异无统计学意义(P>0.05),MPTP组小鼠目标象限停留时间百分比、路程百分比及穿台次数与对照组相比均减少(P均 < 0.01),MPTP+Ace组小鼠目标象限停留时间百分比、路程百分比及穿台次数与MPTP组相比均增加(P均 < 0.01)。该结果表明Ace可部分改善MPTP诱导的亚急性帕金森病模型小鼠的空间学习记忆能力。
|
图 2 Ace对MPTP诱导的亚急性帕金森病模型小鼠Morris水迷宫实验指标的影响 Fig 2 Effect of Ace on indexes of Morris water maze test in mice with subacute Parkinson disease induced by MPTP A: Trajectory of training phase before modeling; B: Swimming speed during training before modeling; C: Training latency before modeling; D: Trajectory of space exploration experiment after modeling; E: Swimming speed in space exploration experiment after modeling; F: Percentage of distance traveled in target quadrant during space exploration experiment after modeling; G: Percentage of time spent in target quadrant during space exploration experiment after modeling; H: Crossing times in space exploration experiment after modeling. In the trajectory diagram, the target quadrant is marked by red circle, the position of the mouse entering water is marked by red dot, and the position of the mouse at the end of observation is marked by blue dot. **P < 0.01 vs control group; △△P < 0.01 vs MPTP group. n=8, x±s. Ace: Acetate; MPTP: 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. |
2.2 Ace对MPTP诱导亚急性帕金森病模型小鼠海马组织小胶质细胞活化的抑制作用
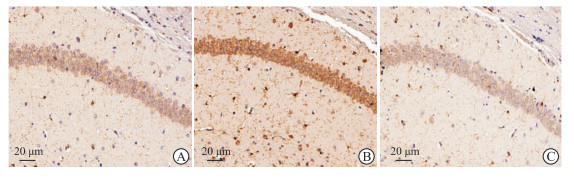
用IBA-1标记海马组织小胶质细胞,通过免疫组织化学染色检测小胶质细胞的活化情况。检测结果(图 3)显示,MPTP组小鼠海马组织中IBA-1标记的小胶质细胞与对照组相比明显增多,呈活化状态;MPTP+Ace组小鼠海马组织中活化小胶质细胞与MPTP组相比明显减少。该结果提示Ace可抑制MPTP诱导的亚急性帕金森病模型小鼠海马组织小胶质细胞活化。
|
图 3 Ace对MPTP诱导的亚急性帕金森病模型小鼠海马小胶质细胞活化的影响 Fig 3 Effect of Ace on hippocampal microglia activation of mice with subacute Parkinson disease induced by MPTP A: Control group; B: MPTP group; C: MPTP+Ace group. The activated hippocampal microglia were labeled with IBA-1 and detected by immunohistochemistry. Ace: Acetate; MPTP: 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; IBA-1: Ionized calcium binding adaptor molecule 1. |
2.3 Ace对MPTP诱导亚急性帕金森病模型小鼠血清与海马组织中促炎因子的抑制作用
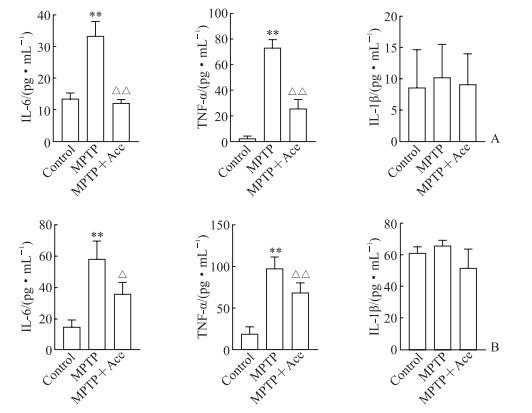
ELISA检测结果(图 4)显示,MPTP组小鼠血清和海马组织中促炎因子TNF-α和IL-6水平均比对照组升高(P均 < 0.01),IL-1β水平与对照组相比差异无统计学意义(P均>0.05);MPTP+Ace组小鼠血清和海马组织中TNF-α和IL-6水平均较MPTP组降低(P均 < 0.05),IL-1β水平与MPTP组相比差异均无统计学意义(P均>0.05)。该结果提示Ace可降低MPTP诱导亚急性帕金森病模型小鼠血清与海马组织中促炎因子TNF-α和IL-6水平,抑制中枢神经炎症反应。
|
图 4 Ace对MPTP诱导的亚急性帕金森病模型小鼠血清和海马组织中炎症因子的影响 Fig 4 Effect of Ace on inflammatory factors in serum and hippocampus of mice with subacute Parkinson disease induced by MPTP A: Serum; B: Hippocampus. The inflammatory factors were detected by enzyme-linked immunosorbent assay. **P < 0.01 vs control group; △P < 0.05, △△P < 0.01 vs MPTP group. n=6, x±s. Ace: Acetate; MPTP: 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; IL: Interleukin; TNF-α: Tumor necrosis factor α. |
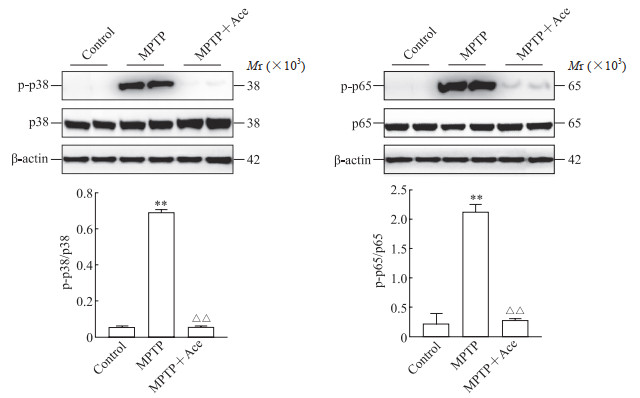
2.4 Ace对MPTP诱导亚急性帕金森病模型小鼠海马组织炎症信号通路的抑制作用
蛋白质印迹法检测结果(图 5)显示,MPTP组小鼠海马组织中磷酸化p38和磷酸化p65蛋白表达水平均较对照组上调(P均 < 0.01),MPTP+Ace组小鼠海马组织中磷酸化p38和磷酸化p65蛋白表达水平均较MPTP组下降(P均 < 0.01)。该结果提示Ace能够抑制MPTP诱导亚急性帕金森病模型小鼠海马组织中NF-κB和MAPK炎症信号通路。
|
图 5 Ace对MPTP诱导的亚急性帕金森病模型小鼠海马组织中炎症通路蛋白的影响 Fig 5 Effect of Ace on inflammatory pathway proteins in hippocampus of mice with subacute Parkinson disease induced by MPTP The proteins were detected by Western blotting. **P < 0.01 vs control group; △△P < 0.01 vs MPTP group. n=6, x±s. Ace: Acetate; MPTP: 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; p-p38: Phosphorylated p38; p-p65: Phosphorylated p65. |
3 讨论
MPTP诱导的帕金森病模型是目前最为经典的帕金森病模型之一。MPTP具有极强的脂溶性,可通过血脑屏障进入中枢神经系统引起氧化应激、线粒体凋亡、炎症等一系列反应,最终导致黑质致密部和纹状体的多巴胺能神经元损伤[13-16]。本研究结果显示连续7 d腹腔注射MPTP的小鼠出现了明显的运动和认知功能障碍,震颤麻痹评分增高,爬杆时间延长,水迷宫实验目标象限停留时间百分比、路程百分比及穿台次数减少,表明MPTP可成功建构亚急性帕金森病模型。
MPTP可诱导中枢神经炎症反应,而小胶质细胞活化在帕金森病神经炎症机制中起着重要作用。小胶质细胞的过度激活可显著增加TNF-α、IL-6等炎症因子的产生,促进多巴胺神经元凋亡,与帕金森病的发生和发展密切相关[17-19]。有研究发现Ace具有调控免疫反应及炎症反应等功能,可抑制小胶质细胞活化,减轻神经炎症反应及氧化应激,从而改善老年小鼠认知功能[11]。因此,我们推测Ace也可改善帕金森病中的中枢神经炎症。本研究发现Ace预处理后再注射MPTP的小鼠震颤麻痹评分下降,爬杆时间缩短,水迷宫实验目标象限停留时间百分比、路程百分比及穿台次数增加,提示Ace可改善MPTP诱导亚急性帕金森病小鼠的运动和认识功能障碍。MPTP诱导帕金森病模型小鼠海马组织活化的小胶质细胞明显增多,血清和海马组织中TNF-α、IL-6水平升高,而Ace可部分抑制小胶质细胞活化状态,明显减少促炎因子TNF-α、IL-6的产生,提示Ace通过抑制小胶质细胞活化、改善中枢神经炎症反应,对MPTP诱导帕金森病模型小鼠的运动和认知功能产生保护作用。有趣的是,本研究中对照组、MPTP组、MPTP+Ace组小鼠血清和海马组织中IL-1β的变化没有统计学意义,可能与MPTP诱导帕金森病的中枢炎症与其他原因导致的中枢炎症不同有关,具体机制仍有待探讨。
既往研究显示NF-κB/MAPK信号通路参与帕金森病等退行性疾病的发病[20]。本研究检测了Ace对帕金森病模型小鼠海马组织NF-κB/MAPK信号通路的影响,结果显示Ace可以抑制NF-κB/MAPK信号通路蛋白p38和p65磷酸化水平。但Ace通过何种途径抑制NF-κB和MAPK信号通路的活化仍需进一步深入探讨。
综上所述,本研究初步证明Ace可通过抑制NF-κB/MAPK炎症信号通路减轻海马组织小胶质细胞活化,抑制中枢神经炎症反应,改善MPTP诱导帕金森病模型小鼠的运动和认知功能障碍。我们后续将对Ace改善帕金森病小鼠运动和认知功能障碍的具体机制进一步研究,为帕金森病的预防和治疗提供潜在的研究方向。
| [1] |
ZHANG Z X, ROMAN G C, HONG Z, WU C B, QU Q M, HUANG J B, et al. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai[J]. Lancet, 2005, 365: 595-597. DOI:10.1016/S0140-6736(05)70801-1 |
| [2] |
ARMSTRONG M J, OKUN M S. Diagnosis and treatment of Parkinson disease: a review[J]. JAMA, 2020, 323: 548-560. DOI:10.1001/jama.2019.22360 |
| [3] |
POEWE W, ANTONINI A. Novel formulations and modes of delivery of levodopa[J]. Mov Disord, 2015, 30: 114-120. DOI:10.1002/mds.26078 |
| [4] |
ALEXOUDI A, ALEXOUDI I, GATZONIS S. Parkinson's disease pathogenesis, evolution and alternative pathways: a review[J]. Rev Neurol (Paris), 2018, 174: 699-704. DOI:10.1016/j.neurol.2017.12.003 |
| [5] |
RANSOHOFF R M. How neuroinflammation contributes to neurodegeneration[J]. Science, 2016, 353: 777-783. DOI:10.1126/science.aag2590 |
| [6] |
TRAVAGLI R A, BROWNING K N, CAMILLERI M. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance[J]. Nat Rev Gastroenterol Hepatol, 2020, 17: 673-685. DOI:10.1038/s41575-020-0339-z |
| [7] |
DALILE B, VAN OUDENHOVE L, VERVLIET B, VERBEKE K. The role of short-chain fatty acids in microbiota-gut-brain communication[J]. Nat Rev Gastroenterol Hepatol, 2019, 16: 461-478. DOI:10.1038/s41575-019-0157-3 |
| [8] |
JACKSON-LEWIS V, PRZEDBORSKI S. Protocol for the MPTP mouse model of Parkinson's disease[J]. Nat Protoc, 2007, 2: 141-151. DOI:10.1038/nprot.2006.342 |
| [9] |
XU M D, JIANG Z Y, WANG C L, LI N, BO L L, ZHA Y P, et al. Publisher correction: acetate attenuates inflammasome activation through GPR43-mediated Ca2+-dependent NLRP3 ubiquitination[J/OL]. Exp Mol Med, 2019, 51: 1. DOI: 10.1038/s12276-019-0296-1.
|
| [10] |
AL-HARBI N O, NADEEM A, AHMAD S F, ALOTAIBI M R, ALASMARI A F, ALANAZI W A, et al. Short chain fatty acid, acetate ameliorates sepsisinduced acute kidney injury by inhibition of NADPH oxidase signaling in T cells[J]. Int Immunopharmacol, 2018, 58: 24-31. DOI:10.1016/j.intimp.2018.02.023 |
| [11] |
WEN C, XIE T, PAN K, DENG Y, ZHAO Z, LI N, et al. Acetate attenuates perioperative neurocognitive disorders in aged mice[J]. Aging (Albany NY), 2020, 12: 3862-3879. |
| [12] |
林臻, 陈洪志, 赵航, 刘辉, 许妍妍, 刘海岩, 张浩. MPTP诱导C57BL/6小鼠帕金森模型的制备和评估[J]. 中国比较医学杂志, 2020, 30: 57-62. |
| [13] |
JACKSON-LEWIS V, PRZEDBORSKI S. Protocol for the MPTP mouse model of Parkinson's disease[J]. Nat Protoc, 2007, 2: 141-151. DOI:10.1038/nprot.2006.342 |
| [14] |
DAUBNER S C, LET, WANG S Z. Tyrosine hydroxylase and regulation of dopamine synthesis[J]. Arch Biochem Biophys, 2011, 508: 1-12. DOI:10.1016/j.abb.2010.12.017 |
| [15] |
SALVATORE M F, MCINNIS T R, CANTU M A, APPLE D M, PRUETT B S. Tyrosine hydroxylase inhibition in substantia nigra decreases movement frequency[J]. Mol Neurobiol, 2019, 56: 2728-2740. DOI:10.1007/s12035-018-1256-9 |
| [16] |
TAN X F, ZHANG L, ZHU H X, QIN J B, TIAN M L, DONG C M, et al. Brn4 and TH synergistically promote the differentiation of neural stem cells into dopaminergic neurons[J]. Neurosci Lett, 2014, 571: 23-28. DOI:10.1016/j.neulet.2014.04.019 |
| [17] |
LIU X M, WANG C K, LIU W W, SONG S, FU J N, HAYASHI T, et al. Oral administration of silibinin ameliorates cognitive deficits of Parkinson's disease mouse model by restoring mitochondrial disorders in hippocampus[J]. Neurochem Res, 2021, 46: 2317-2332. DOI:10.1007/s11064-021-03363-5 |
| [18] |
HUANG Z J, ZHOU T, SUN X W, ZHENG Y F, CHENG B, LI M, et al. Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation[J]. Cell Death Differ, 2018, 25: 180-189. DOI:10.1038/cdd.2017.141 |
| [19] |
LEE E, HWANG I, PARK S, HONG S, HWANG B, CHO Y, et al. MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration[J]. Cell Death Differ, 2019, 26: 213-228. DOI:10.1038/s41418-018-0124-5 |
| [20] |
BARTELS T, DE SCHEPPER S, HONG S. Microglia modulate neurodegeneration in Alzheimer's and Parkinson's diseases[J]. Science, 2020, 370: 66-69. DOI:10.1126/science.abb8587 |
 2022, Vol. 43
2022, Vol. 43


