2. 上海交通大学医学院附属第九人民医院药剂科,上海 200011
2. Department of Pharmacy, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China
肿瘤的发生、发展与其赖以生存的微环境密切相关,立足于肿瘤微环境开展研究有望发现新的肿瘤治疗靶点。肿瘤微环境由多种细胞和细胞外基质构成,这些细胞与基质间的相互联系、相互作用促进了肿瘤的发生、发展[1]。肿瘤微环境有缺氧、低pH、高还原性等特征,其中缺氧是大多数实体瘤的典型特征,并且在不同肿瘤中其发生率和严重程度都不同[2]。有研究表明缺氧使肿瘤细胞发生生物学特性改变进而导致肿瘤细胞恶性增殖,同时缺氧也是导致肿瘤产生治疗抵抗的一个重要原因[3-4]。因此,利用实体肿瘤特殊的缺氧微环境,有望发展出新型高效、高选择性的治疗方法,提高实体肿瘤的治疗效果。
近年来,有研究通过纳米递送系统来调节肿瘤的缺氧微环境,使所递送药物发挥更好的肿瘤治疗作用[5]。纳米递送系统的多功能特性使其在治疗恶性肿瘤方面比传统治疗方法更具优越性。纳米递送载体不仅能通过实体肿瘤的高渗透长滞留效应(enhanced permeadility and retention effect,EPR效应)在肿瘤部位积聚,还可以在表面修饰肿瘤特异性配体使其主动靶向肿瘤细胞,降低治疗药物的系统性毒性,精准治疗肿瘤。纳米递送系统能够克服传统化学治疗药物水溶性差、非靶向毒性和非特异性生物分布等缺陷[6],有望实现肿瘤的高效治疗。基于改善肿瘤缺氧微环境的纳米靶向递送系统的研究已成为当前的研究热点。本文综述了近年来基于缺氧微环境提高肿瘤化学治疗、放射治疗、光动力疗法和免疫疗法的纳米递送载体的研究进展,以期为进一步开发高效、以缺氧为靶点的抗肿瘤纳米递送系统找到新的突破口,为肿瘤的靶向治疗提供新策略。
1 肿瘤缺氧及其分子机制肿瘤缺氧的发生源于组织氧供应和氧消耗之间的不平衡。由肿瘤血管内皮生长因子(vascular endothelial growth factor,VEGF)刺激所产生的血管脉络紊乱和结构不正常会导致血管血流量少,血红蛋白携载氧不充足、氧供应不足;肿瘤细胞的快速增殖扩张需要消耗大量氧以满足代谢,导致氧供需失衡,最终形成肿瘤的缺氧微环境[7]。
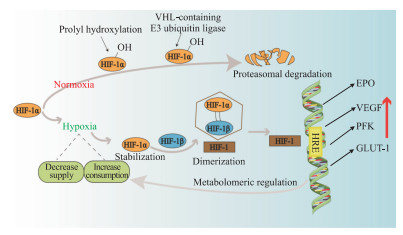
缺氧可诱导肿瘤微环境中的一些细胞因子如促红细胞生成素(erythropoietin,EPO)和相关蛋白如磷酸果糖激酶(phosphofructokinase,PFK)表达的变化,这些改变在肿瘤细胞适应缺氧、能量存储、转移增殖及肿瘤血管生成中起重要作用,最终导致肿瘤细胞代谢异常,也激化了肿瘤的恶性生长和治疗抵抗[8]。缺氧诱导因子1(hypoxia-inducible factor 1,HIF-1)主要介导了上述细胞因子(如EPO)和相关蛋白(如PFK)的改变。HIF-1是具有转录活性的核蛋白,由HIF-1α亚基和HIF-1β亚基组成。在常氧条件下,HIF-1α在泛素化作用下被降解;在缺氧条件下,HIF-1α保持稳定。HIF-1β在细胞内稳定表达,起结构性作用,HIF-1α亚基必须与HIF-1β亚基聚合形成异二聚体才能发挥转录因子的作用。对缺氧作出应激反应的基因称为缺氧应激基因(hypoxia response gene,HRG),在HRG中受HIF-1α调控的基因则为HIF-1α的靶基因。这些靶基因的启动子或增强子内含数量不同的缺氧反应元件(hypoxia response element,HRE),是HIF-1α的结合位点。HIF-1α与HRE结合形成转录起始复合物,启动靶基因转录产生多种产物,引起一系列反应(图 1)。例如,缺氧条件下HIF-1可与VEGF启动子区的HRE结合,引起VEGF表达的上调,增加新生血管的生成,促进肿瘤的生长和转移[9-11]。
|
图 1 肿瘤缺氧的分子机制示意图 Fig 1 Diagrammatic sketch of molecular mechanism of tumor hypoxia HIF-1: Hypoxia-inducible factor 1; VHL: Von Hippel-Lindau disease gene; EPO: Erythropoietin; VEGF: Vascular endothelial growth factor; PFK: Phosphofructokinase; GLUT-1: Glucose transporter 1; HRE: Hypoxia response element |
2 基于肿瘤缺氧微环境的纳米靶向治疗研究 2.1 化学治疗
化学治疗是肿瘤治疗最常用的手段之一,但目前面临严峻的耐药挑战,肿瘤缺氧微环境是导致化学治疗耐药的一个重要原因[12]。为了实现纳米药物在肿瘤部位靶向性积累,科学家引入了许多策略,如采用pH值响应型、温度响应型等纳米递送系统促进药物的传递,设计缺氧响应型纳米递送系统也是促进肿瘤靶向治疗的有效策略之一(图 2)。
|
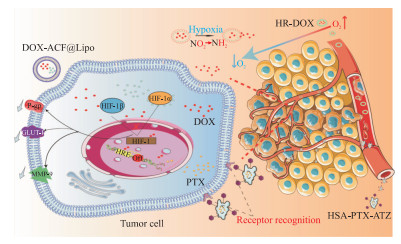
图 2 基于缺氧增强肿瘤化学治疗效果的纳米载药系统靶向递送示意图 Fig 2 Diagrammatic sketch of nano-targeted drugs delivery system based on hypoxia-enhanced tumor chemotherapy DOX-ACF@Lipo, HR-DOX and HSA-PTX-ATZ are nano-targeted drugs. DOX: Doxorubicin; ACF: Acriflavine; Lipo: Liposome; HR: Methoxy poly(ethylene glycol)-block-poly (glutamic acid)-graft-6-(2-nitroimidazole) hexyl amine; HSA: Human serum albumin; PTX: Paclitaxel; ATZ: Acetazolamide; P-gp: P-glycoprotein; GLUT-1: Glucose transporter 1; MMP-9: Matrix metalloproteinase 9; HIF-1: Hypoxia-inducible factor 1; HRE: Hypoxia response element. |
有研究表明,在肿瘤缺氧微环境中,HIF-1可以上调肿瘤耐药相关蛋白,包括P-糖蛋白(P-glycoprotein,P-gp)、葡萄糖转运蛋白1(glucose transporter 1,GLUT-1)和基质金属蛋白酶9(matrix metalloproteinase 9,MMP-9)等,从而导致肿瘤化学治疗耐药[13]。Xu等[13]用脂质体封装HIF-1抑制剂吖啶黄(acriflavine,ACF)和盐酸多柔比星(doxorubicin,DOX)构建了双功能脂质体DOX-ACF@Lipo,所释放的ACF和DOX分别能有效抑制HIF-1功能和DNA复制过程。体内研究结果显示DOX-ACF@Lipo组中P-gp、GLUT-1和MMP-9的表达比脂质体封装DOX(DOX@Lipo)组分别下降32.3%、31.2%和32.4%,表明DOX和ACF的结合可有效抑制化学治疗耐药相关蛋白的表达,提高抗肿瘤效果。Ahmad等[14]利用疏水硝基咪唑衍生物(nitro imidazole derivative,NID)与甲氧基聚乙二醇-嵌段-聚(L-谷氨酸)共价偶合,合成了缺氧响应型纳米粒子甲氧基聚乙二醇-嵌段-聚谷氨酸-接枝-6-(2-硝基咪唑)己胺,DOX被疏水部分包裹在缺氧响应型纳米粒子的胶束核心中。在缺氧条件下,该纳米胶束硝基咪唑环的NO2基团被还原为NH2基团,DOX释放更快,同时具有极高的生物相容性。
利用各种配体识别肿瘤特定生物标志物的靶向药物传递系统也能极大促进选择性给药,增强治疗效果。缺氧微环境下肿瘤细胞表面过表达碳酸酐酶Ⅸ受体,而正常细胞不表达碳酸酐酶Ⅸ受体,因此靶向碳酸酐酶Ⅸ受体可以起到靶向肿瘤的作用。Tatiparti等[15]研制了一种以碳酸酐酶Ⅸ受体的小分子配体乙酰唑胺(acetazolamide,ATZ)和人血清白蛋白(human serum albumin,HAS)为载体输送抗肿瘤药物紫杉醇(paclitaxel,PTX)的纳米粒子HSA-PTX-ATZ,体外研究结果表明HSA-PTX-ATZ在缺氧条件下的细胞杀伤作用比在常氧条件下更高,具有优越的诱导细胞凋亡的能力。
2.2 放射治疗放射治疗是一种有效的肿瘤治疗手段,在临床上应用广泛,然而在放射治疗肿瘤的过程中只有小部分电离辐射能被肿瘤吸收,其抗肿瘤效果受到大大限制。有研究表明,缺氧微环境是限制肿瘤吸收电离辐射的一大重要原因[16],因此,能够缓解肿瘤缺氧的纳米粒子可以有效地提高放射治疗效率。
有许多研究通过纳米递送系统提高肿瘤微环境中的O2水平,以克服肿瘤缺氧,从而实现放射增敏(图 3)。MnO2可以作为一种激活剂触发肿瘤细胞内膜H2O2分解产生O2,以减轻肿瘤缺氧,实现肿瘤放射敏感。Fan等[17]用转换纳米探针锚定MnO2纳米片治疗实体肿瘤,获得很好的抗肿瘤效应。Yi等[18]利用放射增敏剂Au纳米颗粒与活化剂MnO2成功构建核壳Au@MnO2-聚乙二醇(polyethylene glycol,PEG)纳米粒子,其在体外和体内均可显著增强肿瘤放射治疗效率。Tian等[19]将131I标记的HAS与MnO2纳米粒结合,制备的131I-HSA-MnO2纳米粒静脉注射后可通过EPR效应使131I在肿瘤部位蓄积,并可同时缓解肿瘤缺氧微环境,明显提升了放射治疗效果。

|
图 3 基于缺氧增强肿瘤放射治疗效果的纳米载药系统靶向递送示意图 Fig 3 Diagrammatic sketch of nano-targeted drugs delivery system based on hypoxia-enhanced tumor radiotherapy TaOx@PFC-PEG, Au@MnO2-PEG and 131I-HSA-CAT are nano-targeted drugs. PFC: Perfluorocarbon; PEG: Polyethylene glycol; HSA: Human serum albumin; CAT: Catalase. |
此外,研究者们还探索了负载过氧化氢酶(catalase,CAT)促进O2生成或与供氧体结合在肿瘤内释放O2的纳米粒子,以克服缺氧相关放射治疗抵抗[20-22]。Song等[16]用氧化钽(tantalum oxide,TaOx)纳米壳包封CAT增强放射治疗疗效,在该生物纳米反应器中TaOx可将辐射能量集中到肿瘤以加重DNA损伤,同时CAT可快速将H2O2分解成O2以克服缺氧引起的辐射抗性。之后,他们将TaOx纳米粒子装饰在供氧体全氟化碳(perfluorocarbon,PFC)纳米液滴上进行放射增敏,制备的TaOx@PFC-PEG纳米粒子不仅能增加X射线吸收、集中辐射能量,还能逐渐释放O2、提高细胞内O2含量,在小鼠肿瘤模型中显示出较好的放射增敏效果。Chen等[23]构建了由131I标记的HSA和CAT分子组成的生物相容性杂化蛋白纳米反应器,该纳米反应器经静脉注射到荷瘤小鼠体内后表现出高效的肿瘤积聚,显著提高了放射性核素131I的治疗效果。
2.3 光动力疗法(photodynamic therapy,PDT)PDT是一种治疗肿瘤的新方法,光、光敏剂及O2是光动力疗法的三要素。PDT作用机制是用特定波长的光源激发体内肿瘤部位吸收的光敏剂,获得能量后的光敏剂与周围O2或生物分子发生能量传递或电子转移,产生各种活性氧(reactive oxygen species,ROS),从而损伤肿瘤部位蛋白质及核酸等生物分子,导致肿瘤细胞凋亡坏死[24-25]。O2在PDT中非常重要,然而肿瘤部位的缺氧微环境严重限制了O2转化为ROS的效率,从而影响了肿瘤PDT的治疗效果。近年来,随着纳米技术的迅速发展,许多新型纳米材料表现出优良的光学、催化活性及ROS产生能力等性能,被广泛应用于肿瘤治疗领域。目前,可通过以下几种方法缓解肿瘤缺氧:催化肿瘤细胞内高浓度的H2O2产生O2;在光敏治疗体系中共载携氧剂,实现肿瘤组织富氧;升高肿瘤部位温度,加快肿瘤部位血流速度(即热疗),增加O2供给等。Cao等[26]合成了由血红蛋白(hemoglobin,Hb)氧供体、具有双光子吸收性能的双芘[bis(pyrene),BP]和光敏剂孟加拉红(rose Bengal,RB)组装的纳米递送复合体系BP@RB-Hb。此纳米载体旨在增强光对生物组织的穿透力,进而高效杀死肿瘤细胞。随着光照强度的增加,Hb氧供体逐渐由氧合态转变为脱氧态,实现O2的释放,提高PDT的效率。体内外实验证实,该纳米递送系统具有良好的生物相容性、血液相容性、穿透性能和携氧性能。这种多功能纳米复合物有望促进单/双光子激发的PDT在肿瘤治疗中的临床应用。Wang等[27]将光敏剂四羟基苯基卟啉(tetrahydroxy phenyl porphyrin,THPP)和短链PEG交联,通过原位自由基聚合方法对CAT进行包裹,获得了一种CAT-THPP-PEG纳米递送系统。这种CAT-THPP-PEG纳米粒在静脉注射后能够靶向肿瘤,并通过触发肿瘤内源性H2O2的分解产生O2,从而大大缓解肿瘤缺氧。CAT-THPP-PEG递送体系还可以用99mTc4+标记,99mTc4+螯合THPP的卟啉结构,进而可以在体内进行单光子发射计算机断层成像,具有很好的临床应用前景。
由于肿瘤部位扩散受限和灌注受限导致的缺氧与肿瘤的进展、转移和耐药性密切相关。Jiang等[28]通过在氧化石墨炔(graphdiyne oxide,GDYO)纳米片表面包覆iRGD肽修饰的红细胞膜(iRGD peptide-modified red blood membrane,i-RBM)构建仿生超薄GDYO纳米片,利用近红外辐射有效地催化水的氧化,进而释放O2并生成单线态氧(1O2)。另外,GDYO纳米片在实现肿瘤靶向的同时还具有良好的光热转换性能,其光热转换效率为60.8%。该递送体系通过同时实现肿瘤再氧化和血液灌注以增强PDT的抗肿瘤治疗效果。
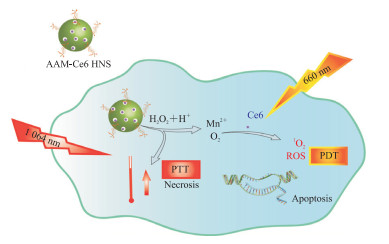
一种将近红外光转化为热量杀死癌细胞的光热疗法(photothermal therapy,PTT)技术也渐渐兴起。Wu等[29]用Au/Ag-MnO2空心纳米球(Au/Ag-MnO2 hollow nanosphere,AAM HNS)制备了一种负载光敏剂Ce6的多功能热敏纳米体系AAM-Ce6 HNS(图 4)。AAM-Ce6 HNS在近红外光照射下具有显著的光热转换能力,释放出大量热量;同时MnO2纳米粒子催化内源性H2O2生成O2,增强PDT对肿瘤组织的杀伤作用;MnO2纳米粒子还降解释放大量的Mn2+,后者具有顺磁性,能够增强MRI信号。研究结果表明,AAM-Ce6 HNS还可实现三模态成像(荧光/光声/MRI)引导的PTT/PDT联合肿瘤治疗[29]。
|
图 4 基于缺氧增强肿瘤PDT/PTT的纳米载药系统靶向递送示意图 Fig 4 Diagrammatic sketch of nano-targeted drugs delivery system based on hypoxia-enhanced tumor PDT/PTT PDT: Photodynamic therapy; PTT: Photothermal therapy; AAM-Ce6 HNS: Au/Ag-MnO2-Ce6 hollow nanosphere; ROS: Reactive oxygen species. |
2.4 免疫治疗
肿瘤的发生、发展是多种因素作用的结果,不仅与细胞本身的基因突变有关,也离不开细胞与免疫系统和微环境的相互作用。人体细胞受到自身免疫系统的监视,这种监视可识别和清除绝大多数的早期恶变细胞和新生肿瘤。某些恶变细胞在发展成肿瘤组织的过程中能够逃避免疫监视,限制了免疫系统对它们的识别和杀伤,称之为肿瘤的免疫逃逸。研究表明,免疫逃逸与缺氧微环境密切相关[30-32]。
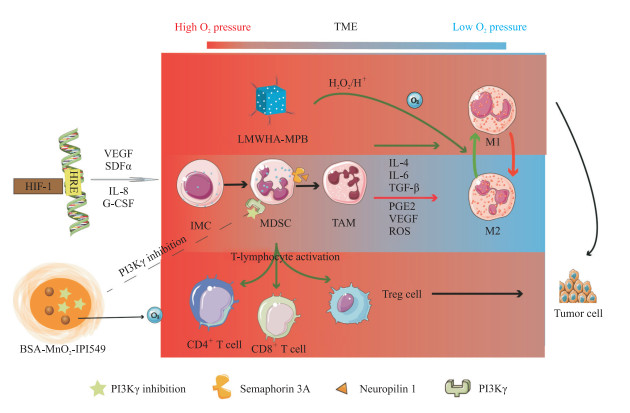
缺氧一方面刺激了HIF家族表达增高,使肿瘤及其微环境分泌多种促血管生成因子,促进异常血管形成;另一方面促使巨噬细胞、中性粒细胞向促瘤表型转化,抑制T细胞和自然杀伤细胞的杀伤作用,使肿瘤细胞具有免疫逃逸能力。肿瘤微环境中的缺氧刺激促进了多种肿瘤逃逸机制包括免疫抑制和肿瘤变异的出现[33-34]。缺氧通过HIF-1诱导产生VEGF、基质细胞衍生因子α(stromal cell-derived factor α,SDFα)、IL-8和粒细胞集落刺激因子等,有助于将未成熟髓系细胞动员和招募到肿瘤微环境中,并转化为肿瘤相关巨噬细胞(tumor-associated macrophage,TAM)和骨髓来源的抑制性细胞(myeloid-derived suppressor cell,MDSC)。这些细胞通常表达信号蛋白3A(semaphorin 3A,SEMA3A),SEMA3A与神经蛋白1结合介导免疫细胞进入肿瘤缺氧区。随后,HIF-1下调SEMA3A表达,使免疫细胞保留并集中在缺氧区域中。在该环境中富集的一些因子,包括IL-4、IL-6、TGF-β、前列腺素E2、VEGF和ROS等,有利于这些细胞分化为抑制性M2型巨噬细胞或成熟MDSC,从而产生肿瘤免疫逃逸[35-37]。免疫抑制细胞在肿瘤的低氧区积累,激活癌细胞免疫耐受机制使其逃避宿主免疫监视,促进肿瘤进展。
针对肿瘤缺氧微环境和免疫抑制微环境设计的纳米递送系统能够增强免疫治疗效果(图 5),有希望实现肿瘤综合治疗。Zhang等[38]用低分子量透明质酸(low molecular weight hyaluronic acid,LMWHA)对介孔普鲁士蓝(mesoporous Prussian blue,MPB)进行表面修饰,合成的LMWHA-MPB纳米粒子可作为原位巨噬细胞转化调节器和O2发生器。不同分子量的透明质酸多糖能促进不同的炎症介质和信号的产生,LMWHA可刺激巨噬细胞产生与经典激活状态相关的促炎介质,从而重塑TAM表型(致瘤型M2→抗瘤型M1),在原位进行巨噬细胞转化调节;MPB不仅是优良的染料,还具有优异的CAT活性,可以催化内源性H2O2产生大量O2以缓解肿瘤缺氧。体内抗肿瘤实验结果显示,LMWHA-MPB负载超声增敏剂血卟啉单甲醚(hematoporphyrin monomethyl ether,HMME)后可通过改善肿瘤缺氧微环境有效抑制4T1肿瘤细胞的增殖和转移。Yu等[39]开发了一种缺氧微环境和肿瘤免疫微环境(tumor immune microenvironment,TIME)响应的多功能纳米递送系统,以具有良好生物相容性的牛血清白蛋白(bovine albumin,BSA)为药物载体,包封MnO2和PI3Kγ抑制剂IPI549构建纳米载体BSA-MnO2-IPI549。MnO2的包封使得纳米粒对肿瘤的酸性微环境和H2O2富集的区域高度敏感,MnO2催化H2O2产生O2使得纳米递送系统崩解并快速释放IPI549,同时产生的O2缓解缺氧所致的细胞程序性死亡配体1(programmed cell death ligand 1,PD-L1)的上调(PD-L1是一种免疫抑制分子的配体,其下调可以缓解免疫抑制)。另外,所释放的IPI549与髓源性抑制细胞上的PI3Kγ结合导致TAM的M1极化和肿瘤抑制性T淋巴细胞的激活,最终达到肿瘤免疫治疗的目的。
|
图 5 基于缺氧增强肿瘤免疫治疗的纳米载药系统靶向递送示意图 Fig 5 Diagrammatic sketch of nano-targeted drugs delivery system based on hypoxia-enhanced tumor immunotherapy BSA-MnO2-IPI549 and LMWHA-MPB are nano-targeted drugs. BSA: Bovine albumin; IPI549: PI3Kγ inhibitor; LMWHA: Low molecular weight hyaluronic acid; MPB: Mesoporous Prussian blue; TME: Tumor microenvironment; HIF-1: Hypoxia-inducible factor 1; HRE: Hypoxia response element; VEGF: Vascular endothelial growth factor; SDFα: Stromal cell-derived factor α; IL: Interleukin; G-CSF: Granulocyte colony-stimulating factor; IMC: Immature myeloid cell; MDSC: Myeloid-derived suppressor cell; TAM: Tumor-associated macrophage; TGF-β: Transforming growth factor β; PGE2: Prostaglandin E2; ROS: Reactive oxygen species; Treg cell: Regulatory T cell; PI3Kγ: Phosphatidylinositide 3-kinase γ. |
2.5 基于改善缺氧重塑肿瘤微环境的纳米靶向递送研究
缺氧作为肿瘤微环境的核心特征,一方面可导致肿瘤细胞代谢重编程(如厌氧呼吸的产生),另一方面还可影响肿瘤细胞的复杂生物学行为(如肿瘤细胞的迁移性)[40-41]。有研究表明逆转肿瘤缺氧微环境有希望实现有效的抗肿瘤治疗。类囊体分布在叶绿体基质和蓝藻细胞中,是单层膜围成的扁平小囊,类囊体膜上含有光合色素和电子传递链组分,可以进行光能向活跃的化学能的转化。Zheng等[42]将类囊体膜包裹在合成纳米粒的表面,构建了一种光触发非生物/生物纳米类囊体(photosynthetic leaf-inspired abiotic/biotic nano-thylakoid,PLANT)O2递送体系。该递送体系在660 nm波长激光照射条件下在小鼠肿瘤部位高效地产生O2,体内外实验结果表明其不仅可以逆转明显的肿瘤缺氧,抑制厌氧呼吸,使肿瘤细胞整个代谢网络恢复正常,而且还可以调节肿瘤血管的异常结构和功能,限制肿瘤细胞的迁移,显著提高PDT或抗肿瘤血管生成治疗的疗效。
3 小结与展望缺氧与肿瘤的发生、转移及耐受息息相关,缺氧微环境在肿瘤治疗过程中发挥着重要作用。随着纳米技术的迅速发展,可以通过纳米递送体系来克服常规肿瘤治疗过程中所遇到的困难,如药物递送效率低、靶向性差和容易耐药等,甚至还可以通过纳米技术靶向肿瘤缺氧微环境来实现精准治疗。众多研究表明,利用纳米技术应对肿瘤缺氧微环境的治疗有希望提高肿瘤治疗的效果。但这些新兴纳米材料大多仍处于基础研究阶段,在临床应用之前还需要进行更严格的临床试验。
| [1] |
THAKKAR S, SHARMA D, KALIA K, TEKADE R K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: a review[J]. Acta Biomater, 2020, 101: 43-68. DOI:10.1016/j.actbio.2019.09.009 |
| [2] |
MICHIELS C, TELLIER C, FERON O. Cycling hypoxia: a key feature of the tumor microenvironment[J]. Biochim Biophys Acta, 2016, 1866: 76-86. |
| [3] |
HUANG X L, ZHUANG J, CHUNG S W, HUANG B W, HALPERT G, NEGRON K, et al. Hypoxiatropic protein nanocages for modulation of tumor- and chemotherapy-associated hypoxia[J]. ACS Nano, 2019, 13: 236-247. DOI:10.1021/acsnano.8b05399 |
| [4] |
BRIZEL D M, SCULLY S P, HARRELSON J M, LAYFIELD L J, BEAN J M, PROSNITZ L R, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma[J]. Cancer Res, 1996, 56: 941-943. |
| [5] |
SAHU A, KWON I, TAE G. Improving cancer therapy through the nanomaterials-assisted alleviation of hypoxia[J/OL]. Biomaterials, 2020, 228: 119578. DOI: 10.1016/j.biomaterials.2019.119578.
|
| [6] |
LIANG J, WU W L, LAI D Y, LI J B, FANG C L. Enhanced solubility and targeted delivery of curcumin by lipopeptide micelles[J]. J Biomater Sci Polym Ed, 2015, 26: 369-383. DOI:10.1080/09205063.2015.1012034 |
| [7] |
JANONIENE A, LIU Z H, BARANAUSKIENE L, MÄKILÄ E, MA M, SALONEN J, et al. A versatile carbonic anhydrase Ⅸ targeting ligand-functionalized porous silicon nanoplatform for dual hypoxia cancer therapy and imaging[J]. ACS Appl Mater Interfaces, 2017, 9: 13976-13987. DOI:10.1021/acsami.7b04038 |
| [8] |
ZHAO J D, DU F, LUO Y, SHEN G S, ZHENG F C, XU B H. The emerging role of hypoxia-inducible factor-2 involved in chemo/radioresistance in solid tumors[J]. Cancer Treat Rev, 2015, 41: 623-633. DOI:10.1016/j.ctrv.2015.05.004 |
| [9] |
WANG G L, SEMENZA G L. Purification and characterization of hypoxia-inducible factor 1[J]. J Biol Chem, 1995, 270: 1230-1237. DOI:10.1074/jbc.270.3.1230 |
| [10] |
WANG G L, JIANG B H, RUE E A, SEMENZA G L. Hypoxia-inducible factor 1 is a basic-helix-loop-helixPAS heterodimer regulated by cellular O2 tension[J]. Proc Natl Acad Sci USA, 1995, 92: 5510-5514. DOI:10.1073/pnas.92.12.5510 |
| [11] |
SEMENZA G L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations[J]. J Clin Invest, 2013, 123: 3664-3671. DOI:10.1172/JCI67230 |
| [12] |
DOKTOROVA H, HRABETA J, KHALIL M A, ECKSCHLAGER T. Hypoxia-induced chemoresistance in cancer cells: the role of not only HIF-1[J]. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 2015, 159: 166-177. DOI:10.5507/bp.2015.025 |
| [13] |
XU L, ZHANG Z C, DING Y W, WANG L, CHENG Y L, MENG L T, et al. Bifunctional liposomes reduce the chemotherapy resistance of doxorubicin induced by reactive oxygen species[J]. Biomater Sci, 2019, 7: 4782-4789. DOI:10.1039/C9BM00590K |
| [14] |
AHMAD Z, LV S X, TANG Z H, SHAH A, CHEN X S. Methoxy poly (ethylene glycol)-block-poly (glutamic acid)-graft-6-(2-nitroimidazole) hexyl amine nanoparticles for potential hypoxia-responsive delivery of doxorubicin[J]. J Biomater Sci Polym Ed, 2016, 27: 40-54. DOI:10.1080/09205063.2015.1107707 |
| [15] |
TATIPARTI K, SAU S, GAWDE K A, IYER A K. Copper-free 'click' chemistry-based synthesis and characterization of carbonic anhydrase- Ⅸ anchored albumin-paclitaxel nanoparticles for targeting tumor hypoxia[J/OL]. Int J Mol Sci, 2018, 19: 838. DOI: 10.3390/ijms19030838.
|
| [16] |
SONG G S, JI C H, LIANG C, SONG X J, YI X, DONG Z L, et al. TaOx decorated perfluorocarbon nanodroplets as oxygen reservoirs to overcome tumor hypoxia and enhance cancer radiotherapy[J]. Biomaterials, 2017, 112: 257-263. DOI:10.1016/j.biomaterials.2016.10.020 |
| [17] |
FAN W P, BU W B, SHEN B, HE Q J, CUI Z W, LIU Y Y, et al. Intelligent MnO2 nanosheets anchored with upconversion nanoprobes for concurrent pH-/ H2O2-responsive UCL imaging and oxygen-elevated synergetic therapy[J]. Adv Mater, 2015, 27: 4155-4161. DOI:10.1002/adma.201405141 |
| [18] |
YI X, CHEN L, ZHONG X Y, GAO R L, QIAN Y T, WU F, et al. Core-shell Au@MnO2 nanoparticles for enhanced radiotherapy via improving the tumor oxygenation[J]. Nano Res, 2016, 9: 3267-3278. DOI:10.1007/s12274-016-1205-8 |
| [19] |
TIAN L, CHEN Q, YI X, CHEN J, LIANG C, CHAO Y, et al. Albumin-templated manganese dioxide nanoparticles for enhanced radioisotope therapy[J/OL]. Small, 2017, 13: 1700640. DOI: 10.1002/smll.201700640.
|
| [20] |
SONG G S, CHEN Y Y, LIANG C, YI X, LIU J J, SUN X Q, et al. Catalase-loaded TaOx nanoshells as bionanoreactors combining high-Z element and enzyme delivery for enhancing radiotherapy[J]. Adv Mater, 2016, 28: 7143-7148. DOI:10.1002/adma.201602111 |
| [21] |
GAO M, LIANG C, SONG X, CHEN Q, JIN Q, WANG C, et al. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy[J/OL]. Adv Mater, 2017, 29: 1701429. DOI: 10.1002/adma.201701429.
|
| [22] |
LU N, FAN W P, YI X, WANG S, WANG Z T, TIAN R, et al. Biodegradable hollow mesoporous organosilica nanotheranostics for mild hyperthermia-induced bubbleenhanced oxygen-sensitized radiotherapy[J]. ACS Nano, 2018, 12: 1580-1591. DOI:10.1021/acsnano.7b08103 |
| [23] |
CHEN J W, LIANG C, SONG X J, YI X, YANG K, FENG L Z, et al. Hybrid protein nano-reactors enable simultaneous increments of tumor oxygenation and iodine-131 delivery for enhanced radionuclide therapy[ J/OL]. Small, 2019, 15: e1903628. DOI: 10.1002/smll.201903628.
|
| [24] |
DOLMANS D E, FUKUMURA D, JAIN R K. Photodynamic therapy for cancer[J]. Nat Rev Cancer, 2003, 3: 380-387. DOI:10.1038/nrc1071 |
| [25] |
SHI X T, ZHANG C Y, GAO J, WANG Z J. Recent advances in photodynamic therapy for cancer and infectious diseases[ J/OL]. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2019, 11: e1560. DOI: 10.1002/wnan.1560.
|
| [26] |
CAO H Q, WANG L, YANG Y, LI J, QI Y F, LI Y, et al. An assembled nano complex for improving both therapeutic efficiency and treatment depth in photodynamic therapy[J]. Angew Chem Int Ed Engl, 2018, 57: 7759-7763. DOI:10.1002/anie.201802497 |
| [27] |
WANG H R, CHAO Y, LIU J J, ZHU W W, WANG G L, XU L G, et al. Photosensitizer-crosslinked in situ polymerization on catalase for tumor hypoxia modulation & enhanced photodynamic therapy[J]. Biomaterials, 2018, 181: 310-317. DOI:10.1016/j.biomaterials.2018.08.011 |
| [28] |
JIANG W, ZHANG Z, WANG Q, DOU J X, ZHAO Y Y, MA Y C, et al. Tumor reoxygenation and blood perfusion enhanced photodynamic therapy using ultrathin graphdiyne oxide nanosheets[J]. Nano Lett, 2019, 19: 4060-4067. DOI:10.1021/acs.nanolett.9b01458 |
| [29] |
WU K, ZHAO H H, SUN Z Q, WANG B, TANG X Y, DAI Y N, et al. Endogenous oxygen generating multifunctional theranostic nanoplatform for enhanced photodynamic-photothermal therapy and multimodal imaging[J]. Theranostics, 2019, 9: 7697-7713. DOI:10.7150/thno.38565 |
| [30] |
CHOUAIB S, NOMAN M Z, KOSMATOPOULOS K, CURRAN M A. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer[J]. Oncogene, 2017, 36: 439-445. DOI:10.1038/onc.2016.225 |
| [31] |
FACCIABENE A, PENG X H, HAGEMANN I S, BALINT K, BARCHETTI A, WANG L P, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells[J]. Nature, 2011, 475: 226-230. DOI:10.1038/nature10169 |
| [32] |
NOMAN M Z, JANJI B, HU S J, WU J C, MARTELLI F, BRONTE V, et al. Tumor-promoting effects of myeloidderived suppressor cells are potentiated by hypoxiainduced expression of miR-210[J]. Cancer Res, 2015, 75: 3771-3787. DOI:10.1158/0008-5472.CAN-15-0405 |
| [33] |
TRIPATHI C, TEWARI B N, KANCHAN R K, BAGHEL K S, NAUTIYAL N, SHRIVASTAVA R, et al. Macrophages are recruited to hypoxic tumor areas and acquire a pro-angiogenic M2-polarized phenotype via hypoxic cancer cell derived cytokines oncostatin M and eotaxin[J]. Oncotarget, 2014, 5: 5350-5368. DOI:10.18632/oncotarget.2110 |
| [34] |
JEONG S K, KIM J S, LEE C G, PARK Y S, KIM S D, YOON S O, et al. Tumor associated macrophages provide the survival resistance of tumor cells to hypoxic microenvironmental condition through IL-6 receptormediated signals[J]. Immunobiology, 2017, 222: 55-65. DOI:10.1016/j.imbio.2015.11.010 |
| [35] |
DU R, LU K, PETRITSCH C, LIU P, GANSS R, PASSEGUÉ E, et al. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion[J]. Cancer Cell, 2008, 13: 206-220. DOI:10.1016/j.ccr.2008.01.034 |
| [36] |
OBERMAJER N, MUTHUSWAMY R, ODUNSI K, EDWARDS R P, KALINSKI P. PGE2-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment[J]. Cancer Res, 2011, 71: 7463-7470. DOI:10.1158/0008-5472.CAN-11-2449 |
| [37] |
CASAZZA A, LAOUI D, WENES M, RIZZOLIO S, BASSANI N, MAMBRETTI M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/ Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity[J]. Cancer Cell, 2013, 24: 695-709. DOI:10.1016/j.ccr.2013.11.007 |
| [38] |
ZHANG H J, ZHANG X G, REN Y P, CAO F, HOU L, ZHANG Z Z. An in situ microenvironmental nanoregulator to inhibit the proliferation and metastasis of 4T1 tumor[J]. Theranostics, 2019, 9: 3580-3594. DOI:10.7150/thno.33141 |
| [39] |
YU M, DUAN X H, CAI Y J, ZHANG F, JIANG S Q, HAN S S, et al. Multifunctional nanoregulator reshapes immune microenvironment and enhances immune memory for tumor immunotherapy[J/OL]. Adv Sci (Weinh), 2019, 6: 1900037. DOI: 10.1002/advs.201900037.
|
| [40] |
ZHENG X C, TANG H, XIE C, ZHANG J L, WU W, JIANG X Q. Tracking cancer metastasis in vivo by using an iridium-based hypoxia-activated optical oxygen nanosensor[J]. Angew Chem Int Ed Engl, 2015, 54: 8094-8099. DOI:10.1002/anie.201503067 |
| [41] |
NAKAZAWA M S, KEITH B, SIMON M C. Oxygen availability and metabolic adaptations[J]. Nat Rev Cancer, 2016, 16: 663-673. DOI:10.1038/nrc.2016.84 |
| [42] |
ZHENG D W, LI B, XU L, ZHANG Q L, FAN J X, LI C X, et al. Normalizing tumor microenvironment based on photosynthetic abiotic/biotic nanoparticles[J]. ACS Nano, 2018, 12: 6218-6227. DOI:10.1021/acsnano.8b02977 |
 2022, Vol. 43
2022, Vol. 43


