2. 广东药科大学附属第一医院临床药学重点专科,广州 510699
2. Key Specialty of Clinical Pharmacy, The First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou 510699, Guangdong, China
各种神经体液因素或物理刺激因素都会引起心脏压力负荷和容量负荷增加,代偿性引起心肌生理性肥厚[1],长期超负荷会造成病理性心肌肥厚和纤维化,这是多种心血管疾病如冠心病、心力衰竭、心肌梗死及其他心功能障碍性疾病的病理基础[2]。在早期的心脏超负荷压力阶段,心肌细胞体积增大、室壁厚度增加和心肌收缩力增强是病理性心肌肥厚的主要特征,随着病理性应激状态的持续,心脏间质纤维化程度升高造成心脏重构、心功能损伤加重,最终发展为心力衰竭,严重威胁患者的健康[3]。目前用于治疗病理性心肌肥厚和纤维化药物的主要药理作用是扩张血管、减少心脏压力负荷和容量负荷,但这些药物对减轻病理性心肌肥厚和纤维化的作用有限[4-5],有必要从新的角度寻找治疗心力衰竭的药物。研究显示,在心脏病理状态下心脏能量代谢底物由脂肪酸β氧化转化为葡萄糖氧化,这种转变与心脏病变进展密切相关[6-7]。
短链酰基辅酶A脱氢酶(short-chain acyl-CoA dehydrogenase,SCAD)能特异性催化短链脂肪酸的β氧化[8]。前期研究发现,在心肌肥厚或心力衰竭过程中心肌SCAD的表达下调,而促进其表达能减轻病理性心肌肥厚、延缓心力衰竭的进程[9-12]。黄素腺嘌呤二核苷酸(flavin adenine dinucleotide,FAD)是SCAD的辅酶[13],参与脂肪酸β氧化的电子传递,在多种氧化还原反应与生物能量学反应中起到重要作用。进一步研究显示,FAD能促进SCAD表达,延缓自发性高血压大鼠心肌肥厚和纤维化的进程[14],而核黄素(维生素B2)是FAD的前体且在人体内可转化为黄素单核苷酸和FAD[15],那么补充核黄素是否能延缓病理性心肌肥厚与纤维化的进程呢?有研究发现,孕妇补充核黄素能降低新生儿先天性心脏病风险[16],核黄素还能改善SCAD缺乏症和FAD基因异常的临床症状[17-18]。我们推测核黄素通过激活SCAD改善病理性心肌肥厚与纤维化。
主动脉弓缩窄(transverse aortic constriction,TAC)手术能快速建立病理性心肌肥厚和纤维化模型[19-20],本研究用TAC手术构建小鼠病理性心肌肥厚和纤维化模型,并以核黄素灌胃干预,探究核黄素在病理性心肌肥厚与纤维化中的作用。
1 材料和方法 1.1 TAC手术与动物分组44只8~10周龄SPF级C57BL/6小鼠,均为雄性,体重20~25 g,购自广东省医学实验动物中心,动物生产许可证号为SYXK(粤)2018-0002。用TAC手术构建小鼠病理性心肌肥厚和纤维化模型,手术采用0.45 mm孔径垫针、6-0结扎线、角针(1/2弧)、4-0缝合线。手术组小鼠行主动脉弓结扎固定,假手术组小鼠仅开胸穿线不结扎。
小鼠随机分为4组:假手术+生理盐水(normal saline,NS)组(n=10)、TAC+NS组(n=12)、假手术+核黄素组(n=10)及TAC+核黄素组(n=12)。核黄素灌胃剂量为20 mg·kg-1·d-1,假手术+NS组和TAC+NS组灌胃等体积NS。各组小鼠手术前给药1周,手术后继续给药4周。TAC+NS组及TAC+核黄素组小鼠在手术过程中和术后各死亡2只,共有40只小鼠用于实验。术后第5周取材进行后续实验。
1.2 试剂与仪器快速RNA提取试剂盒(哈尔滨新海基因检测有限公司,货号213000);通用型SYBR Green qPCR预混液(武汉赛维尔生物科技有限公司,货号2111063);核黄素(美国Sigma公司,货号WXBD2883V);鼠抗GAPDH单克隆抗体(货号10013030)、兔抗SCAD单克隆抗体(货号00012655)、兔抗胶原蛋白Ⅰ单克隆抗体(货号00095883)、兔抗胶原蛋白Ⅲ单克隆抗体(货号00086215)、兔抗α平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)单克隆抗体(货号BC017554)、抗鼠二抗(货号2000216)均购自美国Proteintech公司;抗兔二抗(英国Abcam公司,货号215219536);SCAD酶活性试剂盒(美国GENMED公司,货号1-2020412-10);羟脯氨酸含量测定试剂盒(货号20200103)、游离脂肪酸检测试剂盒(货号20211221)、ATP检测试剂盒(货号20211216)均购自南京建成生物工程研究所;小鼠FAD检测试剂盒(上海双赢生物科技有限公司,货号202112)。心房钠尿肽(atrial natriuretic peptide,ANP)、脑钠肽(brain natriuretic peptide,BNP)、β-肌球蛋白重链(β-myosin heavychain,β-MHC)等引物序列均由生工生物工程(上海)有限公司合成。
血压计SB0890(美国KENT公司);紫外分光光度计(美国Thermo公司);高速冷冻离心机(湖南湘仪离心机仪器有限公司),-80 ℃低温冰箱(安徽中科都菱商用电器股份有限公司);Multiskan FC全自动酶标仪(美国Thermo公司);Nano-1000微量分光光度计(杭州奥盛仪器有限公司);7500 Fast实时荧光定量PCR系统(美国ABI公司);蛋白电泳仪/电转仪(美国Bio-Rad公司);ChemiScope 3000 mini化学发光仪(上海勤翔科学仪器有限公司)。
1.3 小鼠尾动脉收缩压及心脏体重比(heart weight/body weight,HW/BW)、心脏胫骨长度比(heart weight/tibia length,HW/TL)的检测术后第5周测量各组小鼠静息状态尾动脉收缩压。每只小鼠测量6次,间隔2 min,取平均值。收集小鼠HW、BW与TL数据,计算HW/BW和HW/TL。
1.4 超声心动图检查手术后第5周异氟烷吸入麻醉各组小鼠,采用超声多普勒成像系统检测小鼠心脏结构功能指标,实时记录小鼠心动图,取M超曲线。记录各组小鼠舒张末期左心室后壁厚度(left ventricular posterior wall thickness at end-diastole,LVPWd)、收缩末期左心室后壁厚度(left ventricular posterior wall thickness atend-systole,LVPWs)、舒张末期左心室前壁厚度(left ventricular anterior wall thickness at end-diastole,LVAWd)、收缩末期左心室前壁厚度(left ventricular anterior wall thickness at end-systole,LVAWs)、左心室舒张末期内径(left ventricular internal dimension at end-diastole,LVIDd)、左心室收缩末期内径(left ventricular internal dimension at end-systole,LVIDs)、左心室舒张末期容积(left ventricular end-diastolic volume, LVEDV)、左心室收缩末期容积(left ventricular end-systolic volume,LVESV)、左心室射血分数(left ventricular ejectionfraction,LVEF)和左心室短轴缩短率(left ventricularfractional shortening,LVFS)。
1.5 心肌组织形态学检测取出小鼠心脏后,沿最大横切面切开,用4%多聚甲醛溶液固定,石蜡包埋后切片,分别进行SCAD免疫荧光单染、H-E染色、天狼星红染色、麦胚凝集素(wheat germ agglutinin,WGA)染色。
1.6 SCAD酶活性及羟脯氨酸、游离脂肪酸、ATP、FAD含量检测均依据检测说明书进行操作。
1.7 蛋白质印迹法检测取适当大小的小鼠心肌组织,经匀浆、离心、裂解,提取组织总蛋白。测得570 nm波长处光密度,将其代入BCA蛋白定量标准曲线定量后分装,样品蛋白以10% SDS-PAGE分离。电转条件下将蛋白转移到PVDF膜,用TBST洗去膜上电泳液,然后将PVDF膜放入新配制的5%牛血清蛋白(bull serum albumin,BSA)中室温封闭90 min,分割目的条带,分别孵育一抗过夜,洗膜,室温下孵育二抗1 h,化学发光底物结合后在化学发光成像仪下曝光显影。
1.8 qPCR检测提取小鼠心肌组织总RNA,使用微量分光光度计测定RNA纯度与浓度,反转录合成cDNA。依据试剂盒说明书配制cDNA反应扩增体系并检测,采用2-ΔΔCt法计算各样品mRNA表达水平,各样品检测结果以GAPDH均一化。SCAD上游引物序列为5'-TGGCGACG-GTTACACACTGT-3',下游引物序列为5'-CGAG-CTCACCCATCTTCTTAAC-3';ANP上游引物序列为5'-TGGCGACGGTTACACACTGT-3',下游引物序列为5'-AAGCTGTTGCAGCCTAGTCC-3';BNP上游引物序列为5'-TGGGAGGTCACTCCTA-TCCT-3',下游引物序列为5'-GGCCATTTCCTCCG-ACTTT-3';β-MHC上游引物序列为5'-CGGACCTT-GGAAGACCAGAT-3',下游引物序列为5'-GAC-AGCTCCCCATTCTCTGT-3';胶原蛋白Ⅰ上游引物序列为5'-AGGCTTCAGTGGTTTGGATG-3',下游引物序列为5'-CACCAACAGCACCATCGT-TA-3';胶原蛋白Ⅲ上游引物序列为5'-CCCAACC-CAGAGATCCCATT-3',下游引物序列为5'-GA-AGCACAGGAGCAGGTGTAGA-3';α-SMA上游引物序列为5'-TCCAGAGTCCACAATACC-AG-3',下游引物序列为5'-AATGACCCAGATTA-TGTTTGAGACC-3';GAPDH上游引物序列为5'-AGGAGTAAGAAACCCTGGAC-3',下游引物序列为5'-CTGGGATGGAATTGTGAG-3'。
1.9 统计学处理应用ImageJ软件处理切片染色及蛋白质印迹图片结果,用SPSS 21.0软件进行统计分析。计量资料以x±s表示,各组间比较采用单因素方差分析或t检验。检验水准(α)为0.05。
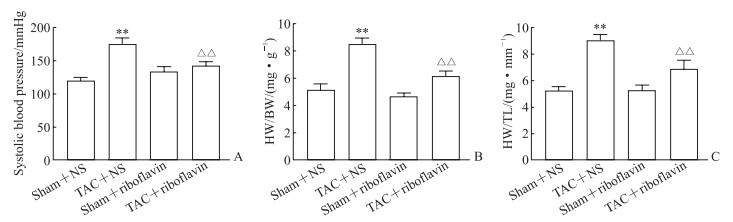
2 结果 2.1 核黄素对小鼠尾动脉收缩压、HW/BW及HW/TL的影响如图 1A所示,TAC手术后小鼠尾动脉收缩压较假手术+NS组升高(P<0.01),核黄素灌胃后TAC+核黄素组小鼠尾动脉收缩压较TAC+NS组下降(P<0.01)。如图 1B、1C所示,TAC手术后小鼠HW/BW和HW/TL升高(P均<0.01),核黄素灌胃后TAC+核黄素组小鼠HW/BW和HW/TL较TAC+NS组下降(P均<0.01)。
|
图 1 各组小鼠尾动脉收缩压、HW/BW、HW/TL的比较 Fig 1 Comparison of tail artery systolic blood pressure and ratios of HW/BW and HW/TL of mice in each group A: Changes of systolic blood pressure; B: Changes of HW/BW ratio; C: Changes of HW/TL ratio. 1 mmHg=0.133 kPa. **P < 0.01 vs sham+NS group; △△P < 0.01 vs TAC+NS group. n=10, x±s. HW: Heart weight; BW: Body weight; TL: Tibia length; NS: Normal saline; TAC: Transverse aortic constriction. |
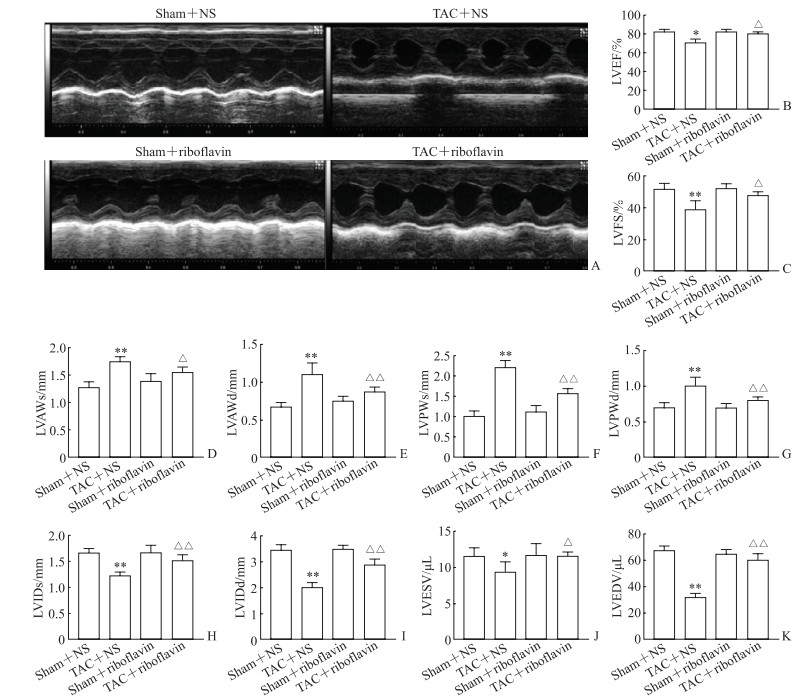
2.2 核黄素对小鼠超声心动图结果的影响
结果如图 2所示,与假手术+NS组相比,TAC手术后小鼠的LVEF、LVFS、LVIDs、LVIDd、LVESV、LVEDV均降低(P<0.05,P<0.01),LVAWs、LVAWd、LVPWs、LVPWd均升高(P均<0.01),表明TAC手术后小鼠发生了病理性心肌肥厚和心脏舒缩功能障碍。与TAC+NS组相比,TAC+核黄素组LVEF、LVFS、LVIDs、LVIDd、LVESV、LVEDV均升高(P<0.05,P<0.01),LVAWs、LVAWd、LVPWs、LVPWd均降低(P<0.05,P<0.01),表明核黄素减轻了TAC手术导致的病理性心肌肥厚和心脏舒缩功能障碍。
|
图 2 各组小鼠超声心动图结果比较 Fig 2 Comparison of echocardiographic results of mice in each group A: Changes of echocardiography; B-K: Changes of specific parameters of echocardiography. *P < 0.05, **P < 0.01 vs sham+NS group; △P < 0.05, △△P < 0.01 vs TAC+NS group. n=10, x±s. NS: Normal saline; TAC: Transverse aortic constriction; LVEF: Left ventricular ejection fraction; LVFS: Left ventricular fractional shortening; LVAWs: Left ventricular anterior wall thickness at end-systole; LVAWd: Left ventricular anterior wall thickness at end-diastole; LVPWs: Left ventricular posterior wall thickness at end-systole; LVPWd: Left ventricular posterior wall thickness at end-diastole; LVIDs: Left ventricular internal dimension at end-systole; LVIDd: Left ventricular internal dimension at end-diastole; LVESV: Left ventricular end-systolic volume; LVEDV: Left ventricular end-diastolic volume. |
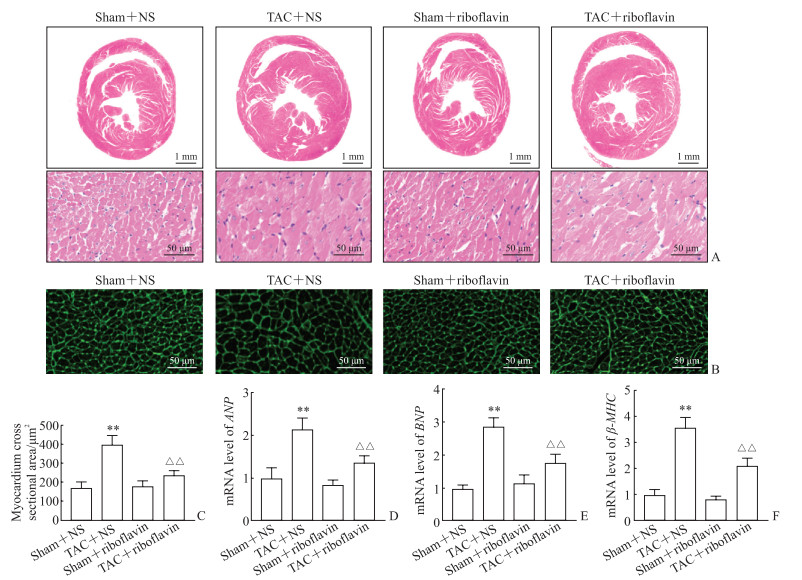
2.3 核黄素对小鼠病理性心肌肥厚的影响
如图 3A所示,与假手术+NS组相比,TAC+NS组小鼠出现病理性心肌肥厚,心室壁增厚、心室腔减小;与TAC+NS组相比,TAC+核黄素组小鼠心室壁变薄、心室腔增大。如图 3B、3C所示,TAC+NS组小鼠心肌细胞横截面积高于假手术+NS组(P<0.01);与TAC+NS组相比,TAC小鼠核黄素灌胃后心肌细胞横截面积减小(P<0.01)。同时,与假手术+NS组相比,TAC+NS组小鼠心肌组织病理性心肌肥厚标志物ANP、BNP和β-MHC的mRNA表达水平增加(P均<0.01),而经核黄素灌胃后,与TAC+NS组相比,TAC+核黄素组小鼠上述标志物mRNA水平降低(P均<0.01,图 3D~3F)。以上结果表明核黄素减轻了TAC手术引起的小鼠病理性心肌肥厚。
|
图 3 各组小鼠心肌肥厚指标比较 Fig 3 Comparison of myocardial hypertrophy indexes among mice in each group A: Hematoxylin-eosin staining; B: Wheat germ agglutinin (WGA) staining; C: Myocardium cross sectionl area detected by WGA staining; D: mRNA levels of ANP in myocardial tissues detected by quantitative polymerase chain reaction; E: mRNA levels of BNP in myocardial tissues detected by quantitative polymerase chain reaction; F: mRNA levels of β-MHC in myocardial tissues detected by quantitative polymerase chain reaction. **P < 0.01 vs sham+NS group; △△P < 0.01 vs TAC+NS group. n=6, x±s. NS: Normal saline; TAC: Transverse aortic constriction; ANP: Atrial natriuretic peptide; BNP: Brain natriuretic peptide; β-MHC: β-myosin heavy chain. |
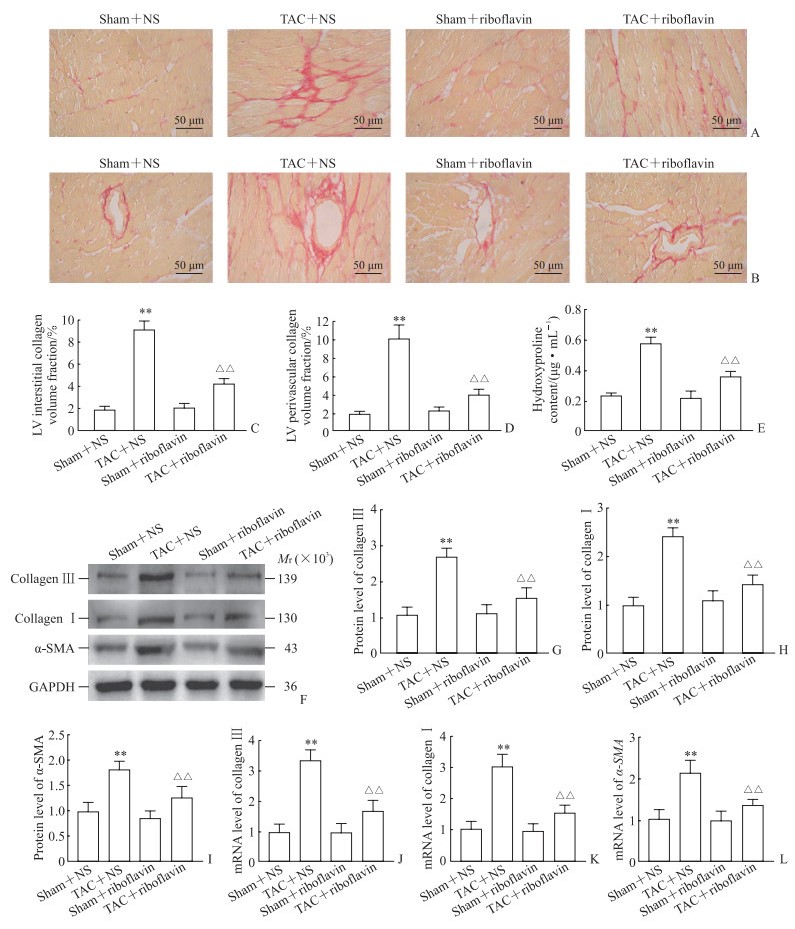
2.4 核黄素对小鼠心肌纤维化的影响
与假手术+NS组相比,TAC手术加重了小鼠心脏间质胶原和血管周围胶原沉积(图 4A、4B);TAC+NS组小鼠左心室间质胶原和血管周围胶原容积分数均高于假手术+NS组(P均<0.01,图 4C、4D);心肌组织羟脯氨酸含量(图 4E),胶原蛋白Ⅰ、胶原蛋白Ⅲ、α-SMA蛋白(图 4F~4I)和mRNA(图 4J~4L)表达水平均高于假手术+NS组(P均<0.01)。与TAC+NS组相比,TAC+核黄素组左心室间质胶原和血管周围胶原容积分数、羟脯氨酸含量,以及胶原蛋白Ⅰ、胶原蛋白Ⅲ、α-SMA的蛋白和mRNA表达水平均降低(P均<0.01)。以上结果表明,核黄素减轻了TAC引起的小鼠心肌纤维化。
|
图 4 各组小鼠心肌纤维化程度比较 Fig 4 Comparison of myocardial fibrosis degree among mice in each group A, C: Cardiac interstitial collagen area and statistical results; B, D: Cardiac perivascular collagen area and statistical results; E: Myocardial hydroxyproline content; F-I: Protein expression levels of collagen Ⅲ, collagen Ⅰ, and α-SMA in myocardium of each group detected by Western blotting; J-L: mRNA expression levels of collagen Ⅲ, collagen Ⅰ, and α-SMA in myocardium detected by quantitative polymerase chain reaction. **P < 0.01 vs sham+NS group; △△P < 0.01 vs TAC+NS group. n=6, x±s. NS: Normal saline; TAC: Transverse aortic constriction; LV: Left ventricle; α-SMA: α-smooth muscle actin; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. |
2.5 核黄素对小鼠心肌组织SCAD表达、酶活性及FAD含量的影响
与假手术+NS组相比,TAC+NS组心肌组织SCAD免疫荧光强度降低(P<0.01,图 5A、5B);核黄素灌胃处理后,与TAC+NS组相比,TAC+核黄素组SCAD荧光强度增加(P<0.01)。TAC导致小鼠心肌组织SCAD蛋白(图 5C、5D)和mRNA(图 5E)水平均降低(P均<0.01),而核黄素灌胃后SCAD蛋白和mRNA表达水平均升高(P均<0.01)。SCAD酶活性(图 5F)和心肌组织SCAD辅酶FAD的含量(图 5G)也出现同样变化趋势。以上结果表明,核黄素改善了TAC手术导致的病理性心肌肥厚和纤维化,可能与其提高心肌组织FAD水平、增强SCAD酶活性、促进心肌组织SCAD表达有关。

|
图 5 各组小鼠心肌组织SCAD表达、酶活性和FAD含量比较 Fig 5 Comparison of myocardial SCAD expression, enzyme activity and FAD content of mice in each group A: Left ventricular SCAD expression levels measured by immunofluorescence staining; B: SCAD immunofluorescence intensity statistics of myocardial tissue; C, D: Protein expression levels of SCAD detected by Western blotting; E: mRNA expression levels of SCAD detected by quantitative polymerase chain reaction; F: SCAD enzyme activity in myocardial tissues; G: FAD content of myocardial tissues. **P < 0.01 vs sham+NS group; △△P < 0.01 vs TAC+NS group. n=6, x±s. SCAD: Short-chain acyl-CoA dehydrogenase; FAD: Flavin adenine dinucleotide; NS: Normal saline; TAC: Transverse aortic constriction; DAPI: 4', 6-diamidino-2-phenylindole; AFI: Average fluorescence intensity; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. |
2.6 核黄素对小鼠心肌脂肪酸β氧化的影响
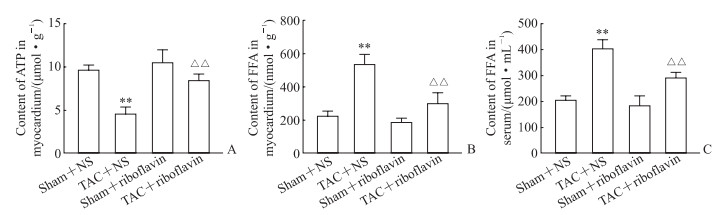
结果如图 6所示,与假手术+NS组相比,TAC手术后小鼠心肌组织ATP含量降低(P<0.01)、血清和心肌游离脂肪酸含量均升高(P均<0.01);与TAC+NS组相比,TAC+核黄素组心肌组织ATP含量升高(P<0.01)、血清和心肌游离脂肪酸含量均降低(P均<0.01)。以上结果表明核黄素可能通过促进心肌脂肪酸β氧化、改善心肌能量代谢,减轻病理性心肌肥厚和纤维化。
|
图 6 各组小鼠心肌组织ATP、游离脂肪酸及血清游离脂肪酸含量的比较 Fig 6 Comparison of myocardial ATP, FFA and serum FFA content of mice in each group A: ATP content in myocardium; B, C: FFA content in myocardium and serum. **P < 0.01 vs sham+NS group; △△P < 0.01 vs TAC+NSgroup. n=6, x±s. ATP: Adenosine triphosphate; FFA: Free fatty acid; NS: Normal saline; TAC: Transverse aortic constriction. |
3 讨论
脂肪酸β氧化对于心血管疾病的发展意义重大。前期研究显示,脂肪酸β氧化限速酶SCAD在病理性心肌肥厚、心肌纤维化和心力衰竭大鼠心肌中的表达下调,通过SCAD重组腺病毒和FAD尾静脉注射都能提高SCAD表达、增加心肌脂肪酸β氧化,改善心肌能量代谢,从而减轻病理性心肌肥厚和纤维化[14, 21-22]。因此促进SCAD和其辅酶FAD在心肌的表达、增强脂肪酸β氧化可能是减轻病理性心肌肥厚与纤维化的途径。
核黄素在线粒体电子传递链、线粒体和过氧化物酶体脂肪酸β氧化、氧化还原动态平衡和支链氨基酸分解代谢等代谢过程中起关键作用[23]。核黄素在胃肠道被吸收,进入血液后被组织特异性核黄素转运蛋白输送到各个器官[24]并转化为黄素酶发挥作用。FAD是核黄素在体内的黄素酶产物之一[25]。研究发现FAD基因突变的患者口服核黄素后临床和生化异常均有改善,包括提高运动耐量和耐力[26];SCAD缺乏症患者经过核黄素治疗后临床症状改善,患者体内的FAD水平提高、SCAD酶活性及蛋白表达水平升高[27]。以上证据表明核黄素能促进SCAD表达,且与心血管疾病密切相关。
小鼠在受到外界刺激时体重容易改变,而在小鼠性成熟后胫骨长不再改变,因此HW/TL常用来衡量心肌肥厚程度和心功能障碍水平[28-29]。本研究发现,在TAC手术诱导压力超负荷病理状态下小鼠HW/BW与HW/TL升高,出现了明显的病理性心肌肥厚和纤维化。
经核黄素灌胃处理后,小鼠心室腔扩大、心室壁厚度降低、心肌胶原含量降低,心脏舒缩功能障碍明显改善,表明核黄素能有效延缓病理性心肌肥厚和纤维化的进程。TAC手术缩窄血管内径会增加心脏压力负荷,导致血压升高,高血压是导致病理性心肌肥厚的因素之一。本研究发现核黄素降低了TAC手术引起的小鼠尾动脉收缩压,从而可减少心肌肥厚的风险。研究发现,核黄素作为体内多种黄素酶的前体可以降低体内血液同型半胱氨酸含量,能降低高血压的风险[30];黄素酶的补充能促进NO释放、舒张血管,从而降低血压[31],以上研究均证实核黄素在心血管疾病中的作用。
在本研究中,TAC组小鼠心肌能量代谢发生了变化:ATP含量减少,游离脂肪酸含量增加,心脏能量供应不足,这种能量代谢的改变会损害心肌功能。核黄素给药后逆转了TAC手术导致的小鼠心肌SCAD表达下调、酶活性降低、游离脂肪酸堆积、ATP含量降低,这提示核黄素改善小鼠病理性心肌肥厚的作用可能通过激活SCAD表达、增强脂肪酸β氧化实现。研究显示补充FAD能促进SCAD基因表达,增强其酶活性[32]。本实验结果表明,核黄素灌胃后小鼠心肌FAD含量增加可能是心肌SCAD表达增加、脂肪酸β氧化增强的原因。
本研究首次发现核黄素可通过提高心脏FAD含量、增强SCAD酶活性、促进SCAD蛋白表达水平、增加脂肪酸β氧化、改善心肌能量代谢减轻病理性心肌肥厚与纤维化。心脏能量代谢变化与各种心血管疾病密切相关,本研究从脂肪酸β氧化代谢出发,为病理性心肌肥厚和纤维化及心力衰竭等心血管疾病的治疗提供了新思路。然而,核黄素减轻病理性心肌肥厚和纤维化的作用是否与激活SCAD确切相关及作用机制还需开展深入的实验研究。
| [1] |
MANCIO J, AZEVEDO D, FRAGAO-MARQUES M, FALCAO-PIRES I, LEITE-MOREIRA A, LUNET N, et al. Meta-analysis of relation of epicardial adipose tissue volume to left atrial dilation and to left ventricular hypertrophy and functions[J]. Am J Cardiol, 2019, 123: 523-531. DOI:10.1016/j.amjcard.2018.10.020 |
| [2] |
PANDEY A, KESHVANI N, AYERS C, CORREA A, DRAZNER M H, LEWIS A, et al. Association of cardiac injury and malignant left ventricular hypertrophy with risk of heart failure in African Americans: the Jacksonheart study[J]. JAMA Cardiol, 2019, 4: 51-58. DOI:10.1001/jamacardio.2018.4300 |
| [3] |
KANAGALA P, CHENG A S H, SINGH A, KHAN J N, GULSIN G S, PATEL P, et al. Relationship between focal and diffuse fibrosis assessed by CMR and clinical outcomes in heart failure with preserved ejection fraction[J]. JACC Cardiovasc Imaging, 2019, 12(11Pt2): 2291-2301. |
| [4] |
LI H, SUREDA A, DEVKOTA H P, PITTALÀ V, BARRECA D, SILVA A S, et al. Curcumin, the golden spice in treating cardiovascular diseases[J/OL]. Biotechnol Adv, 2020, 38: 107343. DOI: 10.1016/j.biotechadv.2019.01.010.
|
| [5] |
MARIAN A J, TAN Y L, LI L L, CHANG J, SYRRIS P, HESSABI M, et al. Hypertrophy regression with N-acetylcysteine in hypertrophic cardiomyopathy (HALT-HCM)[J]. Circ Res, 2018, 122: 1109-1118. DOI:10.1161/CIRCRESAHA.117.312647 |
| [6] |
WANG C, YUAN Y, WU J, ZHAO Y L, GAO X, CHEN Y H, et al. Plin5 deficiency exacerbates pressure overload-induced cardiac hypertrophy and heart failure by enhancing myocardial fatty acid oxidation and oxidative stress[J]. Free Radic Biol Med, 2019, 141: 372-382. DOI:10.1016/j.freeradbiomed.2019.07.006 |
| [7] |
FILLMORE N, MORI J, LOPASCHUK G D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy[J]. Br J Pharmacol, 2014, 171: 2080-2090. DOI:10.1111/bph.12475 |
| [8] |
SCHMIDT S P, CORYDON T J, PEDERSEN C B, BROSS P, GREGERSEN N. Misfolding of short-chain acyl-Co A dehydrogenase leads to mitochondrial fission and oxidative stress[J]. Mol Genet Metab, 2010, 100: 155-162. DOI:10.1016/j.ymgme.2010.03.009 |
| [9] |
HUANG J X, XU L P, HUANG Q J, LUO J N, LIU P Q, CHEN S R, et al. Changes in short-chain acyl-co A dehydrogenase during rat cardiac development and stress[J]. J Cell Mol Med, 2015, 19: 1672-1688. DOI:10.1111/jcmm.12541 |
| [10] |
HUANG Q J, HUANG J X, ZENG Z H, LUO J N, LIU P Q, CHEN S R, et al. Effects of ERK1/2/PPARα/SCAD signal pathways on cardiomyocyte hypertrophy induced by insulin-like growth factor 1 and phenylephrine[J]. Life Sci, 2015, 124: 41-49. DOI:10.1016/j.lfs.2015.01.015 |
| [11] |
ZENG Z H, HUANG Q J, SHU Z H, LIU P Q, CHEN S R, PAN X D, et al. Effects of short-chain acyl-Co A dehydrogenase on cardiomyocyte apoptosis[J]. J Cell Mol Med, 2016, 20: 1381-1391. DOI:10.1111/jcmm.12828 |
| [12] |
廖英勤, 冯静韵, 李忠洪, 臧林泉, 周四桂. 短链酰基辅酶A脱氢酶在老年自发性高血压大鼠慢性心力衰竭中的作用[J]. 广东药科大学学报, 2019, 35: 412-417. |
| [13] |
AUCLAIR O, HAN Y, BURGOS S A. Consumption of milk and alternatives and their contribution to nutrient intakes among Canadian adults: evidence from the 2015 Canadian community health survey-nutrition[J/OL]. Nutrients, 2019, 11: 1948. DOI: 10.3390/nu11081948.
|
| [14] |
MA Z C, QIN X, ZHONG X Y, LIAO Y Q, SU Y S, LIU X, et al. Flavine adenine dinucleotide inhibits pathological cardiac hypertrophy and fibrosis through activating short chain acyl-Co A dehydrogenase[J/OL]. Biochem Pharmacol, 2020, 178: 114100. DOI: 10.1016/j.bcp.2020.114100.
|
| [15] |
DAS R, QUBTY W. Retrospective observational study on riboflavin prophylaxis in child and adolescent migraine[J]. Pediatr Neurol, 2021, 114: 5-8. DOI:10.1016/j.pediatrneurol.2020.09.009 |
| [16] |
SMEDTS H P M, RAKHSHANDEHROO M, VERKLEIJ-HAGOORT A C, DE VRIES J H M, OTTENKAMP J, STEEGERS E A P, et al. Maternal intake of fat, riboflavin and nicotinamide and the risk of having offspring with congenital heart defects[J]. Eur J Nutr, 2008, 47: 357-365. DOI:10.1007/s00394-008-0735-6 |
| [17] |
HO G, YONEZAWA A, MASUDA S, INUI K I, SIM K G, CARPENTER K, et al. Maternal riboflavin deficiency, resulting in transient neonatal-onset glutaric aciduria type 2, is caused by a microdeletion in the riboflavin transporter gene GPR172B[J/OL]. Hum Mutat, 2011, 32: E1976-E1984. DOI: 10.1002/humu.21399.
|
| [18] |
MOSEGAARD S, BRUUN G H, FLYVBJERG K F, BLIKSRUD Y T, GREGERSEN N, DEMBIC M, et al. An intronic variation in SLC52A1 causes exon skipping and transient riboflavin-responsive multiple acyl-Co A dehydrogenation deficiency[J]. Mol Genet Metab, 2017, 122: 182-188. DOI:10.1016/j.ymgme.2017.10.014 |
| [19] |
MOHAMMED S F, STORLIE J R, OEHLER E A, BOWEN L A, KORINEK J, LAM C S P, et al. Variable phenotype in murine transverse aortic constriction[J]. Cardiovasc Pathol, 2012, 21: 188-198. DOI:10.1016/j.carpath.2011.05.002 |
| [20] |
RICHARDS D A, ARONOVITZ M J, CALAMARAS T D, TAM K, MARTIN G L, LIU P W, et al. Distinct phenotypes induced by three degrees of transverse aortic constriction in mice[J/OL]. Sci Rep, 2019, 9: 5844. DOI: 10.1038/s41598-019-42209-7.
|
| [21] |
苏永少, 廖英勤, 钟小艺, 马智超, 刘培庆, 路静, 等. SCAD重组腺病毒治疗大鼠心肌梗死后心力衰竭的实验研究[J]. 中国药理学通报, 2020, 36: 608-615. DOI:10.3969/j.issn.1001-1978.2020.05.005 |
| [22] |
冯静韵, 廖英勤, 钟小艺, 苏永少, 马智超, 秦学, 等. 静脉注射SCAD重组腺病毒减轻自发性高血压大鼠心肌肥厚和纤维化[J]. 中国病理生理杂志, 2021, 37: 809-817. DOI:10.3969/j.issn.1000-4718.2021.05.005 |
| [23] |
ZHOU G Q, PAN Q Q, HU Z Y, QIU J P, YU Z L. Heterologous expression and characterization of flavinadenined inucleotide synthetase from Candida famata for flavin adenine dinucleotide production[J]. Protein Pept Lett, 2021, 28: 229-239. DOI:10.2174/0929866527666200708151327 |
| [24] |
BALASUBRAMANIAM S, CHRISTODOULOU J, RAHMAN S. Disorders of riboflavin metabolism[J]. J Inherit Metab Dis, 2019, 42: 608-619. DOI:10.1002/jimd.12058 |
| [25] |
NAGANO T, AWAI Y, KUWABA S, OSUMI T, MIO K, IWASAKI T, et al. Riboflavin transporter SLC52A1, a target of p53, suppresses cellular senescence by activating mitochondrial complex Ⅱ[J/OL]. Mol Biol Cell, 2021, 32: br10. DOI: 10.1091/mbc.E21-05-0262.
|
| [26] |
SCHIFF M, VEAUVILLE-MERLLIÉ A, SU C H, TZAGOLOFF A, RAK M, OGIER DE BAULNY H, et al. SLC25A32 mutations and riboflavin-responsive exercise intolerance[J]. N Engl J Med, 2016, 374: 795-797. DOI:10.1056/NEJMc1513610 |
| [27] |
VAN MALDEGEM B T, DURAN M, WANDERS R J A, WATERHAM H R, WIJBURG F A. Flavin adenine dinucleotide status and the effects of high-dose riboflavin treatment in short-chain acyl-Co A dehydrogenase deficiency[J]. Pediatr Res, 2010, 67: 304-308. DOI:10.1203/PDR.0b013e3181cbd57b |
| [28] |
盖迎利, 徐明, 白燕, 吕志珍, 张幼怡, 李昭屏. SD大鼠生长发育期心脏结构及功能的超声心动图评价[J]. 中国分子心脏病学杂志, 2008, 8: 12-16. |
| [29] |
ZHANG Q L, CAO Y G, LUO Q, WANG P, SHI P L, SONG C, et al. The transient receptor potential vanilloid-3 regulates hypoxia-mediated pulmonary artery smooth muscle cells proliferation via PI3K/AKT signaling pathway[J/OL]. Cell Prolif, 2018, 51: e12436. DOI: 10.1111/cpr.12436.
|
| [30] |
WILSON C P, WARD M, MCNULTY H, STRAIN J J, TROUTON T G, HORIGAN G, et al. Riboflavin offers a targeted strategy for managing hypertension in patients with the MTHFR 677TT genotype: a 4-y follow-up[J]. Am J Clin Nutr, 2012, 95: 766-772. DOI:10.3945/ajcn.111.026245 |
| [31] |
HASHMI-HILL M P, SANDOCK K, BATES J N, ROBERTSON T P, LEWIS S J. Flavin adenine dinucleotide may release preformed stores of nitrosyl factors from the vascular endothelium of conscious rats[J]. J Cardiovasc Pharmacol, 2007, 50: 142-154. DOI:10.1097/FJC.0b013e31805c1646 |
| [32] |
LUCAS T G, HENRIQUES B J, RODRIGUES J V, BROSS P, GREGERSEN N, GOMES C M. Cofactors and metabolites as potential stabilizers of mitochondrial acyl-Co A dehydrogenases[J]. Biochim Biophys Acta, 2011, 1812: 1658-1663. DOI:10.1016/j.bbadis.2011.09.009 |
 2022, Vol. 43
2022, Vol. 43


