2. 内蒙古医科大学研究生院,呼和浩特 010058;
3. 内蒙古医科大学附属医院神经内科,呼和浩特 010059
2. Graduate School, Inner Mongolia Medical University, Hohhot 010058, Inner Mongolia Autonomous Region, China;
3. Department of Neurology, The Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010059, Inner Mongolia Autonomous Region, China
帕金森病(Parkinson’s disease,PD)是患病率仅低于阿尔茨海默病的神经系统变性疾病,严重影响患者的运动功能和生活质量[1]。多巴胺能神经元变性坏死是PD经典病理改变,Braak等[2]提出PD发展病理分期假说,为寻找临床前诊断PD的生物标志物提供了宝贵的线索。研究者们发现,线粒体功能异常、氧化应激、神经炎症及细胞凋亡均可能引起细胞丢失和异常蛋白聚集,参与PD发生和发展,因此神经炎症和细胞凋亡成为PD发病机制研究的热点[3]。
人神经母细胞瘤细胞SH-SY5Y具有多巴胺能神经元的特征,广泛用于研究PD。P2X4受体(P2X4 receptor,P2X4R)是脑内分布最广泛的P2X受体,主要在小胶质细胞、神经元及内皮细胞上表达,在神经病理性疼痛、脑缺血损伤及阿尔茨海默病的发生、发展中发挥重要作用[4]。P2X4R参与PD小胶质细胞激活、介导神经炎症反应,进而造成黑质多巴胺能神经元凋亡[5],但具体作用机制不明。
泛连接蛋白1(pannexin 1,PANX1)是重要的缝隙连接蛋白,在中枢神经系统中高表达,在脑内主要分布在皮质、纹状体、嗅球及海马等部位,参与炎症因子释放和细胞凋亡;它通过激活小胶质细胞上的P2X12受体引起小胶质细胞的吞噬作用,加速细胞死亡。Toll样受体(Toll-like receptor,TLR)2是TLR家族的重要成员,在神经系统中广泛表达,主要集中于小胶质细胞和星型胶质细胞,参与神经炎症反应。P2X4R、PANX1和TLR2均主要分布在小胶质细胞并参与神经炎症和细胞凋亡,三者是否协同参与PD的发生目前尚未见相关报道。
为此,本研究采用6-羟基多巴胺(6-hydroxy-dopamine,6-OHDA)制备PD SH-SY5Y细胞模型,并沉默P2X4R,通过改变PANX1和TLR2表达研究caspase 3及cleaved caspase 3蛋白表达的变化、检测细胞活性和凋亡情况,探讨P2X4R在PD细胞模型中的作用和途径,为治疗PD寻找新的靶点。
1 材料和方法 1.1 细胞及主要试剂、仪器人神经母细胞瘤细胞SH-SY5Y(货号AC217,上海泽叶生物科技有限公司);全反式视黄酸(all-trans-retinoic acid,atRA;货号R2625,美国Sigma公司);FBS(货号10099141,美国Gibco公司);6-OHDA(货号H4381,100 mg;美国Sigma公司);P2X4R siRNA(上海吉玛制药技术有限公司);CCK-8检测试剂盒(货号C008-3,上海七海复泰生物科技有限公司);caspase 3活性检测试剂盒(货号BC3830,北京索莱宝科技有限公司);PANX1抗体(货号91137,100 µL;美国CST公司);P2X4R抗体(货号ab77434,100 µL;英国Abcam公司);TLR2抗体(货号ab213676,100 µL;英国Abcam公司);caspase 3抗体(货号9662,100 µL;美国CST公司);cleaved caspase 3抗体(货号9661,100 µL;美国CST公司);反转录试剂盒(货号K1622,美国Thermo公司);细胞凋亡检测试剂盒(货号A005-3,上海七海复泰生物科技有限公司)。
酶标仪(型号iMark,美国Bio-Rad公司);超微量分光光度计(型号DS-11,美国DeNovix公司);实时荧光定量PCR仪(型号MyGo Pro,英国IT-IS公司);化学发光凝胶成像一体机(型号WSE-6100,日本ATTO公司)。
1.2 构建PD细胞模型将SH-SY5Y细胞铺于96孔板(1×105/孔),用终浓度为10 µmol/L的atRA处理细胞4 d,将细胞形态从三角形诱导成长梭形。处理组分别更换含50、100和150 µmol/L 6-OHDA的培养基,继续培养24 h;细胞培养基更换为无血清培养基,并设置不含细胞的空白对照,在培养箱继续孵育1 h,用酶标仪检测450 nm处的光密度(D)值,CCK-8法检测细胞存活率。
1.3 检测细胞中P2X4R的表达将SH-SY5Y细胞铺于6孔板(1×106/孔),用atRA进行细胞诱导,然后分别用50、100和150 µmol/L 6-OHDA处理,胰酶消化并收集细胞后分别进行P2X4R蛋白和mRNA水平检测。P2X4R蛋白检测采用蛋白质印迹法,先提取细胞总蛋白,蛋白凝胶电泳后转膜,封闭后分别加入一抗和二抗进行孵育,采用化学发光法在蛋白凝胶成像系统下显影。P2X4R mRNA检测采用qPCR法,首先提取样品RNA并在超微量分光光度计上测定RNA浓度和纯度,RNA反转录后进行qPCR检测。
1.4 沉默P2X4R由上海吉玛制药技术有限公司合成3种P2X4R siRNA,分别为P2X4R siRNA-540、P2X4R siRNA-792和P2X4R siRNA-1401。确定转染条件为转染试剂3 μL+siRNA 6 μL,将诱导后的细胞进行转染并分组,更换正常完全培养基继续培养24 h,收集细胞,采用蛋白质印迹法和qPCR法分别检测细胞中P2X4R蛋白和mRNA的表达。
1.5 检测P2X4R沉默及P2X4R抑制剂对PD模型细胞的影响将SH-SY5Y细胞铺于96孔板(1×105/孔)并用终浓度为10 µmol/L的atRA进行细胞诱导,细胞分组转染或加入P2X4R抑制剂三羰基二氯代钌(Ⅱ)二聚物[tricarbonyldichlororuthenium (Ⅱ) dimer,CORM2](288144-500 mg,美国Sigma公司)并造模,采用CCK-8法检测细胞存活率,通过流式细胞术检测细胞凋亡情况,用蛋白质印迹法检测细胞中caspase 3、cleaved caspase 3和PANX1的表达。
1.6 检测TLR2过表达对PD模型细胞的影响将SH-SY5Y细胞铺于96孔板(1×105/孔),用终浓度为10 µmol/L的atRA进行细胞诱导,转染试剂3 μL+siRNA 6 μL对转染组转染后进行细胞造模,胰酶消化并收集细胞。细胞分为3组:PD+P2X4R siRNA(P2X4R沉默组)、PD+P2X4R siRNA+pCMV3-NCV(空质粒对照组)和PD+P2X4R siRNA+pCMV3-TLR2(TLR2过表达组),采用CCK-8法检测细胞存活率,通过流式细胞术检测细胞凋亡情况,用蛋白质印迹法检测细胞中caspase 3和cleaved caspase 3的表达。
1.7 统计学处理应用SPSS 19.0软件进行统计学分析。符合正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验,3组及以上组间比较采用单因素方差分析并通过Bonferroni法进行多重比较。检验水准(α)为0.05。
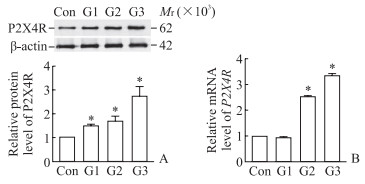
2 结果 2.1 PD模型细胞中P2X4R蛋白和mRNA表达水平结果显示,50、100和150 µmol/L 6-OHDA处理的SH-SY5Y细胞中P2X4R蛋白表达水平均高于对照组,且呈剂量依赖性(P均<0.05,图 1A);100 µmol/L和150 µmol/L 6-OHDA组P2X4R mRNA表达水平高于对照组(P均<0.05,图 1B)。
|
图 1 不同浓度6-OHDA对SH-SY5Y细胞中P2X4R蛋白(A)和mRNA(B)表达的影响 Fig 1 Effect of different concentrations of 6-OHDA on the expression of P2X4R protein (A) and mRNA (B) in SH-SY5Y cells *P < 0.05 vs control (Con) group. n=3, x±s. G1: 50 µmol/L 6-OHDA; G2: 100 µmol/L 6-OHDA; G3: 150 µmol/L 6-OHDA. 6-OHDA: 6-hydroxydopamine; P2X4R: P2X4 receptor. |
2.2 P2X4R沉默细胞系的筛选
P2X4R siRNA-540、P2X4R siRNA-792和P2X4R siRNA-1401组SH-SY5Y细胞中P2X4R蛋白和mRNA的表达水平均低于对照组(P均<0.05),其中P2X4R siRNA-540组表达水平最低,沉默效果最好(图 2)。故选择P2X4R siRNA-540用于后续实验。

|
图 2 P2X4R沉默后SH-SY5Y细胞中P2X4R蛋白(A)和mRNA(B)的表达 Fig 2 Expression of P2X4R protein (A) and mRNA (B) in SH-SY5Y cells after P2X4R silencing *P < 0.05 vs control (Con) group. n=3, x±s. G1: P2X4R siRNA-540; G2: P2X4R siRNA-792; G3: P2X4R siRNA-1401. P2X4R: P2X4 receptor; X-T: X-tremeGENEHP DNA transfection reagent; NC: Negative control; siRNA: Small interfering RNA. |
2.3 抑制P2X4R对PD模型细胞的影响
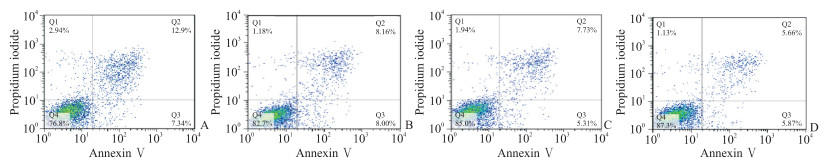
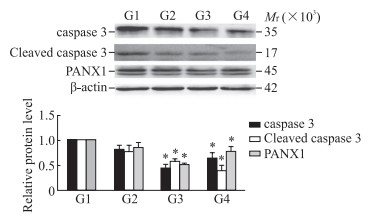
P2X4R沉默组(PD+P2X4R siRNA组)及P2X4R抑制剂组(PD+CORM2组)的细胞凋亡少于PD组(图 3),细胞存活率高于PD组[(113.03±6.81)%、(117.16±5.31)% vs(100.00±4.25)%],细胞中caspase 3、cleaved caspase 3和PANX1蛋白表达水平均低于PD组(P均<0.05,图 4)。
|
图 3 流式细胞术检测P2X4R抑制后PD模型细胞凋亡情况 Fig 3 Apoptosis of PD model cells after P2X4R inhibition detected by flow cytometry A: PD group; B: PD+NC group; C: PD+P2X4R siRNA group; D: PD+CORM2 group. P2X4R: P2X4 receptor; PD: Parkinson's disease; NC: Negative control; siRNA: Small interfering RNA; CORM2: Tricarbonyldichlororuthenium (Ⅱ) dimer. |
|
图 4 蛋白质印迹法检测P2X4R抑制后PD模型细胞中caspase 3、cleaved caspase 3和PANX1蛋白的表达 Fig 4 Expression of caspase 3, cleaved caspase 3 and PANX1 proteins in PD model cells detected by Western blotting after P2X4R inhibition *P < 0.05 vs G1 group. n=3, x±s. G1: PD; G2: PD+NC; G3: PD+P2X4R siRNA; G4: PD+CORM2. P2X4R: P2X4 receptor; PD: Parkinson's disease; caspase: Cysteine aspartic acid specific protease; PANX1: Pannexin 1; NC: Negative control; siRNA: Small interfering RNA; CORM2: Tricarbonyldichlororuthenium (Ⅱ) dimer. |
2.4 改变TLR2蛋白表达对PD模型细胞的影响
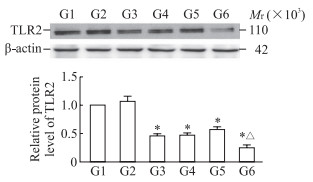
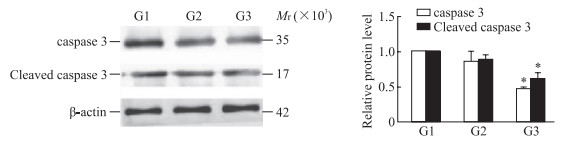
P2X4R沉默组和P2X4R抑制剂组细胞中TLR2蛋白的表达水平均低于PD组(P均<0.05);PANX1过表达后细胞中TLR2蛋白表达水平低于P2X4R沉默组(P<0.05),见图 5。TLR2过表达后细胞存活率增高[(101.75±8.10)% vs(113.40±6.81)%]、细胞凋亡减少[(41.10±0.71)% vs(31.80±4.38)%],细胞中caspase 3和cleaved caspase 3蛋白表达水平均降低(P均<0.05,图 6)。
|
图 5 蛋白质印迹法检测P2X4R抑制和PANX1过表达后PD模型细胞中TLR2蛋白的表达 Fig 5 Expression of TLR2 protein in PD model cells detected by Western blotting after P2X4R inhibition and PANX1 overexpression *P < 0.05 vs G1 group; △P < 0.05 vs G3 group. n=3, x±s. G1: PD; G2: PD+NC; G3: PD+P2X4R siRNA; G4: PD+CORM2; G5: PD+P2X4R siRNA+pCMV3-NCV; G6: PD+P2X4R siRNA+pCMV3-PANX1. P2X4R: P2X4 receptor; PANX1: Pannexin 1; PD: Parkinson's disease; TLR2: Toll-like receptor 2; NC: Negative control; siRNA: Small interfering RNA; CORM2: Tricarbonyldichlororuthenium (Ⅱ) dimer; pCMV3-NCV: pCMV3-untagged negative control vector. |
|
图 6 蛋白质印迹法检测TLR2过表达后PD模型细胞中caspase 3和cleaved caspase 3蛋白的表达 Fig 6 Expression of caspase 3 and cleaved caspase 3 proteins in PD model cells detected by Western blotting after TLR2 overexpression *P < 0.05 vs G1 group. n=3, x±s. G1: PD+P2X4R siRNA; G2: PD+P2X4R siRNA+pCMV3-NCV; G3: PD+P2X4R siRNA+pCMV3-TLR2. TLR2: Toll-like receptor 2; PD: Parkinson's disease; caspase: Cysteine aspartic acid specific protease; P2X4R: P2X4 receptor; siRNA: Small interfering RNA; pCMV3-NCV: pCMV3-untagged negative control vector. |
3 讨论
我国已逐步进入老龄化社会,PD患病率和患病人数均呈现逐年增加趋势。研究证实P2X4R间接参与多巴胺神经传递[6-7],直接调节多巴胺水平,影响多巴胺的合成和转运,并增加多巴胺受体水平,改变多巴胺能神经元功能和神经传递[5]。神经炎症是PD发病机制的研究热点,小胶质细胞在神经炎症中发挥着极为重要的作用[8]。既往研究发现P2X受体参与多种神经系统疾病的发生和发展[9-10],P2X4R在中枢神经系统炎症及阿尔茨海默病中发挥作用[4, 11]。PD和阿尔茨海默病同属于神经系统变性疾病,推测P2X4R可能在PD中发挥重要作用[7]。本实验发现,6-OHDA诱导的PD细胞模型的P2X4R蛋白和mRNA表达水平均高于对照组,且呈剂量依赖性,表明P2X4R可能参与PD神经细胞的变性坏死。本实验筛选P2X4R沉默效果最好的细胞并检测细胞活性和凋亡情况,结果显示P2X4R沉默后细胞活性明显提高、细胞凋亡减少,说明P2X4R影响了PD模型细胞的活性和凋亡。
caspase是生物体内重要的蛋白酶,参与神经炎症和细胞凋亡,其中caspase 3是执行细胞凋亡最重要的蛋白[12]。魏丽萍等[13]研究发现,P2X4R过表达会导致PD大鼠脑黑质中IL-1β及α-突触核蛋白表达上调,增加多巴胺能神经元的坏死。本实验结果显示,P2X4R沉默后caspase 3和cleaved caspase 3蛋白表达水平降低、细胞凋亡减少,说明P2X4R可能通过调控caspase 3蛋白而影响了PD模型细胞的活性和凋亡,其具体作用途径有待进一步研究。
PANX1是在神经系统具有较高表达的缝隙连接蛋白泛连接蛋白家族重要成员[14-15],有研究发现,星形胶质细胞上PANX1通道能够控制ATP跨膜转运及调节Ca2+内流,参与神经细胞能量代谢,影响星形胶质细胞激活[16],还能与P2X受体形成复合体参与神经炎症和细胞凋亡[17-18]。PANX1与P2X7受体和caspase 1构成炎性复合物,可通过抑制IL-1等炎症因子释放减少细胞凋亡[19]。Wilkaniec等[20]用外源α-突触核蛋白处理神经突触小体,发现激活的P2X7受体与PANX1结合并通过降低ATP活性影响细胞能量代谢。本实验也发现,P2X4R沉默后PANX1蛋白表达降低,提示其在PD发病中可能发挥重要作用。
与P2X4R和PANX1相同,TLR2在中枢神经系统广泛表达,尤其是在小胶质细胞和星形胶质细胞,而小胶质细胞是神经炎症的主要效应细胞[21]。Parzych等[18]研究发现,在人类单核细胞中TLR2和TLR4均可诱导IL-1β释放,而TLR2需要PANX1和P2X7受体激活以刺激IL-1β释放,但它们激活caspase 1的机制有所不同。Dunton等[22]用P2X受体激活caspase 1后发现,压力诱导的尿路上皮细胞通过P2X7受体激发ATP释放,进而激活P2X4R,后者介导caspase 1的炎症反应。有研究证实PANX1与P2X4R形成复合体参与细胞能量代谢和神经炎症,推测三者之间可能通过某种联系共同影响细胞能量代谢和凋亡[23]。本实验结果表明,6-OHDA处理细胞后P2X4R表达水平升高,P2X4R表达下调后PANX1蛋白表达水平也降低,进一步过表达PANX1后TLR2蛋白表达下降,过表达TLR2后caspase 3蛋白表达降低、细胞凋亡减少、细胞活性增高。当P2X4R沉默后,TLR2蛋白表达低于PD组,提示细胞凋亡增多、细胞活性降低,推测P2X4R可能通过影响TLR2蛋白表达而影响细胞凋亡,PANX1和TLR2可能是P2X4R影响细胞活性和凋亡的靶点与途径[24]。
本实验通过PD细胞模型观察caspase 3、PANX1和TLR2蛋白表达,研究P2X4R的作用和途径,结果显示P2X4R通过调控PANX1和TLR2蛋白的表达影响细胞凋亡和细胞活性,为寻找PD新的治疗靶点提供了依据。
| [1] |
PRINGSHEIM T, JETTE N, FROLKIS A, STEEVES T D L. The prevalence of Parkinson's disease: a systematic review and meta-analysis[J]. Mov Disord, 2014, 29: 1583-1590. DOI:10.1002/mds.25945 |
| [2] |
BRAAK H, DEL TREDICI K, RüB U, DE VOS R A I, JANSEN STEUR E N H, BRAAK E. Staging of brain pathology related to sporadic Parkinson's disease[J]. Neurobiol Aging, 2003, 24: 197-211. DOI:10.1016/S0197-4580(02)00065-9 |
| [3] |
PRADHAN S, ANDREASSON K. Commentary: Progressive inflammation as a contributing factor to early development of Parkinson's disease[J]. Exp Neurol, 2013, 241: 148-155. DOI:10.1016/j.expneurol.2012.12.008 |
| [4] |
BURNSTOCK G, KENNEDY C. P2X receptors in health and disease[J]. Adv Pharmacol, 2011, 61: 333-372. |
| [5] |
KHOJA S, SHAH V, GARCIA D, ASATRYAN L, JAKOWEC M W, DAVIES D L. Role of purinergic P2X4 receptors in regulating striatal dopamine homeostasis and dependent behaviors[J]. J Neurochem, 2016, 139: 134-148. DOI:10.1111/jnc.13734 |
| [6] |
XIAO C, ZHOU C Y, LI K X, DAVIES D L, YE J H. Purinergic type 2 receptors at GABAergic synapses on ventral tegmental area dopamine neurons are targets for ethanol action[J]. J Pharmacol Exp Ther, 2008, 327: 196-205. DOI:10.1124/jpet.108.139766 |
| [7] |
KRüGEL U, KITTNER H, FRANKE H, ILLES P. Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo[J]. Synapse, 2003, 47: 134-142. DOI:10.1002/syn.10162 |
| [8] |
FAHMY A M, BOULAIS J, DESJARDINS M, MATHEOUD D. Mitochondrial antigen presentation: a mechanism linking Parkinson's disease to autoimmunity[J]. Curr Opin Immunol, 2019, 58: 31-37. DOI:10.1016/j.coi.2019.02.004 |
| [9] |
YAMAMOTO K, SOKABE T, MATSUMOTO T, YOSHIMURA K, SHIBATA M, OHURA N, et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice[J]. Nat Med, 2006, 12: 133-137. DOI:10.1038/nm1338 |
| [10] |
SAVIO L E B, DE ANDRADE MELLO P, DA SILVA C G, COUTINHO-SILVA R. The P2X7 receptor in inflammatory diseases: angel or demon? [J/OL]. Front Pharmacol, 2018, 9: 52. DOI: 10.3389/fphar.2018.00052.
|
| [11] |
陈汉泽, 田力, 薛维爽, 滕伟禹. 阿尔茨海默病中炎症反应的研究进展[J]. 中国临床神经科学, 2017, 25: 342-347. DOI:10.3969/j.issn.1008-0678.2017.03.019 |
| [12] |
SHALINI S, DORSTYN L, DAWAR S, KUMAR S. Old, new and emerging functions of caspases[J]. Cell Death Differ, 2015, 22: 526-539. DOI:10.1038/cdd.2014.216 |
| [13] |
魏丽萍, 薛莉, 许文帅, 谢安木. P2X4R过表达对帕金森病模型鼠脑黑质中白介素-1β、α突触核蛋白及多巴胺能神经元的影响[J]. 中国临床神经科学, 2019, 27: 617-623. |
| [14] |
IGLESIAS R, DAHL G, QIU F, SPRAY D C, SCEMES E. Pannexin 1: the molecular substrate of astrocyte "hemichannels"[J]. J Neurosci, 2009, 29: 7092-7097. DOI:10.1523/JNEUROSCI.6062-08.2009 |
| [15] |
YEUNG A K, PATIL C S, JACKSON M F. Pannexin-1 in the CNS: emerging concepts in health and disease[J]. J Neurochem, 2020, 154: 468-485. DOI:10.1111/jnc.15004 |
| [16] |
DíAZ E F, LABRA V C, ALVEAR T F, MELLADO L A, INOSTROZA C A, OYARZúN J E, et al. Connexin 43 hemichannels and pannexin-1 channels contribute to the α-synuclein-induced dysfunction and death of astrocytes[J]. Glia, 2019, 67: 1598-1619. |
| [17] |
SANDILOS J K, CHIU Y H, CHEKENI F B, ARMSTRONG A J, WALK S F, RAVICHANDRAN K S, et al. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region[J]. J Biol Chem, 2012, 287: 11303-11311. DOI:10.1074/jbc.M111.323378 |
| [18] |
PARZYCH K, ZETTERQVIST A V, WRIGHT W R, KIRKBY N S, MITCHELL J A, PAUL-CLARK M J. Differential role of pannexin-1/ATP/P2X7 axis in IL-1βrelease by human monocytes[J]. FASEB J, 2017, 31: 2439-2445. DOI:10.1096/fj.201600256 |
| [19] |
SILVERMAN W R, DE RIVERO VACCARI J P, LOCOVEI S, QIU F, CARLSSON S K, SCEMES E, et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes[J]. J Biol Chem, 2009, 284: 18143-18151. DOI:10.1074/jbc.M109.004804 |
| [20] |
WILKANIEC A, GĄSSOWSKA M, CZAPSKI G A, CIEŚLIK M, SULKOWSKI G, ADAMCZYK A. P2X7 receptor-pannexin 1 interaction mediates extracellular alpha-synuclein-induced ATP release in neuroblastoma SH-SY5Y cells[J]. Purinergic Signal, 2017, 13: 347-361. DOI:10.1007/s11302-017-9567-2 |
| [21] |
WANG Y C, WANG P F, FANG H, CHEN J, XIONG X Y, YANG Q W. Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury[J]. Stroke, 2013, 44: 2545-2552. DOI:10.1161/STROKEAHA.113.001038 |
| [22] |
DUNTON C L, PURVES J T, HUGHES F M Jr, JIN H X, NAGATOMI J. Elevated hydrostatic pressure stimulates ATP release which mediates activation of the NLRP3 inflammasome via P2X4 in rat urothelial cells[J]. Int Urol Nephrol, 2018, 50: 1607-1617. DOI:10.1007/s11255-018-1948-0 |
| [23] |
周金凤, 覃朝燕, 赖炜明, 杜昌升. 嘌呤P2受体的免疫调节功能[J]. 中国细胞生物学学报, 2015, 37: 1151-1157. DOI:10.11844/cjcb.2015.08.0125 |
| [24] |
JURGA A M, PIOTROWSKA A, MAKUCH W, PRZEWLOCKA B, MIKA J. Blockade of P2X4 receptors inhibits neuropathic pain-related behavior by preventing MMP-9 activation and, consequently, pronociceptive interleukin release in a rat model[J/OL]. Front Pharmacol, 2017, 8: 48. DOI: 10.3389/fphar.2017.00048.
|
 2022, Vol. 43
2022, Vol. 43


