2. 重庆医科大学附属儿童医院儿科学重庆市重点实验室,重庆 400014;
3. 重庆医科大学附属儿童医院儿童发育疾病研究教育部重点实验室,重庆 400014;
4. 重庆医科大学附属儿童医院泌尿外科,重庆 400014;
5. 重庆医科大学附属儿童医院儿童发育重大疾病国家国际科技合作基地,重庆 400014;
6. 重庆医科大学附属儿童医院国家儿童健康与疾病临床医学研究中心,重庆 400014
2. Chongqing Key Laboratory of Pediatrics, Children's Hospital of Chongqing Medical University, Chongqing 400014, China;
3. Key Laboratory of Child Development and Disorders of Ministry of Education, Children's Hospital of Chongqing Medical University, Chongqing 400014, China;
4. Department of Urology, Children's Hospital of Chongqing Medical University, Chongqing 400014, China;
5. China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Children's Hospital of Chongqing Medical University, Chongqing 400014, China;
6. National Clinical Research Center for Child Health and Disorders, Children's Hospital of Chongqing Medical University, Chongqing 400014, China
尿道下裂是指阴茎腹侧发育缺陷导致的尿道口异位开口,发病率为1/1 000~1/100[1],是小儿泌尿系统最常见的发育缺陷之一[2]。尿道下裂病因仍不明确,可能由基因、环境和内分泌等多因素共同作用所致[1, 3],探究其发病机制十分必要。雄激素驱动外生殖器雄性化是阴茎正常发育的关键[4]。然而,在胚胎外生殖结节性别分化的过程中,雄激素受体(androgen receptor,AR)有哪些协同转录因子尚不清楚。v-maf肌筋膜纤维肉瘤癌基因同源物B(v-maf musculoaponeurotic fibrosarcoma oncogene homolog B,Mafb)是一种碱性亮氨酸拉链(basic-leucine zipper protein,bZIP)转录因子,属于活化蛋白1(activator protein 1,AP-1)超家族成员,表达广泛并参与多种生物学事件,如细胞分化、凋亡、增殖和迁移等[5-7]。除在颌面部、胰腺和肾脏等组织表达并发挥重要作用[8-10]外,Mafb在正常雄性个体尿道板上皮细胞中亦有显著表达,这是雄激素干预雄性尿道分化的重要靶区[11-12]。有研究通过器官培养实验发现,雄激素5α-双氢睾酮(5α-dihydrotestosterone,DHT)干预后的雄性和雌性小鼠生殖结节中Mafb表达明显增加[13]。本课题组前期研究发现,尿道下裂患儿较正常儿童包皮组织中Mafb表达显著下降[14]。这一系列的研究结果表明Mafb受雄激素信号通路调控,可能在雄性外生殖结节发育和尿道下裂的发病机制中起到至关重要的作用。此外,本课题组前期通过免疫荧光技术及透射电子显微镜证实,上皮-间质转化(epithelial-mesenchymal transition,EMT)在大鼠尿道发育中发挥重要作用[15]。本研究拟构建Mafb基因敲除小鼠,观察其是否有尿道下裂表型,并关注EMT标志物上皮钙黏蛋白(epithelial cadherin,E-cadherin)和α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)在此模型尿道处的表达,为后续探讨Mafb基因对尿道发育的影响及相关机制奠定基础。
1 材料和方法 1.1 主要材料 1.1.1 实验动物采用成簇的规律间隔的短回文重复序列(clustered regularly interspaced short palindromic repeats,CRISPR)及其相关蛋白9(CRISPR-associated protein 9,Cas9)介导的基因编辑技术构建以C57BL/6J为遗传背景的Mafb基因敲除小鼠,由本课题组与上海南方模式生物科技股份有限公司[实验动物生产许可证号:SCXK(沪)2017-0010]合作完成。野生型C57BL/6J小鼠购自重庆医科大学实验动物中心[实验动物生产许可证号:SCXK(渝)2017-0001]。实验动物均在重庆医科大学附属儿童医院动物实验中心SPF级动物房饲养[实验动物使用许可证号:SYXK(渝)2017-0012]。实验操作符合实验动物伦理学要求并通过伦理审查(CHCMU-IACUC20200424018)。
1.1.2 试剂EmeraldAmp PCR Master Mix、DNA marker[宝日医生物技术(北京)有限公司],引物[生工生物工程(上海)股份有限公司],蛋白酶K(大连美仑生物技术有限公司),兔抗Mafb多克隆抗体、小鼠抗E-cadherin单克隆抗体(英国Abcam公司),兔抗α-SMA单克隆抗体(成都正能生物技术有限责任公司),Hoechst 33342、抗荧光淬灭封片液(上海碧云天生物技术有限公司),Cy3-山羊抗兔二抗、Cy3-山羊抗小鼠二抗(北京康为世纪生物科技有限公司),Triton X-100(美国Sigma公司),Tris、EDTA、十二烷基硫酸钠(sodium dodecyl sulfate,SDS)(美国Genview公司)。组织裂解液配制:3 mol/L NaCl 66.7 μL、1 mol/L Tris-HCl(pH=8.0)100 μL、0.5 mol/L EDTA 10 μL、10% SDS 20 μL、蛋白酶K 10 μL,双蒸水定容至1 mL。
1.1.3 仪器T100 PCR仪(美国Bio-Rad公司),光学显微成像系统和荧光显微成像系统(日本Nikon公司),Inspect S50扫描电子显微镜(美国FEI公司),组织切片机(德国Leica公司)。
1.2 Mafb基因敲除小鼠的构建和基因型鉴定 1.2.1 Mafb基因敲除小鼠的构建根据Ensembl数据库(http://ensemblgenomes.org/)中的小鼠基因组序列信息,小鼠Mafb基因仅存在转录本Mafb-201(ENSMUST00000099126.5),且仅有一个外显子(exon 1)。通过Crispor在线软件(http://crispor.tefor.net/),针对exon 1的5'-非翻译区(untranslated region,UTR)和3'-UTR分别设计2条特异性高且更靠近编码区域的指导RNA(guide RNA,gRNA),其中gRNA1的序列为5'-GGGAAAACTTTGCGGCCGGC-3'(前间隔序列邻近基序为CGG),gRNA2的序列为5'-TCTGGGCCAGGGCAAGGGCG-3'(前间隔序列邻近基序为GGG)。通过体外转录的方式获得Cas9 mRNA和gRNA,将其显微注射到C57BL/6J小鼠的受精卵中,通过非同源末端连接(non-homologous end joining,NHEJ)途径进行基因沉默,获得F0代小鼠(图 1)。经PCR扩增及上海南方模式生物科技股份有限公司测序鉴定阳性的F0代小鼠与野生型C57BL/6J小鼠交配获得6只F1代小鼠。将鉴定为Mafb基因敲除杂合子(Mafb+/-)的F1代小鼠按照雌雄比2∶1于傍晚合笼,次日清晨在雌鼠阴道口寻找阴栓,发现阴栓当天视为妊娠日(gestation day,GD)0.5 d,GD 18.5 d剖宫产取出胎鼠,采用PCR鉴定Mafb和性别基因型。
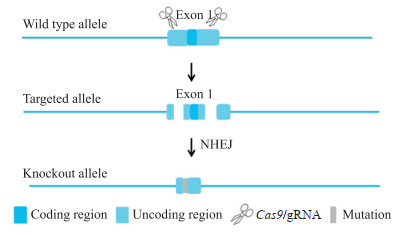
|
图 1 Mafb基因敲除小鼠构建策略 Fig 1 Construction strategy of Mafb knockout mice The Cas9 protein bound to the target site under the guidance of gRNA resulting in the deletion of the sequence of exon 1, and finally obtained Mafb knockout mice. Mafb: V-maf musculoaponeurotic fibrosarcoma oncogene homolog B; Cas9: Clustered regularly interspaced short palindromic repeats-associated protein 9; gRNA: Guide RNA; NHEJ: Non-homologous end joining. |
1.2.2 PCR鉴定基因型
取胎鼠尾组织,加入组织裂解液500 μL,56 ℃水浴过夜;次日以13 000×g离心15 min,取上清液至1.5 mL EP管中,加等体积异丙醇,混匀后13 000×g离心10 min;弃上清,经75%乙醇洗涤后用DEPC水溶解DNA,放入-20 ℃冰箱备用。基因型鉴定引物序列由上海南方模式生物科技股份有限公司设计(表 1),由生工生物工程(上海)股份有限公司合成。Mafb敲除和野生型基因型鉴定PCR扩增条件:95 ℃ 5 min;95 ℃ 15 s,60 ℃ 15 s,72 ℃ 2 min,循环35次;72 ℃ 5 min,12 ℃保持。性别基因型鉴定PCR扩增条件:95 ℃ 2 min;95 ℃ 30 s,60 ℃ 30 s,72 ℃30 s,循环35次;72 ℃ 2 min,12 ℃保持。取PCR产物10 μL进行1.5%琼脂糖凝胶电泳。
|
|
表 1 基因型鉴定引物序列 Tab 1 Primer sequences for genotype identification |
1.3 Mafb-/-胎鼠阴茎组织表型观察
收集雄性Mafb-/-和野生型胎鼠阴茎组织标本,一部分放入4%多聚甲醛溶液中固定以备石蜡包埋,一部分放入2.5%戊二醛溶液中固定进行扫描电子显微镜观察。
1.3.1 免疫荧光技术检测Mafb、E-cadherin和α-SMA蛋白表达固定完成的Mafb-/-和野生型胎鼠阴茎组织由重庆医科大学儿科研究所免疫组织化学室统一脱水处理。充分浸蜡后,将阴茎远端垂直向下进行包埋,凝固后连续切片,厚度为4 μm,由远及近顺序编号。切片放入60 ℃烤箱烘烤24 h,室温保存。取出保存的切片,放入60 ℃烤箱预热30 min,脱蜡后置于枸橼酸溶液中用微波炉高火加热修复抗原,冷却后经0.2% Triton X-100溶液打孔、0.5%牛血清白蛋白(bovine serum albumin,BSA)溶液封闭后,加入Mafb抗体、E-cadherin抗体和α-SMA抗体(稀释比例为1∶200,0.5% BSA配制)4 ℃过夜孵育。室温复温30 min,PBS清洗后加入荧光二抗(稀释比例为1∶200,0.5% BSA配制)、Hoechst 33342(稀释比例为1∶200,PBS配制)孵育。使用抗荧光淬灭封片液封片,通过荧光显微成像系统采集图像。
1.3.2 扫描电子显微镜和H-E染色观察阴茎组织形态将固定完成的胎鼠阴茎组织用PBS清洗后,用4%蔗糖溶液洗涤5 min,梯度乙醇脱水;样本粘于导电胶上,临界点干燥,真空喷镀,在扫描电子显微镜下观察。选取阴茎组织石蜡切片,脱蜡后经H-E染色,中性树脂封片,在光学显微镜下观察。
2 结果 2.1 小鼠繁殖情况获得的Mafb+/-小鼠饮食、发育等正常,皮肤、毛色与野生型小鼠相比无明显差异,无外观畸形(图 2A、2B);Mafb+/-雌鼠妊娠期为19.5 d,哺乳期为21 d,合笼后每胎孕5~8只,可正常生长繁殖。Mafb-/-鼠出生正常,但很快出现发绀,并在出生2 h内死亡(图 2C、2D)。GD 18.5 d的Mafb-/-胎鼠外观与野生型胎鼠未见明显差异,未出现唇腭裂、四肢缺损、脊柱裂等外观畸形(图 2E~2H)。
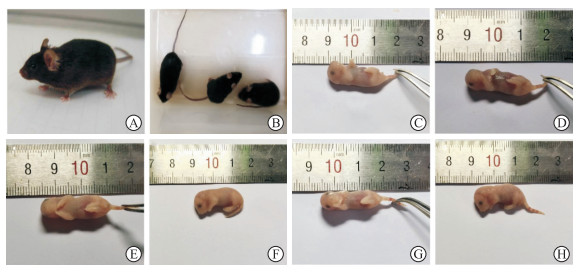
|
图 2 Mafb基因敲除小鼠大体形态学观察 Fig 2 General morphological observation of Mafb knockout mice A: Adult Mafb+/- mouse; B: Adult Mafb+/- mouse (the left one) and wild type mice (the right two); C: Newborn wild type mouse; D: Newborn Mafb-/- mouse; E, F: Fetal wild type mouse at GD 18.5 d; G, H: Fetal Mafb-/- mouse at GD 18.5 d. Mafb: V-maf musculoaponeurotic fibrosarcoma oncogene homolog B; GD: Gestation day. |
2.2 小鼠基因型鉴定结果
P1引物为Mafb基因缺失型引物,P2引物为Mafb基因野生型引物,P3引物为雄性基因型引物。根据PCR结果判定基因型,野生型小鼠仅有P2引物扩增产物约406 bp的条带,Mafb+/-小鼠会同时出现P1和P2引物扩增产物约604 bp和约406 bp的条带,Mafb-/-小鼠仅出现P1引物扩增产物约604 bp的条带(图 3A);雄性小鼠出现P3引物扩增产物约250 bp的条带,雌性小鼠则未出现条带(图 3B)。
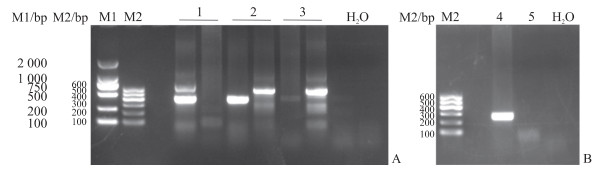
|
图 3 Mafb基因敲除小鼠基因型鉴定结果 Fig 3 Results of genotype identification of Mafb knockout mice A: Genotype identification of Mafb; B: Genotype identification of SRY. M1: DL 2000 marker; M2: DNA marker Ⅰ; 1: Genotype identification of wild type mice, only 406 bp band; 2: Genotype identification of Mafb+/- mice, both 406 bp and 604 bp bands; 3: Genotype identification of Mafb-/- mice, only 604 bp band; 4: Genotype identification of male mice; 5: Genotype identification of female mice; H2O: Negative control. Mafb: V-maf musculoaponeurotic fibrosarcoma oncogene homolog B; SRY: Sex determining region Y. |
2.3 Mafb基因敲除小鼠阴茎组织中Mafb蛋白表达情况
免疫荧光检测结果显示,Mafb蛋白在野生型胎鼠阴茎组织中主要表达在尿道上皮细胞,而在Mafb-/-胎鼠阴茎组织中几乎没有表达(图 4),证明Mafb基因敲除小鼠模型成功建立。
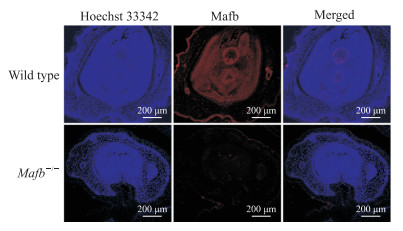
|
图 4 免疫荧光检测Mafb-/-胎鼠阴茎组织中Mafb蛋白的表达 Fig 4 Mafb protein expression in penile tissues of fetal Mafb-/- mice detected by immunofluorescence Mafb: V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. |
2.4 Mafb基因敲除小鼠阴茎组织形态
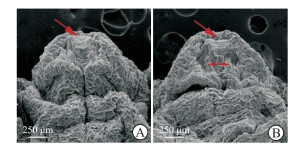
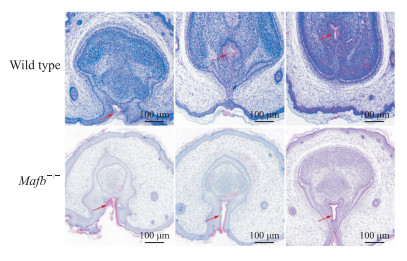
扫描电子显微镜下观察可见,雄性C57BL/6J小鼠的阴茎在GD 18.5 d已基本发育成型,野生型胎鼠阴茎组织形态完整,尿道皱褶在腹侧融合,冠状沟清晰,尿道并入龟头(图 5A);Mafb-/-胎鼠阴茎组织形态异常,尿道皱褶融合障碍、腹侧包皮部分缺如,呈现“V”字形,尿道开口在阴茎腹侧位置(图 5B)。H-E染色结果显示,野生型胎鼠阴茎组织尿道板已充分融合,中心尿道腔清晰;Mafb-/-胎鼠阴茎腹侧包皮发育缺损,尿道板未完全融合(图 6)。
|
图 5 扫描电子显微镜下观察Mafb-/-胎鼠阴茎组织形态 Fig 5 Morphology of penile tissues of fetal Mafb-/- mice observed under scanning electron microscopy A: Fetal wide type mouse; B: Fetal Mafb-/- mouse. The single-head arrows indicate urethral meatus, and the double-headed arrow indicates failure of urethral fold fusion in fetal Mafb-/-mouse. Mafb: V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. |
|
图 6 Mafb-/-胎鼠阴茎组织连续切片病理学观察 Fig 6 Pathology of serial sections of penile tissues in fetal Mafb-/- mice Hematoxylin-eosin staining. The sections from left to right are the distal to proximal end of the penis. The arrows indicate urethral meatus. Mafb: V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. |
2.5 Mafb基因敲除小鼠阴茎组织E-cadherin和α-SMA表达情况
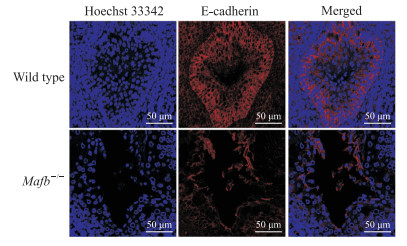
免疫荧光检测结果显示,与野生型胎鼠相比,Mafb-/-胎鼠阴茎组织尿道缝处细胞E-cadherin表达明显下降(图 7),α-SMA表达明显增加(图 8)。
|
图 7 免疫荧光检测Mafb-/-胎鼠阴茎组织中E-cadherin的表达 Fig 7 E-cadherin expression in penile tissues of fetal Mafb-/- mice detected by immunofluorescence Mafb: V-maf musculoaponeurotic fibrosarcoma oncogene homolog B; E-cadherin: Epithelial cadherin. |

|
图 8 免疫荧光检测Mafb-/-胎鼠阴茎组织中α-SMA的表达 Fig 8 α-SMA expression in penile tissues of fetal Mafb-/-mice detected by immunofluorescence Mafb: V-maf musculoaponeurotic fibrosarcoma oncogene homolog B; α-SMA: α-smooth muscle actin. |
3 讨论
尿道下裂是男性最常见的先天性畸形之一,主要表现为腹侧包皮发育不全和尿道口异位等症状[16-17]。目前尿道下裂的主要治疗手段仍是外科手术,通常需要多次手术以达到功能和外观上可接受的结果,但术后并发症如尿道瘘、尿道狭窄等常不能避免,特别是重型尿道下裂患者,甚至可出现终身排尿困难和性功能障碍,并有增加心理问题发生的风险[18-19]。因此,尿道下裂作为一个重要的健康问题,探究其发病机制尤为重要。目前大多数尿道下裂患者病因仍不明确,而现阶段主要的研究方向是遗传易感性和环境内分泌干扰物[20-21],其中,对外生殖器发育具有重要调控功能的雄激素信号通路逐渐成为关注重点。
外生殖器的发育涉及多种信号通路调控,雄激素信号通路在男性生殖发育过程中发挥着重要作用[4]。动物胚胎研究发现,在小鼠中,直到GD 14.5 d,不同性别外生殖器的解剖学差异才显著,而生殖器结节的性别分化始于GD 16.5 d,此时尿道板管状化形成尿道,男性尿道并入龟头,这一系列过程依赖于雄激素信号通路[22]。在胚胎组织或成体组织中局部产生的雄激素包括睾酮和DHT,这2种激素在生殖结节器官发生中通过AR介导其重要作用[23-24],而雄激素可诱导Mafb的表达。有研究表明Mafb在AR基因条件性敲除小鼠的雄性生殖结节中表达显著下调[25],结合本课题组前期使用DHT干预正常儿童包皮成纤维细胞发现Mafb表达显著上调、而AR拮抗剂氟他胺干预会显著下调Mafb的表达[26],提示转录因子Mafb为雄激素反应靶基因,可能参与调节男性外生殖器形成。
Mafb是AP-1家族的重要成员,作为启动基因转录的开关,其在肾脏、胰腺、肺和脑等多个组织中广泛表达[5],该基因缺失可能会导致生长发育过程中部分功能减弱或障碍,例如,Mafb基因突变与跖骨溶解综合征相关,Mafb和Maf双敲除可导致睾丸血管增生、睾丸间质细胞缺陷和生殖细胞数量减少等[27-29]。在与尿道发育过程类似的颌面部发育研究中,已证实小鼠胚胎颅面腭融合过程中Mafb在腭架周围上皮和内侧缘上皮中表达强烈,表达模式与唇腭发育中作用一致,表明Mafb在唇腭形态发生中发挥作用[30]。本研究构建了Mafb基因敲除小鼠,发现Mafb-/-胎鼠阴茎尿道板融合不完全,腹侧包皮融合障碍,出现明显的尿道下裂表型,说明Mafb是男性外生殖器形成过程中的重要一环。但Mafb的下游基因有哪些及如何通过靶基因影响尿道发育的具体机制目前仍不明确,需进一步研究。EMT在胚胎发育中,尤其在神经嵴发育、腭部形成等过程中的关键作用已有相关文献报道[31-32],本课题组前期证实EMT与大鼠胚胎阴茎发育关系密切[15],故本研究挑选EMT关键蛋白E-cadherin和α-SMA检测,发现Mafb-/-胎鼠尿道缝处细胞表达与野生型小鼠相比出现明显差异,提示间质细胞可能存在异常增殖,推测尿道融合障碍可能与Mafb表达下降导致尿道缝处EMT异常有关,其机制有待进一步研究。
CRISPR/Cas9系统通过gRNA和Cas9蛋白的参与,将待编辑的细胞基因组DNA看作病毒或外源DNA,精确剪切预设的DNA位点造成DNA双链断裂,诱导其发生缺失突变[33],具有合成简便、特异性高、可对靶基因多个位点或多个基因同时敲除、更容易预测可能的突变位点等优点[34]。相较于此前研究使用的Cre-loxp系统构建模型[25, 35-36],本研究利用CRISPR-Cas9技术通过NHEJ高效沉默基因,成功构建了Mafb基因敲除小鼠,Mafb+/-小鼠生长良好、能够正常繁育,而Mafb-/-小鼠在出生时正常、但很快出现发绀并死亡,这与2003年的一项研究结果相印证,此研究分析Mafb-/-小鼠会由于呼吸节律缺陷和致命性的中枢性呼吸暂停导致出生后立即死亡[35]。本研究取材的GD 18.5 d小鼠阴茎组织几乎已发育完整[37],因此Mafb-/-小鼠出生后的致命性缺陷对本研究结果几乎没有影响。本研究采用免疫荧光技术检测Mafb-/-胎鼠阴茎组织形态,结果表明几乎没有Mafb蛋白表达,说明敲除成功,且与PCR基因型鉴定结果一致。
本研究利用CRISPR/Cas9技术成功构建了Mafb基因敲除小鼠,通过对Mafb基因敲除小鼠的繁育和基因型的鉴定及Mafb-/-胎鼠尿道下裂表型的初步研究,证实该基因敲除可导致小鼠出现尿道下裂表型及尿道缝处细胞中E-cadherin和α-SMA的表达差异,这为进一步研究Mafb在尿道下裂发生机制中的作用奠定了基础。
| [1] |
MANSON J M, CARR M C. Molecular epidemiology of hypospadias: review of genetic and environmental risk factors[J]. Birth Defects Res A Clin Mol Teratol, 2003, 67: 825-836. DOI:10.1002/bdra.10084 |
| [2] |
WOOD D, BAIRD A, CARMIGNANI L, DE WIN G, HOEBEKE P, HOLMDAHL G, et al. Lifelong congenital urology: the challenges for patients and surgeons[J]. Eur Urol, 2019, 75: 1001-1007. DOI:10.1016/j.eururo.2019.03.019 |
| [3] |
SKAKKEBAEK N E, RAJPERT-DE MEYTS E, BUCK LOUIS G M, TOPPARI J, ANDERSSON A M, EISENBERG M L, et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility[J]. Physiol Rev, 2016, 96: 55-97. DOI:10.1152/physrev.00017.2015 |
| [4] |
MATSUSHITA S, SUZUKI K, MURASHIMA A, KAJIOKA D, ACEBEDO A R, MIYAGAWA S, et al. Regulation of masculinization: androgen signalling for external genitalia development[J]. Nat Rev Urol, 2018, 15: 358-368. DOI:10.1038/s41585-018-0008-y |
| [5] |
TSUCHIYA M, MISAKA R, NITTA K, TSUCHIYA K. Transcriptional factors, Mafs and their biological roles[J]. World J Diabetes, 2015, 6: 175-183. DOI:10.4239/wjd.v6.i1.175 |
| [6] |
PAI EL, CHEN J, FAZEL DARBANDI S, CHO F S, CHEN J, LINDTNER S, et al. Maf and Mafb control mouse pallial interneuron fate and maturation through neuropsychiatric disease gene regulation[J/OL]. Elife, 2020, 9: e54903. DOI: 10.7554/eLife.54903.
|
| [7] |
LABOTT A T, LOPEZ-PAJARES V. Epidermal differentiation gene regulatory networks controlled by MAF and MAFB[J]. Cell Cycle, 2016, 15: 1405-1409. DOI:10.1080/15384101.2016.1172148 |
| [8] |
YUAN Q, BLANTON S H, HECHT J T. Association of ABCA4 and MAFB with non-syndromic cleft lip with or without cleft palate[J]. Am J Med Genet A, 2011, 155A: 1469-1471. |
| [9] |
XIAFUKAITI G, MAIMAITI S, OGATA K, KUNO A, KUDO T, SHAWKI H H, et al. MafB is important for pancreatic β-cell maintenance under a MafA-deficient condition[J/OL]. Mol Cell Biol, 2019, 39: e00080-19. DOI: 10.1128/MCB.00080-19
|
| [10] |
USUI T, MORITO N, SHAWKI H H, SATO Y, TSUKAGUCHI H, HAMADA M, et al. Transcription factor MafB in podocytes protects against the development of focal segmental glomerulosclerosis[J]. Kidney Int, 2020, 98: 391-403. DOI:10.1016/j.kint.2020.02.038 |
| [11] |
NISHIDA H, MIYAGAWA S, MATSUMARU D, WADA Y, SATOH Y, OGINO Y, et al. Gene expression analyses on embryonic external genitalia: identification of regulatory genes possibly involved in masculinization processes[J]. Congenit Anom (Kyoto), 2008, 48: 63-67. DOI:10.1111/j.1741-4520.2008.00180.x |
| [12] |
向涵, 王绍, 周玥, 刘星, 沈炼桔, 龙春兰, 等. 邻苯二甲酸二乙酯诱导的大鼠尿道下裂与Mafb的表达相关: 基于转录组测序结果[J]. 南方医科大学学报, 2019, 39: 456-463. |
| [13] |
MATSUSHITA S, SUZUKI K, OGINO Y, HINO S, SATO T, SUYAMA M, et al. Androgen regulates Mafb expression through its 3' UTR during mouse urethral masculinization[J]. Endocrinology, 2016, 157: 844-857. DOI:10.1210/en.2015-1586 |
| [14] |
KONG X, LUO J, XIANG H, WANG S, SHEN L, LONG C, et al. Expression of Mafb is down-regulated in the foreskin of children with hypospadias[J/OL]. J Pediatr Urol, 2021, 17: 70. e1-70. e6. DOI: 10.1016/j.jpurol.2020.10.006.
|
| [15] |
ZHOU Y, LIU X, HUANG F, LIU Y, CAO X, SHEN L, et al. Epithelial-mesenchymal transformation and apoptosis in rat urethra development[J]. Pediatr Res, 2017, 82: 1073-1079. DOI:10.1038/pr.2017.185 |
| [16] |
RÜBBEN I, STEIN R. Hypospadias: insights and challenges[J]. Urologe A, 2017, 56: 1256-1265. DOI:10.1007/s00120-017-0498-x |
| [17] |
BASKIN L S, EBBERS M B. Hypospadias: anatomy, etiology, and technique[J]. J Pediatr Surg, 2006, 41: 463-472. DOI:10.1016/j.jpedsurg.2005.11.059 |
| [18] |
WIENER J S, HUCK N, BLAIS A S, RICKARD M, LORENZO A, DI CARLO H N M, et al. Challenges in pediatric urologic practice: a lifelong view[J]. World J Urol, 2021, 39: 981-991. DOI:10.1007/s00345-020-03203-1 |
| [19] |
DEMARCO R T. Hypospadias surgery: search for elusive perfection-should we re-define surgical success to improve outcomes and provide reasonable postoperative expectations?[J]. J Urol, 2021, 206: 505-506. DOI:10.1097/JU.0000000000001926 |
| [20] |
FOSTER W G, EVANS J A, LITTLE J, ARBOUR L, MOORE A, SAUVE R, et al. Human exposure to environmental contaminants and congenital anomalies: a critical review[J]. Crit Rev Toxicol, 2017, 47: 59-84. DOI:10.1080/10408444.2016.1211090 |
| [21] |
JOODI M, AMERIZADEH F, HASSANIAN S M, ERFANI M, GHAYOUR-MOBARHAN M, FERNS G A, et al. The genetic factors contributing to hypospadias and their clinical utility in its diagnosis[J/OL]. J Cell Physiol, 2019, 234: 5519-5523. DOI: 10.1002/jcp.27350.
|
| [22] |
SUZUKI H, SUZUKI K, YAMADA G. Systematic analyses of murine masculinization processes based on genital sex differentiation parameters[J]. Dev Growth Differ, 2015, 57: 639-647. DOI:10.1111/dgd.12247 |
| [23] |
SUZUKI H, MATSUSHITA S, SUZUKI K, YAMADA G. 5α-Dihydrotestosterone negatively regulates cell proliferation of the periurethral ventral mesenchyme during urethral tube formation in the murine male genital tubercle[J]. Andrology, 2017, 5: 146-152. DOI:10.1111/andr.12241 |
| [24] |
COOKE P S, WALKER W H. Male fertility in mice requires classical and nonclassical androgen signaling[ J/OL]. Cell Rep, 2021, 36: 109557. DOI: 10.1016/j.celrep.2021.109557.
|
| [25] |
SUZUKI K, NUMATA T, SUZUKI H, RAGA D D, IPULAN L A, YOKOYAMA C, et al. Sexually dimorphic expression of Mafb regulates masculinization of the embryonic urethral formation[J]. PNAS, 2014, 111: 16407-16412. DOI:10.1073/pnas.1413273111 |
| [26] |
王绍, 周玥, 向涵, 刘星, 沈炼桔, 龙春兰, 等. 雄激素受体干预对包皮成纤维细胞Mafb基因表达的影响[J]. 重庆医科大学学报, 2019, 44: 1003-1009. |
| [27] |
LI S Y, GU X, HEINRICH A, HURLEY E G, CAPEL B, DEFALCO T. Loss of Mafb and Maf distorts myeloid cell ratios and disrupts fetal mouse testis vascularization and organogenesis[J]. Biol Reprod, 2021, 105: 958-975. DOI:10.1093/biolre/ioab098 |
| [28] |
RUSSELL R, CARNESE P P, HENNINGS T G, WALKER E M, RUSS H A, LIU J S, et al. Loss of the transcription factor MAFB limits β-cell derivation from human PSCs[J/OL]. Nat Commun, 2020, 11: 2742. DOI: 10.1038/s41467-020-16550-9.
|
| [29] |
ZANKL A, DUNCAN E L, LEO P J, CLARK G R, GLAZOV E A, ADDOR M C, et al. Multicentric carpotarsal osteolysis is caused by mutations clustering in the amino-terminal transcriptional activation domain of MAFB[J]. Am J Hum Genet, 2012, 90: 494-501. DOI:10.1016/j.ajhg.2012.01.003 |
| [30] |
BEATY T H, MURRAY J C, MARAZITA M L, MUNGER R G, RUCZINSKI I, HETMANSKI J B, et al. A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4[J]. Nat Genet, 2010, 42: 525-529. DOI:10.1038/ng.580 |
| [31] |
NAKAJIMA A, F SHULER C, GULKA AOD, HANAI J I. TGF-β signaling and the epithelial-mesenchymal transition during palatal fusion[J/OL]. Int J Mol Sci, 2018, 19: 3638. DOI: 10.3390/ijms19113638.
|
| [32] |
KAWACHI T, TADOKORO R, TAKAHASHI Y. Cell lineage, self-renewal, and epithelial-to-mesenchymal transition during secondary neurulation[J]. J Korean Neurosurg Soc, 2021, 64: 367-373. DOI:10.3340/jkns.2021.0054 |
| [33] |
CROTEAU F R, ROUSSEAU G M, MOINEAU S. The CRISPR-Cas system: beyond genome editing[J]. Med Sci, 2018, 34: 813-819. |
| [34] |
HSU P D, LANDER E S, ZHANG F. Development and applications of CRISPR-Cas9 for genome engineering[J]. Cell, 2014, 157: 1262-1278. DOI:10.1016/j.cell.2014.05.010 |
| [35] |
BLANCHI B, KELLY L M, VIEMARI J C, LAFON I, BURNET H, BÉVENGUT M, et al. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth[J]. Nat Neurosci, 2003, 6: 1091-1100. DOI:10.1038/nn1129 |
| [36] |
PARK J G, TISCHFIELD M A, NUGENT A A, CHENG L, DI GIOIA S A, CHAN W M, et al. Loss of MAFB function in humans and mice causes Duane syndrome, aberrant extraocular muscle innervation, and inner-ear defects[J]. Am J Hum Genet, 2016, 98: 1220-1227. |
| [37] |
GEORGAS K M, ARMSTRONG J, KEAST J R, LARKINS C E, MCHUGH K M, SOUTHARDSMITH E M, et al. An illustrated anatomical ontology of the developing mouse lower urogenital tract[J]. Development, 2015, 142: 1893-1908. DOI:10.1242/dev.117903 |
 2022, Vol. 43
2022, Vol. 43


